Professional Documents
Culture Documents
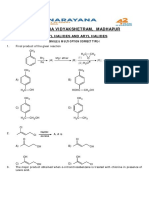
Alkanes
Alkanes
Uploaded by
QwertyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alkanes
Alkanes
Uploaded by
QwertyCopyright:
Available Formats
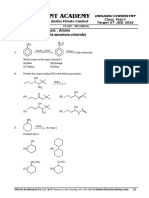
Assignment
FIITJEE ALKANES
1. Propane can be best prepare by the reaction:
Et2O HO
(A) CH3 CH2I CH3I Na (B) CH3 CH2 COONa CH3 COONa 2
Electrolysis
(C) CH3CH2Br CH3 2 CuLi
Et2O
(D) CH3 CH2CH2COONa
NaOH
CaO,
2. The reactivity of alkyl halides for Wurtz reaction is:
(A) 10 20 30 (B) 30 20 10 (C) 20 30 10 (D) 10 30 20
3. Which of the following reactions has zero activation energy?
h
(A) CH4 C CH3 HC (B) C C 2C
(C) CH3 CH3 CH3 CH3 (D) CH3 C C CH3 C C
4. Which statement is incorrect about free radical halogenation of alkanes?
(A) The number of product molecules formed by one photon is very high
(B) If O2 is added, initially the rate of reaction decreases, then it increases
(C) Inhibitors combine with free radical and terminate the chain reaction
(D) Presence of Ph C O O C Ph inhibit the free radical reaction.
O O
5.
Br2 / h
Products?
How many mono brominated products will be obtained by above reaction?
(A) 6 (B) 4 (C) 5 (D) 3
6. How many alkane of molecular weight 100 are chiral?
(A) 1 (B) 2 (C) 3 (D) 4
7. Ph CH CH2 CH3
C 2
h
Pr oducts
Fractional
Distillation
Fraction,
|
CH3
No. of products and no. of fractions are respectively:
(A) 6, 5 (B) 6, 4 (C) 5, 4 (D) 6, 3
CH
H2, Pd Products of the above reaction will be
8.
CH3
(A) Racemic mixture (B) Diastereomers (C) meso (D) Structural isomer
D
NH 2 - NH2
9. Product;
H2 O 2
D
D
H
(A) (B)
D
H
D
H
(C) (D) Both (b) and (c)
H
D
FIITJEE (Hyderabad Classes) Limited.
Assignment
FIITJEE ALKANES
H2 ( 1 Mole)
10. Product :
(A) (B)
(C) (D) None of these
11. On heating CH3COONa with soda lime the gas evolved will be
(A) C 2 H 2 (B) CH 4 (C) C 2 H 6 (D) C 2 H 4 .
12. Which of the following will have least hindered rotation about carbon-carbon bond ?
(A) Ethane (B) Ethylene (C) Acetylene (D) hexa chloro ethane.
–1
13. A sample of 1.79 mg of a compound of molar mass 90 g mol when treated with CH3Mgl releases 1.34 ml
of a gas at STP. The number of active hydrogen in the molecule is
(A) 1 (B) 2 (C) 3 (D) 4
14. What is the chief product obtained when n-butane is treated with bromine in the presence of light at 130°C ?
CH3
(A) H3C Br (B) H3C
Br
H3C CH3
Br
H3C C CH2 Br
(C) (D)
CH3 CH3
15. The treatment of C2 H5 Mgl with water produces
(A) Methane (B) Ethane (C) Ethanal (D) Ethanol.
16. Br
When isobutene is brominated, the percentage of would be
H3C CH3
CH3
(A) 0% (B) 83% (C) 10% (D) 100%
17. Which of the following is used for aromatization of n-hexane?
(A) AlCl3 (B) Na in liquid NH3
(C) Cr2O3/Al2O3 with heat (D) Wilkinson’s catalyst
18. Which of the following reactants suitable for preparation of CH 4 and ethane by using one step only
(A) CH 2 CH 2 (B) CH 3OH (C) CH 3 Br (D) CH 3 CH 2 OH
19. Which among the following alkanes cannot be prepared by reduction of alkyne:
A) Methane B) Isobutane C) Neopentane D) Ethane
20. By which of the following reagent butanoic acid can be converted into butane:
A) HI/P/ B) NaOH/CaO C) CH3MgBr D) All of these
FIITJEE (Hyderabad Classes) Limited.
