Professional Documents
Culture Documents
Untitled Document
Untitled Document
Uploaded by
Renelyn Rocreo0 ratings0% found this document useful (0 votes)
2 views3 pages Here are the steps to solve this problem:
a. Maximum height (dy) = vi^2/2g = (10 m/s)^2 / (2 * -9.8 m/s^2) = 50 m
b. Time to reach maximum height (t) = vi/g = 10 m/s / -9.8 m/s^2 = 1 s
c. Time of flight (T) = 2t = 2 * 1 s = 2 s
d. Final velocity just before hitting ground (vf) = vi - gt = 10 m/s - 9.8 m/s^2 * 2 s = -19.6 m/s (moving downward)
e
Original Description:
Original Title
Untitled document (4)
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document Here are the steps to solve this problem:
a. Maximum height (dy) = vi^2/2g = (10 m/s)^2 / (2 * -9.8 m/s^2) = 50 m
b. Time to reach maximum height (t) = vi/g = 10 m/s / -9.8 m/s^2 = 1 s
c. Time of flight (T) = 2t = 2 * 1 s = 2 s
d. Final velocity just before hitting ground (vf) = vi - gt = 10 m/s - 9.8 m/s^2 * 2 s = -19.6 m/s (moving downward)
e
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
2 views3 pagesUntitled Document
Untitled Document
Uploaded by
Renelyn Rocreo Here are the steps to solve this problem:
a. Maximum height (dy) = vi^2/2g = (10 m/s)^2 / (2 * -9.8 m/s^2) = 50 m
b. Time to reach maximum height (t) = vi/g = 10 m/s / -9.8 m/s^2 = 1 s
c. Time of flight (T) = 2t = 2 * 1 s = 2 s
d. Final velocity just before hitting ground (vf) = vi - gt = 10 m/s - 9.8 m/s^2 * 2 s = -19.6 m/s (moving downward)
e
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 3
Accuracy refers to the closeness of a .
• Subatomic- The subatomic scale is the
measured value to a standard or known domain of physical size that encompasses
value. For example, if you obtain a weight objects smaller than an atom.
measurement of 3.2 kg for a given Atoms of the same element are identical in
substance, but the actual or known weight is physical and chemical properties and have
10 kg, then your measurement is not the same mass. Atoms of different elements
accurate. In this case, your measurement is differ in physical and chemical properties
not close to the known value. and have different masses
Precision refers to the closeness of two or Electrons • First subatomic particle to be
more measurements to each other. Using discovered. • Discovered by J.J Thompson •
the example above, if you weigh a given An electron is a negatively charged
substance five times, and get 3.2 kg each subatomic particle. It can be either free (not
time, then your measurement is very attached to any atom), or bound to the
precise. Precision is independent of nucleus of an atom. • Electrons in atoms
accuracy. You can be very precise but exist in spherical shells of various radii,
inaccurate, as described above. You can representing energy levels. The larger the
also be accurate but imprecise. spherical shell, the higher the energy
contained in the electron.
b. Protons • Discovered by Eugene
Goldstein in 1886. • A stable subatomic
particle occurring in all atomic nuclei, with a
A good analogy for understanding accuracy positive electric charge equal in magnitude
and precision is to imagine a basketball to that of an electron, but of opposite sign.
player shooting baskets. If the player shoots c. Neutron • Discovered by James
with accuracy, his aim will always take the
Chadwick in 1932 • a subatomic particle of
ball close to or into the basket. If the player
about the same mass as a proton but
shoots with precision, his aim will always
without an electric charge, present in all
take the ball to the same location which may
atomic nuclei except those of ordinary
or may not be close to the basket. A good
hydrogen
player will be both accurate and precise by
• Isotopes – variants of a particular
shooting the ball the same way each time
and each time making it into the basket chemical element that differ in neutron
Molecules- a group of atoms bonded number, and consequently in nucleon
number.
together, representing the smallest
Mass number ➢ Is the number of protons and
fundamental unit of a chemical compound
that can take part in a chemical reaction. • neutrons contained in the nucleus of an atom.
