Professional Documents
Culture Documents
Evans Christian Rosal - Gen Chem Finals
Evans Christian Rosal - Gen Chem Finals
Uploaded by
Evans Christian Velasquez RosalOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Evans Christian Rosal - Gen Chem Finals
Evans Christian Rosal - Gen Chem Finals
Uploaded by
Evans Christian Velasquez RosalCopyright:
Available Formats
I.
II. Give some applications of the following chemical reactions.
a. Decomposition Reaction
1. Photographic films have a coating of silver bromide, which on exposure to light
splits into silver and bromine.
2. While baking a cake with baking powder (sodium bicarbonate), decomposition
leads to the formation of carbon dioxide and sodium hydroxide.
3. When a soda bottle is opened, carbonic acid breaks down to produce water
and carbon dioxide, which causes the fizz.
b. Double replacement reaction
1. As a cleaning agent, especially to clean the water disposal pipes in the
kitchen and bathroom sinks.
2. Can be collected and used as a chemical fire extinguisher
3. To make a chemical volcano.
III. Give the application of the following gas laws and explain Boyle’s Law or
Charles’ Law behind it.
a. Boyle’s Law
1. Deep Sea Fish– The average depth of oceans is around 3000 meters. At
such depths, new lifeforms are evolved. The pressure at such depths is
tremendous, which makes a normal life survival impossible. But deep-sea
creatures are evolved and get accustomed to such harsh environments.
When these lifeforms are brought to the surface of oceans, relatively low-
pressure environment, the gases inside their bodies will expand as per
Boyle's law, and they immediately collapse.
The same is true for us. If a human being is dropped into oceans, its body will
be crushed by the external pressure at great depths.
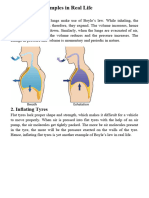
2. Bicycle Pump – A hand bicycle pump works similar to a syringe. When the
handle of a pump is pushed down, the pressure inside the pump will increase
momentarily. In other words, the gas inside is compressed. As a result, the
pressurized gas is forced inside the tire of a vehicle.
3.The bends – The bends are decompression sickness experienced by scuba
divers when they ignore Boyle's law. As a diver dives deep inside water, the
surrounding pressure of the water increases. The high pressures increase the
solubility of the bodily gases in the blood of the diver.
Note: Every 10 meters of depth, pressure increases by 1 atm or 14.7 psi.
The deeper a diver goes, the more the quantity of gases dissolves inside the
blood of the diver. When he/she ascends, the dissolved gases start
expanding since the pressure gets lower. A well-trained diver will always
ascend slowly. But if he/she somehow makes a rapid ascent, the diver will
suffer in severe pain. The gases in the body of the diver will expand quickly,
making the blood a foamy solution. Moreover, the gases between joints will
also expand, which will cause the diver's body to blend. This sometimes can
be a life-threatening condition.
b. Charles’ Law
1. Bakery – Charle’s Law finds its way into our kitchens as well. In case you
have ever tried your hand at baking, you might be familiar with the substance
most commonly used in cooking, i.e., the yeast. Yeast is often used in baking
to make the bakery products fluffy. Yeast is responsible for releasing carbon
dioxide bubbles. These carbon dioxide bubbles expand further with high
temperature. The expansion of the carbon dioxide bubbles with an increase in
temperature works as a leavening agent and cause the bakery products to
become fluffy.
2. Hot Air Balloon – You might have wondered about the working of the hot
air balloon. Charle’s Law describes that temperature and volume are directly
proportional to each other. When a gas is heated, it expands. As the
expansion of the gas takes place, it becomes less dense and the balloon is
lifted in the air. The warm is less dense than the cold air, which means that it
is lighter than the cold air. Also, the warm air has less mass per unit volume.
3. Deodorant Spray Bottle – If you get a chance to read the instructions on a
bottle of deodorant, you might have read the warning signs indicating the
bottle to be kept away from the sunlight and high temperature. Ever wondered
why? The answer lies in Charle’s Law. Under high temperatures, the air
molecules inside the bottle will expand which can lead to the bursting of the
deodorant bottle.