Assignment
FIITJEE ALKANES
21. Which among the following compounds will give Wurtz reaction:
A) CH2 = CH - Br B) C6H5 - Br C) CH2 = CH - CH2 - Br D) None of these
22. Which among the following methods give pure alkane from alkyl halides:
A) Wurtz reaction B) Corey-House synthesis
C) Frankland reaction D) All of these
23. Which among the following is main source to get methane?
A) Wurtz reaction B) Reduction of CH2Cl2
C) Liquification of natural gas D) Kolbe electrolysis
24. What is the chief product when n-butane is treated with bromine in the presence of light at 130oC?
CH 3 CH 2 CH Br
A) CH 3 CH 2 CH 2 CH 2 Br B) |
CH 3
CH 3
CH 3 CH CH 2 Br |
C) | D) CH 3 C CH 2 Br
CH 3 |
CH 3
25. The highest boiling point is expected for
A) iso-Octane B) n-Octane C) 2,2,3,3-Trimethylbutane D) n-butane
26. The major product formed by mono bromination of Methylcyclopentane is
CH 3
|
27. CH 3 CH KMnO
4 A
|
CH 3
CH 3
CH 3 CH 3 |
A) | (B) | (C) CH 3 C OH (D) CH 3 COCH3
CH 3 CH COOH CH 3 C CH 2 |
CH 3
28. A mixture of ethyl iodide and n-propyl iodide is subjected to Wurtz reaction. The hydrocarbon which will not be
formed is
A) Butane B) Propane C) Pentane D) Hexane
29. Which of the following can be used for the preparation of propane ?
1.B2H 6 1. Mg/ ether
A) CH3CH = CH2 B) CH3CH2CH2Cl
2. CH3COOH 2. H2O
HI / / 1500 C NaOH /( CuO)
C) CH3CH2CH2I
D) CH3CH2CH2COONa
30. Which of the following reactions can be used to prepare methane ?
A) Clemmensen B) Wurtz reaction
C) Catalytic hydrogenation of methyl iodide
D) Reduction of methyl iodide by using a zinc-copper couple
FIITJEE (Hyderabad Classes) Limited.
Assignment
FIITJEE ALKANES
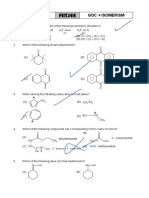
31. Which of the following compounds cannot be prepared by the Wurtz reaction ?
32. Which of the following react with Cl 2 and Br2 at room temperature and in the absence of diffused sunlight to
produce dihalogen derivatives ?
A) Cyclopropane B) Cyclobutane C) Cyclopentane D) Cyclohexane
33. Which of the reactions take place via free radicals as intermediates ?
Peroxides
A) CH3CH = CH2 + HBr
4 CCl
B) CH3CH = CH2 + Br2
Diffused
C) CH3CH2CH3 + Br2
Sun light
D) CH3CH = CH2 + HBr
34. Starting from which of the following compounds methane as well as ethane can be prepared in separate single
steps ?
A) Methyl bromide B) Sodium acetate
C) Ethyl bromide D) Ethyl magnesium bromide
35. Which of the following molecules of alkane will give only one mono halogenated product on reaction with
halogen in presence of sunlight?
(A) H2C CH3 (B) H3C CH2 CH3
(C) H3C4 C (D) CH 2
36. Which of the following methods yield saturated hydrocarbon/?
R CH CH2
BH3
(A) CH COOH (B) R CH CH2
CH2N2
3
COOH
(C) Br Br
Na / ether
(D) NaOH CaO
37. Which of the following alkanes cannot be synthesized by the Wurtz reaction in good yield?
(A) (B)
(C) (D)
38. Which of the following reactions produce the same product?
(A) Mg/ether Na/ether (B) NaOH Electrolysis
COOH
COOH
Mg/ether CH 3Br Red P HI
(C) (D)
Br HOOC
39. Which is/are true statements/reactions?
(A) Al4C3 + H2O CH4 (B) CaC2 + H2O C2H2
(C) Mg2C3 + H2O CH3C CH (D) Holme signal uses mixture of Ca3P2 and CaC2
FIITJEE (Hyderabad Classes) Limited.
Assignment
FIITJEE ALKANES
40.