Mass number = number of protons + number of
Particles- a small localized object or entity
electrons.
to which can be ascribed several physical or
a. FeO = Ferrous Oxide (the oxidation
chemical properties such as volume,
number of Fe is +2)
density, or mass
b. Fe2O3 = Ferric Oxide (the oxidation
number of Fe is +3)
Empirical Formula – it is the simplest 5. Time of flight (T)
positive integer ratio of atoms present in a This is twice as t if we are referring to the time
compound. it took the object to reach the maximum height and back
Chemical equation – the symbolic
to the horizontal position where the object was thrown.
representation of a chemical reaction in the
Mathematically expressed as T = 2t.
form of symbols and formulae, wherein the
reactant entities are given on the left-hand
Kinematic quantities of Objects in
side and the product entities on the right-
Projectile Motion I (Vertical)
hand side.
• Reactants – a substance that takes part in 1. Acceleration due to gravity (ag) – It is
equal to 9.8 m / s2 since object will speed up
and undergoes change during a reaction. •
as it moves downward.
Products – a substance that is formed as
the result of a chemical reaction. 2. Height (dy) – It is the vertical displacement
component of the trajectory. Most often, it is given
PHYSICS in the word problem.
3. Time (t) – It is the time from the point of release
1. Acceleration
up the end of the trajectory. As mentioned earlier,
The object’s thrown upward decelerate at – 9.8
the ball dropped and thrown horizontally will hit the
m/s2. This means the object is slowing down as it moves
same ground. Using this information, the time could
upward.
be computed using
2. Initial velocity (vi)
For objects to move upward, the 4. Vertical Initial Velocity (viy) – Since the
initial velocity is always necessary. initial velocity is horizontal, there is no vertical
Initial velocity is not equal to zero component for its initial velocity, hence, it is zero (v iy
(v ≠ 0)
= 0)
3. Maximum height (dy)
This is the topmost portion the moving object 5. Vertical final Velocity (vfy) – It is the
could reach. Since vf is zero (vf = 0), this could be vertical component of the projectile’s velocity. This
computed using where ag is the acceleration due to is just equal to the final velocity of a free-falling
body with zero (0) initial velocity mathematically
gravity equal to (-9.8 m / s2 ). expressed as vfy = agt
4. Time (t)
This refers to the time it will take for the
Kinematic quantities of Objects in
moving object to reach the maximum height. This could Projectile Motion I (Horizontal)
1.. Range (dx) – horizontal distance component of
be computed using the trajectory mathematically expressed as
dx = vxt
2. Horizontal Velocity (vx) – velocity equal to
initial horizontal velocity since it is in constant
motion.
1. A metal ball was thrown vertically upward
from a horizontal with an initial velocity of 10 m
/ s.
a. What is the maximum height the metal ball
could reach?
b. How long will it take for the ball to reach the
maximum height?
c. What is the time of the flight?
d. Using the time of flight, how fast is the ball
just before it hits the ground?
e. How fast is the ball at 1.5 s? Was it moving
upward or downward?