You might also like
- Dat Test Sample ItemsDocument67 pagesDat Test Sample ItemsKickHannaNo ratings yet
- Is 383 - 2016Document21 pagesIs 383 - 2016L V Laxmipathi Rao83% (40)
- Chemicals and Chemical Processes in Forensic StudiesDocument19 pagesChemicals and Chemical Processes in Forensic StudiesFrooti Dubey86% (7)
- Method Statement For Installation of Water Spray Deluge System - LPG TankDocument13 pagesMethod Statement For Installation of Water Spray Deluge System - LPG Tankpunk cm100% (2)
- SWMS 001 Drilling Soil BoresDocument3 pagesSWMS 001 Drilling Soil BoresEngr Faheem AkhtarNo ratings yet
- Introduction To Openhole Log InterpretationDocument208 pagesIntroduction To Openhole Log InterpretationFrancisco de la Cruz100% (2)
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Scuba Diving and Gas LawDocument17 pagesScuba Diving and Gas LawTie Ing HuiNo ratings yet
- Uses and Applications of The Different Gas LawsDocument8 pagesUses and Applications of The Different Gas LawsWin7Addict100% (30)
- Boyles LawDocument1 pageBoyles LawRevilloNo ratings yet
- Ocean Acidification LabDocument4 pagesOcean Acidification Labapi-237699934No ratings yet
- Gas Laws Form 4 Exercise and ExamplesDocument6 pagesGas Laws Form 4 Exercise and ExampleszahidahoseinNo ratings yet
- Laboratory Activity 6 Analysis of Anions - Carbonate/ BicarbonateDocument2 pagesLaboratory Activity 6 Analysis of Anions - Carbonate/ BicarbonateNikoh Anthony EwayanNo ratings yet
- GAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidDocument9 pagesGAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidMichiiee BatallaNo ratings yet
- AsignmentUlo2aBCHE 1Document5 pagesAsignmentUlo2aBCHE 1Mikaela AstaNo ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- SCRAPBOOKDocument19 pagesSCRAPBOOKJulius Michael Guinto100% (1)
- If You Play-WPS OfficeDocument1 pageIf You Play-WPS Officeawitg031No ratings yet
- 3 Applications of Boyle S, Charle S and Gay-Lussac S LawDocument7 pages3 Applications of Boyle S, Charle S and Gay-Lussac S LawJeric MaribaoNo ratings yet
- Candle Experiment UnmaskedDocument13 pagesCandle Experiment UnmaskedVijay TrivediNo ratings yet
- Elephant ToothpasteDocument4 pagesElephant ToothpasteAmin DeanNo ratings yet
- Applications of Charles LawDocument2 pagesApplications of Charles LawHelmaNo ratings yet
- Week5 AbcdDocument7 pagesWeek5 AbcdCookie MonsterNo ratings yet
- Chemistry Signature Assignment and ReflectionDocument4 pagesChemistry Signature Assignment and Reflectionapi-291557491No ratings yet
- Carbon Dioxide-GLOBAL WARMING-FACTS AND PREDICTIONSDocument1 pageCarbon Dioxide-GLOBAL WARMING-FACTS AND PREDICTIONSNishant SainiNo ratings yet
- Thermody Lab About Egg Chuchu PDFDocument4 pagesThermody Lab About Egg Chuchu PDFGievel Enoroba LopezNo ratings yet
- BoyleDocument1 pageBoylePASTEURSYNCH GELBOLINGO, ERNIE IINo ratings yet
- CHEM II-MatterDocument10 pagesCHEM II-MatterAlyssa Jana Meneses TonogbanuaNo ratings yet
- Gas Lab With QuestionsDocument3 pagesGas Lab With Questionsallan oparaNo ratings yet
- 12 Rousseau Week5 AbcdDocument6 pages12 Rousseau Week5 AbcdCookie MonsterNo ratings yet
- Plants Which Store Food in Roots Are: Carrot and ReddishDocument3 pagesPlants Which Store Food in Roots Are: Carrot and ReddishJyoti GabaNo ratings yet
- Applications of Gas LawsDocument3 pagesApplications of Gas LawsCharm GorospeNo ratings yet
- CO2 SolubilityDocument4 pagesCO2 SolubilityGlory Kolade (gg)No ratings yet