O
This change can be carried out using:
(A) NH2NH2, glycol/OH– (B) Zn-Hg / conc. HCl (C) P/HI (D) NH3/LiAlH4
41. CH4 gas is also called/content of:
(A) CNG (B) Marsh gas (C) water gas (D) producer gas
PASSAGE – 1 (Q.No: 42 to 46)
Our understanding of multi step reaction is incomplete; we cannot realistically predict rates unless we know which step
is rate-determining. One experimental method for finding the rate-determining step is using isotope effects, which are
changes in reaction rates resulting from using different isotopes. The isotope most commonly used is deuterium (D),
the isotope of hydrogen with mass number 2, which has a proton and a neutron in its nucleus. Although the chemistry
of deuterium is nearly identical with that of hydrogen, bonds to deuterium are slightly stronger than bonds to hydrogen,
and the activation energy for abstraction of a deuterium atom is slightly higher than for a hydrogen atom.
42. Select correct statement(s)
If all hydrogen atoms in methane are replaced by deuterium atoms
A) rate of chlorination is decreased B) rate of chlorination is increased
C) activation energy of formation of free radical is decreased
D) activation energy of formation of free radical is increased
43. What is the product of the following reaction of single halogenation CH 2 D2 Cl2
A) CHClD2 B) CH 2 DCl C) both (A) and (B) D) None of these
44. Consider following reaction:
Cl2
CH 3 CD2 CH 3
ha
Most stable intermediate of this reaction is:
A) CH 3CD2 CH 2 B) CH C DCH C) CH 3CDCH 3 D) CH 3CDCH 2 Cl
3 | 2
Cl
45. Rate of halogenation of an alkane is in order:
A) F2 Cl2 Br2 I 2 B) Cl2 F2 Br2 I 2 C) I 2 F2 Cl2 Br2 D)
I 2 Br2 Cl2 F2
46. Consider the following reaction:
Select correct statement(s)
FIITJEE (Hyderabad Classes) Limited.
Assignment
FIITJEE ALKANES
A) A and B are formed in equal amounts
B) intermediate from which B is formed first changes to more stable 20 free radical by migration of D and
then Cl2 reacts
C) only A is formed D) Only B is formed
PASSAGE – 2 (Q.No: 47 to 50)
Chlorination of methane involves three steps: chain-initiating, chain-propagating and chain-terminating.
Cl2
Heat or light
2Cl Chain initiating
CH 4 Cl
CH 3 HCl
Chain propagating
CH 3 Cl 2
CH 3 Cl Cl
When oxygen is passed through the reaction mixture, chlorination of methane slows down temporarily
47. Chain-propagating steps
(A) consume reactive species and form another reactive species
(B) do not produce reactive species
(C) absorb energy and produce reactive species
(D) are not always the part of chain-reaction mechanism
48. Chain-terminating step may involve the formation of
(A) Chlorine (B) Methyl chloride (C) Ethane (D) All the three
49. Although chlorination of methane is an exothermic, the reaction requires high temperature because
(A) Activation energy is low (B) heat of reaction is negative
(C) Chain-initiating step is endothermic (D) Chain-terminating step is endothermic
50. Temporary slow down of chlorination of methane in presence of oxygen in due to the formation of
(A) CH3OO which is highly unstable and decomposes easily
(B) CH3OO which is less reactive than CH3
(C) ClO which is highly reactive
(D) a diradical ClO
KEY
1. C 2. A 3. C 4. D 5. D
6. D 7. A 8. B 9. C 10. B
11. B 12. D 13. C 14. B 15. B
16. D 17. C 18. C 19. A 20. A
21. C 22. B 23. C 24. B 25. B
26. D 27. C 28. A 29. A,B,C,D 30. C,D
31. B, C 32. A, B 33. A, B 34. A, D 35. A,C,D
36. A,B,C,D 37. A, C, D 38. A,B,C,D 39. A, B, C, D 40. A, B, C
41. A, B 42. B, D 43. A 44. C 45. D
46. A 47. A 48. D 49. C 50. B
FIITJEE (Hyderabad Classes) Limited.