You might also like
- Third Periodical Test in Math 5Document8 pagesThird Periodical Test in Math 5fritz78% (9)
- Science 8 Reviewer (Physics) : A. Forces and MotionDocument5 pagesScience 8 Reviewer (Physics) : A. Forces and Motionp r i n c e s s i t a88% (8)
- Kaeser Service ManualDocument126 pagesKaeser Service ManualGabrielNo ratings yet
- Reviewer in Science1Document6 pagesReviewer in Science1Cailin Loraine VibarNo ratings yet
- 9702 Grade Booster-A2-FAQsDocument17 pages9702 Grade Booster-A2-FAQskhanmahera796No ratings yet
- EP 204 Classical Mechanics: Colm BrackenDocument31 pagesEP 204 Classical Mechanics: Colm BrackenNiamh BrowneNo ratings yet
- IGCSEDocument14 pagesIGCSEButter CatNo ratings yet
- Measure The Length of A WireDocument90 pagesMeasure The Length of A Wire译然杨No ratings yet
- Why Gravity Is TrueDocument12 pagesWhy Gravity Is TrueKevin Van HornNo ratings yet
- Physics Questions Sem..Document14 pagesPhysics Questions Sem..VinitgaNo ratings yet
- Chapter3 Motion in 1DDocument32 pagesChapter3 Motion in 1DSüleymanNo ratings yet
- Physics (DONE)Document17 pagesPhysics (DONE)austinchan0728No ratings yet
- Unit 4 Quantume Mechanics Lecture 5Document31 pagesUnit 4 Quantume Mechanics Lecture 5sushank yadavNo ratings yet
- ChemistryDocument65 pagesChemistryDan Sebastian TilaoNo ratings yet
- U 5 T - M P: Knowledge /45 Thinking /20 Application /15 Communication /5 Total /85Document9 pagesU 5 T - M P: Knowledge /45 Thinking /20 Application /15 Communication /5 Total /85Mohammad NiyaifarNo ratings yet
- Dynamics LectureDocument220 pagesDynamics LectureShiro Emiya0% (2)
- PHYSICSDocument17 pagesPHYSICSErica Mae GuzmanNo ratings yet
- Y10 Science Notes Semester 1Document3 pagesY10 Science Notes Semester 1Oscar ReinitzNo ratings yet
- Science ReviewerDocument5 pagesScience ReviewerMitchell CatulongNo ratings yet
- Chapt2 General PhysDocument61 pagesChapt2 General PhysmesayNo ratings yet
- EportDocument6 pagesEportapi-295812958No ratings yet
- Electroceramics Prof. Ashish Garg Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture - 32Document36 pagesElectroceramics Prof. Ashish Garg Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture - 32JATCNo ratings yet
- Mechanics BookletDocument65 pagesMechanics BookletZachNo ratings yet
- JEE Main Atomic Structure - pdf-98Document12 pagesJEE Main Atomic Structure - pdf-98Zameer AnsariNo ratings yet
- GenPhysics 1Document4 pagesGenPhysics 1Mary Harry Dela CruzNo ratings yet
- Wave and Particles: - Wave:: Unit-Iii Quantum MechancsDocument9 pagesWave and Particles: - Wave:: Unit-Iii Quantum MechancsHarshini NNo ratings yet
- Pssutc: Academy of Sciences of Turkmenistan Oguz Han Engineering and Technology University of TurkmenistanDocument12 pagesPssutc: Academy of Sciences of Turkmenistan Oguz Han Engineering and Technology University of TurkmenistanHayko SpitakciNo ratings yet
- GenPhysics2 - Q2 Module 5Document20 pagesGenPhysics2 - Q2 Module 5Jasmin SorianoNo ratings yet
- Gen Chem ReviewerDocument3 pagesGen Chem ReviewerAece CaneteNo ratings yet
- SCIENCEDocument3 pagesSCIENCEdzveaNo ratings yet
- Physics Notes Gr.11Document28 pagesPhysics Notes Gr.11iyermagsNo ratings yet