- Revised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarDocument11 pagesRevised Module 10 Boyles and Charles Law Ma. Lourdes B. AguilarSaysain UkayNo ratings yet
- Gas Laws Opt OutDocument39 pagesGas Laws Opt OutTahir HussainNo ratings yet
- Basic Principles of ExplosivesDocument37 pagesBasic Principles of ExplosivesMaximiliano Muñoz García100% (1)
- Boyles Law CompleteDocument4 pagesBoyles Law CompleteLincoln PiaoanNo ratings yet
- Real Life Application of Boyles LawDocument12 pagesReal Life Application of Boyles LawChelsie Rose GalangcoNo ratings yet
- Changes in Dissolved Oxygen Due To Salinity, Temperature, and LightDocument8 pagesChanges in Dissolved Oxygen Due To Salinity, Temperature, and Lightapi-300732604No ratings yet
- Behavior of GasesDocument44 pagesBehavior of Gasesapi-668571149No ratings yet
- Chem Pt..Document10 pagesChem Pt..Anne Paulene SobretodoNo ratings yet
- Science of Diving: How Science Helps Navy Divers Stay Safe Science Topic: Physiology and PhysicsDocument35 pagesScience of Diving: How Science Helps Navy Divers Stay Safe Science Topic: Physiology and PhysicsFernando Cadena DuqueNo ratings yet
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Air Around Us NBW (1) - Converted - 230124 - 225639Document5 pagesAir Around Us NBW (1) - Converted - 230124 - 225639KAMAKSHI DHIMANNo ratings yet
- Exercise 2-AnswerDocument3 pagesExercise 2-AnswerleorozarioooNo ratings yet
- D I V I N G P H y S I C S A N D C H e M I S T R yDocument23 pagesD I V I N G P H y S I C S A N D C H e M I S T R yErnad HusremovićNo ratings yet
- (Download PDF) Oops Pounce Quick Run An Alphabet Caper Mike Twohy Ebook Online Full ChapterDocument53 pages(Download PDF) Oops Pounce Quick Run An Alphabet Caper Mike Twohy Ebook Online Full Chaptercupintamre100% (4)
- Science Form 1-Chapter 3Document37 pagesScience Form 1-Chapter 3Mohamad Tarmizi100% (2)
- Changes That Materials UndergoDocument47 pagesChanges That Materials UndergoShiella Mariz BinotapaNo ratings yet
- Health and Safety AdviceDocument11 pagesHealth and Safety AdviceacobosaNo ratings yet
- Water and The Aquatic EnvironmentDocument30 pagesWater and The Aquatic EnvironmentRacine CariahNo ratings yet
- Charles LawDocument12 pagesCharles LawLerr Real RelleNo ratings yet
- Photosynthesis and The Carbon Cycle HA&S 220a: C H O + 6O - 6CO 6H ODocument4 pagesPhotosynthesis and The Carbon Cycle HA&S 220a: C H O + 6O - 6CO 6H OSpecsy GuyNo ratings yet
- Siyensikula-Boyle's LawDocument3 pagesSiyensikula-Boyle's Lawrolando dumlaoNo ratings yet
- Carbon: Building Block and Fuel SourceDocument4 pagesCarbon: Building Block and Fuel SourceJL V. AdrianoNo ratings yet
- Excerpt From "Storm in A Teacup" by Helen Czerski.Document25 pagesExcerpt From "Storm in A Teacup" by Helen Czerski.OnPointRadio100% (1)
- Scanned TBK Notes - L2-Oxides of Carbon - Co2, Co - L3-Carbon N Its Allotropes - Compounds - 2021-2022Document21 pagesScanned TBK Notes - L2-Oxides of Carbon - Co2, Co - L3-Carbon N Its Allotropes - Compounds - 2021-2022Nitya RamchandaniNo ratings yet
- Pages From Glencoe - Chemistry - Matter and Change Mcgraw 2008 CH 1Document26 pagesPages From Glencoe - Chemistry - Matter and Change Mcgraw 2008 CH 1api-261034721No ratings yet
- C01 - Introduction To Chemistry PDFDocument28 pagesC01 - Introduction To Chemistry PDF喜助浦原No ratings yet
- Applications of Boyle's LaawDocument2 pagesApplications of Boyle's Laawcherrymaeregalario2001No ratings yet