You might also like
- Quality Control Baking Soda Lab ReportDocument22 pagesQuality Control Baking Soda Lab ReportKatrina Le100% (6)
- JEE Advanced Aldehyde and Ketones Important QuestionsDocument23 pagesJEE Advanced Aldehyde and Ketones Important QuestionsthisissubhaNo ratings yet
- Alkyl Halides and Aryl HalidesDocument16 pagesAlkyl Halides and Aryl Halidesvardesh100% (1)
- Organic QuestionsDocument51 pagesOrganic Questionshemab30851No ratings yet
- Sheet-3-Hydro CarbonDocument8 pagesSheet-3-Hydro CarbonZooper lNo ratings yet
- Basara Vidyakshetram, Madhapur: Na/dry - Et ODocument8 pagesBasara Vidyakshetram, Madhapur: Na/dry - Et OvardeshNo ratings yet
- Jumbo Home Test-1 - FinalDocument59 pagesJumbo Home Test-1 - FinalSayali SachinNo ratings yet
- Hydrocarbon and Alkyl Halide-1Document10 pagesHydrocarbon and Alkyl Halide-1Aarya Vardhan ShandilyaNo ratings yet
- Alcohol Phenol Either and Probability 12Document23 pagesAlcohol Phenol Either and Probability 12jiknown6No ratings yet
- Carboxylic Acid CPPDocument24 pagesCarboxylic Acid CPPGulshan kumarNo ratings yet
- Time: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNDocument6 pagesTime: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNlakshmi.vedanarayanan7785No ratings yet
- Carbonyl CompoundsDocument10 pagesCarbonyl Compoundssudheerpabbati999No ratings yet
- Class Test-1 - Amine (Benzene-Dia-Azonium Chloride) - WithoutDocument8 pagesClass Test-1 - Amine (Benzene-Dia-Azonium Chloride) - WithoutshouryatrialNo ratings yet
- 1-50 QuestionsDocument48 pages1-50 Questionsbolla reddyNo ratings yet
- CL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDocument12 pagesCL H CL H CL H CL H P) Q) : X H Monohalogenated ProductDivya KhandelwalNo ratings yet
- Aliphatic HydrocarbonsDocument11 pagesAliphatic HydrocarbonsRishabhNo ratings yet
- Jumbo Test-2Document5 pagesJumbo Test-2prithvijeetopNo ratings yet
- Sheet 1Document11 pagesSheet 1Zooper lNo ratings yet
- Aldehydes & KetonesDocument9 pagesAldehydes & Ketoneskrishna janamNo ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- Chemistry Test PaperDocument12 pagesChemistry Test Papersougata_rintu9598No ratings yet
- Carbene and NitreneDocument20 pagesCarbene and NitreneKumarNo ratings yet
- Alchols, Phenols, EthersDocument6 pagesAlchols, Phenols, Ethersneshya5339No ratings yet
- Exe 3Document29 pagesExe 3AkashGauravNo ratings yet
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- Pdf&rendition 1Document11 pagesPdf&rendition 1Ishita AgarwalNo ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Hydrocarbon Level:I KVPYDocument2 pagesHydrocarbon Level:I KVPYRAGHUL MNo ratings yet
- Alcohol Phenols and Ether ImportantDocument13 pagesAlcohol Phenols and Ether ImportantHafdoon MuhammedNo ratings yet
- Class XII Haloalkanes, Alcohol, Phenol & EtherDocument4 pagesClass XII Haloalkanes, Alcohol, Phenol & EtherGourango NayakNo ratings yet
- Chemistry Alcohol, Ether & Phenol CPP Combine PDFDocument10 pagesChemistry Alcohol, Ether & Phenol CPP Combine PDFVanshika LudhaniNo ratings yet
- AIEEE Sample Paper-2Document21 pagesAIEEE Sample Paper-2aditya_kumar_meNo ratings yet
- Halogen Derivatives PDFDocument32 pagesHalogen Derivatives PDFRaju Singh100% (1)
- Chemistry Advanced Level Problem Solving (ALPS-1) - PaperDocument15 pagesChemistry Advanced Level Problem Solving (ALPS-1) - PaperAnanmay ChauhanNo ratings yet