- EMERSON COY - PHD - THESISDocument23 pagesEMERSON COY - PHD - THESISAtifAwanNo ratings yet
- Module 2 PhysicsDocument245 pagesModule 2 PhysicsEmre ÖzerNo ratings yet
- General Science UPDocument8 pagesGeneral Science UPvignesh11252002No ratings yet
- Sherman Module 1 SpaceDocument20 pagesSherman Module 1 SpaceYousef YohannaNo ratings yet
- Chapter 2: The Structure of The Atom A MatterDocument9 pagesChapter 2: The Structure of The Atom A MatterMSKNo ratings yet
- Science Exam ReviewerDocument13 pagesScience Exam ReviewerManoli MontinolaNo ratings yet
- Inbound 2217592035381457554Document142 pagesInbound 2217592035381457554Vincent PaulNo ratings yet
- Quick Revision For o Level PhysicsDocument28 pagesQuick Revision For o Level PhysicsRegie Sacil EspiñaNo ratings yet
- Unit - I - Quantum Mechanics PhysicsDocument34 pagesUnit - I - Quantum Mechanics Physicsabisheik942No ratings yet
- Uts Efp Vivi RJDocument5 pagesUts Efp Vivi RJVivi Pipi RJNo ratings yet
- 10th Science 1Document8 pages10th Science 1Daulatrao ShindeNo ratings yet
- Grade 8 Science Quarter 3 ReviewerDocument6 pagesGrade 8 Science Quarter 3 Reviewerasuit9135No ratings yet
- Guia Physics ChrisDocument2 pagesGuia Physics ChrisAndres AlvaradoNo ratings yet
- Basic Physics: Some Concepts For Geodesy: James R. Clynch, 2003Document10 pagesBasic Physics: Some Concepts For Geodesy: James R. Clynch, 2003Rendi Adi FebrianNo ratings yet
- ChemistryDocument4 pagesChemistryKent RosimaNo ratings yet
- Uniformly Accelerated Motion in Vertical DimentaionDocument11 pagesUniformly Accelerated Motion in Vertical DimentaionEricka Pallon CamayudoNo ratings yet
- ACT Science Outside Information Cheat Sheet WT 1Document15 pagesACT Science Outside Information Cheat Sheet WT 1aaravNo ratings yet
- Essential Quantum Mechanics For Chemistry Students The Odd Behavior of MatterDocument4 pagesEssential Quantum Mechanics For Chemistry Students The Odd Behavior of MatterDeepalNo ratings yet
- Exam 4 Part 1Document10 pagesExam 4 Part 1KaylaNo ratings yet
- Periodic Classification of ElementsDocument25 pagesPeriodic Classification of Elementsindubaisakhare12345No ratings yet
- Chemistry 1atomic StructureDocument10 pagesChemistry 1atomic StructureRosely PaquiteNo ratings yet
- Physics NotesDocument11 pagesPhysics NotesnadineNo ratings yet
- RT Intro LectureDocument4 pagesRT Intro LectureIvennaif InvokerNo ratings yet
- IB Physics Circular Motion and GravityDocument9 pagesIB Physics Circular Motion and GravityuzairNo ratings yet
- Physic Form 4/5 DefinitionDocument6 pagesPhysic Form 4/5 DefinitionKa Mun LeongNo ratings yet
- Science 8 1Document18 pagesScience 8 1Hannah Leigh CoronelNo ratings yet
- Physics: Chapter 2 (F4) Linear MotionDocument4 pagesPhysics: Chapter 2 (F4) Linear MotionKai YuanNo ratings yet
- AP Physics: Algebra Based - Study GuideDocument67 pagesAP Physics: Algebra Based - Study GuideThomas LaiNo ratings yet
- IX PHY Motion NotesDocument10 pagesIX PHY Motion NotesFaraz khanNo ratings yet
- RadphysicsDocument143 pagesRadphysicspia toledoNo ratings yet
- Jurnal Spektrofotometri Serapan AtomDocument13 pagesJurnal Spektrofotometri Serapan Atom2OO11O39 Chenia NandiniNo ratings yet