- Dơnload Shadow Reaper 1st Edition Gimpel Ann Full ChapterDocument24 pagesDơnload Shadow Reaper 1st Edition Gimpel Ann Full Chaptersommeianciha100% (2)
- Submarine Air TreatmentDocument16 pagesSubmarine Air Treatmentprabhuarunkumar85No ratings yet
- Evans Christian Rosal - Energy Sources-SummativeDocument2 pagesEvans Christian Rosal - Energy Sources-SummativeEvans Christian Velasquez RosalNo ratings yet
- Performance Task Energy Action Plan FinalDocument11 pagesPerformance Task Energy Action Plan FinalEvans Christian Velasquez RosalNo ratings yet
- Chapter 3-Chapter TestDocument1 pageChapter 3-Chapter TestEvans Christian Velasquez RosalNo ratings yet
- Evans Christian Rosal - Untitled DocumentDocument2 pagesEvans Christian Rosal - Untitled DocumentEvans Christian Velasquez RosalNo ratings yet
- Product MakingDocument10 pagesProduct MakingEvans Christian Velasquez RosalNo ratings yet
- Performance Task (Decomposition Reaction)Document3 pagesPerformance Task (Decomposition Reaction)Evans Christian Velasquez RosalNo ratings yet
- Bloombox Technology PDFDocument8 pagesBloombox Technology PDFImran KhanNo ratings yet
- Sa 213Document11 pagesSa 213gst ajahNo ratings yet
- Interatomic Bonding: Chapter 2 OutlineDocument27 pagesInteratomic Bonding: Chapter 2 OutlineAdrian G. ClaritoNo ratings yet
- Mechanical Design of Overhead Lines PDFDocument70 pagesMechanical Design of Overhead Lines PDFEng-Ahmad Abo-AledousNo ratings yet
- Clearfire - Model CFV: 9.5 - 60 HP Steam Vertical BoilerDocument39 pagesClearfire - Model CFV: 9.5 - 60 HP Steam Vertical Boilerfauzi endraNo ratings yet
- Black Pepper PDFDocument13 pagesBlack Pepper PDFssimisimi100% (5)
- Chapter 3 Plane TrussDocument15 pagesChapter 3 Plane TrussKishan PurohitNo ratings yet
- Design For Plastics Unit 7Document10 pagesDesign For Plastics Unit 7Harinath GowdNo ratings yet
- ROXOL ProposalDocument9 pagesROXOL ProposalAldren Delina RiveraNo ratings yet
- DWSIM Abstract PDFDocument3 pagesDWSIM Abstract PDFredagalihNo ratings yet
- Fire - Fighting Training Manual-GtsDocument27 pagesFire - Fighting Training Manual-GtsNoor Muddassir Khan80% (5)
- قائمة الأدوية الغير وصفيةDocument6 pagesقائمة الأدوية الغير وصفيةDr HbNo ratings yet
- Arsenic Contamination in Groundwater 2.0Document12 pagesArsenic Contamination in Groundwater 2.0Thao LyNo ratings yet
- 43618lecture Note 1 Polymer Chemistry 6th Sem SEC 4T Chem HonsDocument28 pages43618lecture Note 1 Polymer Chemistry 6th Sem SEC 4T Chem HonsFaraj HaiderNo ratings yet
- NiO MethaneDocument13 pagesNiO Methanetrsmurf9911No ratings yet
- Advertisement: Read The Advertisement Below and Answer Questions 3 To 4Document5 pagesAdvertisement: Read The Advertisement Below and Answer Questions 3 To 4Fix Strong VerryNo ratings yet
- Callister Solutions of Ch07Document52 pagesCallister Solutions of Ch07Malika NavaratnaNo ratings yet
- Bright Bar: What We Do, Where To Find UsDocument8 pagesBright Bar: What We Do, Where To Find UsindicomNo ratings yet
- Amedeo AvogadroDocument8 pagesAmedeo AvogadroSameh AhmedNo ratings yet
- 3 Protein: Classification of Proteins Simple ProteinsDocument2 pages3 Protein: Classification of Proteins Simple ProteinsKara AshleighNo ratings yet
- Chemical 8 JuliDocument1 pageChemical 8 JuliAndhy AlfharoNo ratings yet
- Lab Report 5Document13 pagesLab Report 5Jhon CarvajalNo ratings yet
- Api RP 505 2018 Part16pdf - CompressDocument2 pagesApi RP 505 2018 Part16pdf - CompressTeknik Marina100% (1)
- Segetis ProfileDocument18 pagesSegetis ProfileSiti RamadhaniNo ratings yet