- Chem QP PH-V Pet-3 Jee-M DT 11042024 - Test Anal - 240412 - 142616Document20 pagesChem QP PH-V Pet-3 Jee-M DT 11042024 - Test Anal - 240412 - 142616nagavali200006No ratings yet
- Alcohols IIT PACEDocument19 pagesAlcohols IIT PACEDushyant GuptaNo ratings yet
- Chem AlcoholsDocument2 pagesChem Alcoholspinnaacleclasses salemNo ratings yet
- NEET Haloalkanes and Haloarenes Important QuestionsDocument24 pagesNEET Haloalkanes and Haloarenes Important QuestionsDr. Shreya RoushanNo ratings yet
- Haloalkanes: Target Iit-JeeDocument44 pagesHaloalkanes: Target Iit-JeeHarsh VardhanNo ratings yet
- 13 DPP 04P Elimination Excel 1664524624713Document6 pages13 DPP 04P Elimination Excel 1664524624713Jatin SindhwaniNo ratings yet
- Alde & Ket-2&3Document14 pagesAlde & Ket-2&3ayesha sheikhNo ratings yet
- Alde & Ket-6&7Document15 pagesAlde & Ket-6&7ayesha sheikhNo ratings yet
- MOCK TEST - I, JEEM Shift-II, 4-11-2023Document3 pagesMOCK TEST - I, JEEM Shift-II, 4-11-2023shashwat.gupta.707No ratings yet
- Single Correct: Class: Adv - CC Time: 45 Min Class Test-3: OzonolysisDocument4 pagesSingle Correct: Class: Adv - CC Time: 45 Min Class Test-3: Ozonolysisbruh pogNo ratings yet
- Practice Paper - Phase 3Document6 pagesPractice Paper - Phase 3Dhairya VinayakNo ratings yet
- Nsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020Document13 pagesNsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020KritikaNo ratings yet
- Additions To Alkenes and Alkynes: Multiple Choice QuestionsDocument6 pagesAdditions To Alkenes and Alkynes: Multiple Choice QuestionsMuhammad barakatNo ratings yet
- Adv. Prev + Extra Edge 68-74 (Exercise 5 & 6)Document7 pagesAdv. Prev + Extra Edge 68-74 (Exercise 5 & 6)Aditya ShahNo ratings yet
- Ah 003 Qa20Document6 pagesAh 003 Qa20Eklavya GoyalNo ratings yet
- Rits-21 1Document13 pagesRits-21 1Muhammad HamzaNo ratings yet
- Aep - CPP - 2Document11 pagesAep - CPP - 2ayesha sheikhNo ratings yet
- Time: 1 Hrs Max. Marks: 87 Single Correct: OH OH OH OHDocument5 pagesTime: 1 Hrs Max. Marks: 87 Single Correct: OH OH OH OHlakshmi.vedanarayanan7785No ratings yet
- Home Assignment-3Document32 pagesHome Assignment-3ansh guptaNo ratings yet
- PB 003 Alkyl HalideDocument12 pagesPB 003 Alkyl HalideEklavya GoyalNo ratings yet
- Halogen Derivative of Alkanes & Arenes (Level - Iii & Iv) : Choh Socl CHCL So HCLDocument4 pagesHalogen Derivative of Alkanes & Arenes (Level - Iii & Iv) : Choh Socl CHCL So HCLAbhi WanwadeNo ratings yet
- JEE Advanced Hydrocarbons Important QuestionsDocument21 pagesJEE Advanced Hydrocarbons Important QuestionsSai SreyanNo ratings yet
- Alkyl Halide PDFDocument37 pagesAlkyl Halide PDFlingarajugowdaNo ratings yet
- KVPY SA 2017 Chemistry Question Answerkey SolutionsDocument11 pagesKVPY SA 2017 Chemistry Question Answerkey SolutionsPRITHISH HAZRANo ratings yet
- CBSE Class 11 Chemistry WorksheetDocument1 pageCBSE Class 11 Chemistry WorksheetHakim Abbas Ali PhalasiyaNo ratings yet
- 2019 - ISO-24444 - Sun Protection Test Methods - in Vivo Determination of The Sun Protection Factor (SPF)Document28 pages2019 - ISO-24444 - Sun Protection Test Methods - in Vivo Determination of The Sun Protection Factor (SPF)Dummy CipawNo ratings yet
- iGCSE Chemistry Revision SheetsDocument26 pagesiGCSE Chemistry Revision SheetsPanagiotis ScordisNo ratings yet
- PROBLEMS W IT H ARTICLES - : Structure and Written ExpressionDocument6 pagesPROBLEMS W IT H ARTICLES - : Structure and Written ExpressionSarahNo ratings yet