- MATHS Project-3Document14 pagesMATHS Project-3PULAKALA RAJESWARINo ratings yet
- DS-AJ7824S Series Storage EnclosureDocument2 pagesDS-AJ7824S Series Storage EnclosureCORAL ALONSO JIMÉNEZNo ratings yet
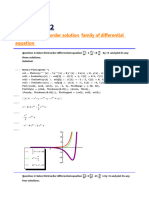
- Practical-2: Plotting of Third Order Solution Family of Differential EquationDocument4 pagesPractical-2: Plotting of Third Order Solution Family of Differential EquationnidhikoshaliaNo ratings yet
- Chapter 4 HPCNDocument32 pagesChapter 4 HPCNmuluken getenetNo ratings yet
- STS Prelims ReviewerDocument6 pagesSTS Prelims ReviewerMarian TorreonNo ratings yet
- VivaDocument149 pagesVivaA DNo ratings yet
- 6.5a Strong and Weak Acids and BasesDocument14 pages6.5a Strong and Weak Acids and BasesEricka GalangNo ratings yet
- 3rd Assessment Mathematics 5Document5 pages3rd Assessment Mathematics 5Raselle Alfonso Palisoc100% (1)
- Coordinate Geometry and Calculus. TIME: 3hrs Max. Marks.75Document16 pagesCoordinate Geometry and Calculus. TIME: 3hrs Max. Marks.75Tarigonda GiridharNo ratings yet
- Denon DCD 1520ae CD Player Review Test LoresDocument7 pagesDenon DCD 1520ae CD Player Review Test LorespetrosNo ratings yet
- Certificat DVR 4 Canale - Vidy 4Document1 pageCertificat DVR 4 Canale - Vidy 4lucian popNo ratings yet
- Signals and Systems I-EEE 203: Lecture Notes 2bDocument31 pagesSignals and Systems I-EEE 203: Lecture Notes 2bplaystationNo ratings yet
- Determination of Free Phosphoric Acid in Superphosphate PDFDocument7 pagesDetermination of Free Phosphoric Acid in Superphosphate PDFJaldasrinivasraoNo ratings yet
- 1 - копияDocument2 pages1 - копияYaroslav ZaglyadinNo ratings yet
- Education: A315/5, IIT Kanpur - India (+91) 9660998600 - Yashbth@iitk - Ac.in - /in/yashbth - /yashbthDocument2 pagesEducation: A315/5, IIT Kanpur - India (+91) 9660998600 - Yashbth@iitk - Ac.in - /in/yashbth - /yashbthVikas SrivastavNo ratings yet
- Ncert ch2 Physics Class 11Document23 pagesNcert ch2 Physics Class 11Karan ManglaNo ratings yet
- Energy Absorption of Safety Nets in Building ConstructionDocument9 pagesEnergy Absorption of Safety Nets in Building ConstructionJorge ChavezNo ratings yet
- T. C. Powers - 1958Document7 pagesT. C. Powers - 1958Rudiele Schankoski0% (1)
- Weld Defects RepairsDocument48 pagesWeld Defects RepairszainalNo ratings yet
- MOMENT EndplateDocument8 pagesMOMENT Endplatemoseslugtu6324No ratings yet
- Lecture 2 - Bearing Capacity of Shallow FoundationsDocument64 pagesLecture 2 - Bearing Capacity of Shallow FoundationsanilNo ratings yet
- Pre-Assessment Questions: Java Binary Input/Output (I/O) Stream ClassesDocument25 pagesPre-Assessment Questions: Java Binary Input/Output (I/O) Stream Classesajay_anavNo ratings yet
- Orion Starblast: Instruction ManualDocument12 pagesOrion Starblast: Instruction ManualAndrew LoNo ratings yet
- Lean To WeldingDocument10 pagesLean To Weldingkarthiks12008658No ratings yet
- Logcat Home Fota Update LogDocument119 pagesLogcat Home Fota Update LogAlanNo ratings yet
- Mineralogía DescriptivaDocument38 pagesMineralogía DescriptivaJorge Rios RNo ratings yet
- Post Practicum Survey Questionnair 2Document3 pagesPost Practicum Survey Questionnair 2Rhea FrancisNo ratings yet