- Comparison of Mechanical Properties of Natural Rubber Vulcanizates Filled With Hybrid Fillers (Carbon Black/Palm Kernel Shell and Palm Kernel Shell/Sandbox Seed Shell)Document6 pagesComparison of Mechanical Properties of Natural Rubber Vulcanizates Filled With Hybrid Fillers (Carbon Black/Palm Kernel Shell and Palm Kernel Shell/Sandbox Seed Shell)Aakash YadavNo ratings yet
- Hind 1954Document12 pagesHind 1954jgNo ratings yet
- Electrolysis of Water: Shaik Shahid Sl. No No. DiagramDocument12 pagesElectrolysis of Water: Shaik Shahid Sl. No No. DiagramShaik ShahidNo ratings yet
- 75 Long Answer Questions in GCSE Science v2Document88 pages75 Long Answer Questions in GCSE Science v2Alex DNo ratings yet
- Determination of Aflatoxin B in Medical Herbs: Interlaboratory StudyDocument11 pagesDetermination of Aflatoxin B in Medical Herbs: Interlaboratory StudySarmilaNo ratings yet
- CCB BrochureDocument50 pagesCCB Brochureelias aouadNo ratings yet
- CIE AS Biology (9700) 2019 2021 CIE AS Biology (9700) 2019 2021 2.2.1 Biological Molecules Key Terms SaveMyExamsDocument32 pagesCIE AS Biology (9700) 2019 2021 CIE AS Biology (9700) 2019 2021 2.2.1 Biological Molecules Key Terms SaveMyExamsHudaNo ratings yet
- Tu Dien Vat Ly Anh VietDocument540 pagesTu Dien Vat Ly Anh ViettiemNo ratings yet
- Edufever: Du PHD in ChemistryDocument19 pagesEdufever: Du PHD in ChemistrybhuvneshNo ratings yet
- Unit 03 HW PacketDocument21 pagesUnit 03 HW Packetanabel mañoNo ratings yet
- Determination of Total Carbohydrates in Algal Biomass: Laboratory Analytical Procedure (LAP)Document17 pagesDetermination of Total Carbohydrates in Algal Biomass: Laboratory Analytical Procedure (LAP)MadhanNo ratings yet
- Drilling Fluids Technology Drilling FluidesDocument27 pagesDrilling Fluids Technology Drilling FluidesSouadHadjadjNo ratings yet
- How To Make Thermite - A Fiery Mix of Iron Oxide and AluminumDocument8 pagesHow To Make Thermite - A Fiery Mix of Iron Oxide and Aluminumalnoorb524No ratings yet
- Effect of Addition of Separan As Flocculant On Quality of Sugarcane JuiceDocument4 pagesEffect of Addition of Separan As Flocculant On Quality of Sugarcane JuiceMolly0630No ratings yet
- Zeolite Cures Cancer Page 2Document9 pagesZeolite Cures Cancer Page 2atpfacebookNo ratings yet
- 3D Porous Cu-NPs g-C3N4 Foam With Excellent CO2 Adsorption and SchottkyDocument10 pages3D Porous Cu-NPs g-C3N4 Foam With Excellent CO2 Adsorption and SchottkyAlejandro SifuentesNo ratings yet
- Date Thermometer 1 'C Thermometer 2 'C TimeDocument2 pagesDate Thermometer 1 'C Thermometer 2 'C Timerose_scribdNo ratings yet
- Sensitive and Selective Detection of MulDocument7 pagesSensitive and Selective Detection of MulquimicosorioNo ratings yet
- Chemical Changes and ReactionsDocument8 pagesChemical Changes and ReactionsHarshit KukrejaNo ratings yet
- Consequetive or Sequential Reaction: Chemical KineticsDocument19 pagesConsequetive or Sequential Reaction: Chemical Kineticsrishabh mishraNo ratings yet
- Basic General Knowledge of Chemistry PDF: Follow UsDocument15 pagesBasic General Knowledge of Chemistry PDF: Follow UsPawanNo ratings yet
- Grade 11assignment 2Document6 pagesGrade 11assignment 2mbusiwezaNo ratings yet
- Hexion Starting Formulation 8014Document4 pagesHexion Starting Formulation 8014uzzy2No ratings yet
- Polymer Phyiscs GeddeDocument301 pagesPolymer Phyiscs Geddeunknown159No ratings yet
- Kapitel 09 DINO Techn Teil PDFDocument11 pagesKapitel 09 DINO Techn Teil PDFAkash KulkarniNo ratings yet