Professional Documents
Culture Documents
America
America
Uploaded by
JOSE HERNANDO JOYA HERERA0 ratings0% found this document useful (0 votes)
21 views4 pagesThe document discusses cardiac action potential propagation. It states that propagation relies on the sodium current (INa) which depends on factors like sodium channel availability and the sodium electrochemical gradient. A large INa is needed to ensure propagation continues under different conditions. Tissues with high sodium channel concentrations like Purkinje fibers have fast conduction. Reduced excitability lowers INa and leads to slower conduction velocity. When safety factor for conduction falls below 1, conduction block occurs. Gap junctions are also important as they electrically couple cells and allow propagation through direct current spread. Variations in gap junction properties help determine conduction differences between tissues.
Original Description:
Original Title
america
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses cardiac action potential propagation. It states that propagation relies on the sodium current (INa) which depends on factors like sodium channel availability and the sodium electrochemical gradient. A large INa is needed to ensure propagation continues under different conditions. Tissues with high sodium channel concentrations like Purkinje fibers have fast conduction. Reduced excitability lowers INa and leads to slower conduction velocity. When safety factor for conduction falls below 1, conduction block occurs. Gap junctions are also important as they electrically couple cells and allow propagation through direct current spread. Variations in gap junction properties help determine conduction differences between tissues.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
21 views4 pagesAmerica
America
Uploaded by
JOSE HERNANDO JOYA HERERAThe document discusses cardiac action potential propagation. It states that propagation relies on the sodium current (INa) which depends on factors like sodium channel availability and the sodium electrochemical gradient. A large INa is needed to ensure propagation continues under different conditions. Tissues with high sodium channel concentrations like Purkinje fibers have fast conduction. Reduced excitability lowers INa and leads to slower conduction velocity. When safety factor for conduction falls below 1, conduction block occurs. Gap junctions are also important as they electrically couple cells and allow propagation through direct current spread. Variations in gap junction properties help determine conduction differences between tissues.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 4
[dV/dt]).
These factors depend on the amplitude of INa, which, in
turn,
is directly related to the Em at the time of stimulation, the
availability
of Na+ channels for stimulation, and the size of the Na+
electrochemical
potential gradient across the cell membrane.
Normally, the charge flow across depolarizing ion channels (INa) is
significantly larger than the charge needed to excite the same cell,
providing
sufficient extra stimulatory current to drive propagation forward.
This property (referred to as “propagation reserve” or “safety of
propagation”)
helps to maintain action potential propagation under different
physiological and pathophysiological conditions.23 Working atrial
and
ventricular myocardium and, in particular, Purkinje fibers have high
concentrations of Na+ channels (Purkinje fibers contain up to 1
million
Na+ channels per cell), which help to generate a large depolarizing
current flow (INa) during the action potential. The large INa spreads
quickly within and between cells to support rapid conduction.
Reduction of membrane excitability leads to a reduction in the rate
or amplitude of depolarization (INa) during phase 0 of the action
potential.
Conduction velocity decreases monotonically with progressive
reduction of membrane excitability. When the safety factor for
conduction
falls to less than 1 (i.e., the source current becomes less than the
current necessary for excitation of downstream tissue), conduction
can
no longer be sustained, and failure (conduction block) occurs.
In tissues with slow response action potentials (sinus and AV
nodes),
the upstroke of the action potential is formed by ICaL instead of INa.
Because ICaL has lower amplitude and slower activation kinetics
than
INa, slow response action potentials exhibit reduced amplitudes and
upstroke velocities. Hence slow conduction (approximately 0.1 to
0.2 m/s)
and prolonged refractoriness are characteristic features of nodal
tissues.
These cells also have a reduced safety factor for conduction, which
means that the stimulating efficacy of the propagating impulse is
low,
and conduction block occurs easily.
Intercellular Propagation
Propagation of action potentials from one cell to adjacent cells is
achieved
by direct ionic current spread (without electrochemical synapses) via
specialized, low resistance intercellular connections (gap junctional
channels) located mainly in arrays within the intercalated disks. Gap
junctions facilitate impulse propagation throughout the heart, so that
the heart behaves electrically as a functional syncytium, resulting in
a
coordinated mechanical function.22
Gap junctional channels connect neighboring cells and allow
biochemical
and low-resistance electrical coupling. Although the resistivity
of the gap junctional membrane for the passage of ions and small
molecules and for electrical propagation is several orders of
magnitude
lower compared with uncoupled cell membranes, gap junction
coupling
provides a resistance pathway that is several orders of magnitude
higher
than the cytoplasmic intracellular resistivity (conduction delay is
approximately
0.21 to 0.27 milliseconds at gap junctions, and 0.05 to 0.1
milliseconds
at the cell membrane).24 As a consequence, impulse propagation
along single cell chains of cardiomyocytes is saltatory, in which the
high-resistance intercellular junctions alternate with the low
cytoplasmic
resistance. However, this feature is lost in intact multicellular tissue
due
to lateral gap junctional coupling which serves to average local
small
differences in activation times of individual cardiomyocytes at the
excitation
wavefront.19
The number, size, and molecular composition of the gap junction
channels contribute to the specific propagation properties of a given
tissue. Tissue-specific connexin expression and gap junction spatial
distribution, as well as the variation in the structural composition of
gap junction channels, allow for a greater versatility of gap junction
physiological features and enable disparate conduction properties in
cardiac tissue.25
pacemaking cells may not be recovered by the end of phase 3 of the
action potential and full restoration of maximum diastolic potential
because L-type Ca2+ channels require longer time to recovery from
inactivation to be able to mediate the upstroke of a new action
potential.
In other words, sinus and AV nodal cells remain refractory for a
time
interval that is longer than the time it takes for full membrane
repolarization
to occur, a phenomenon termed postrepolarization refractoriness.
This can also occur in working myocardium during some disease
states such as myocardial infarction.
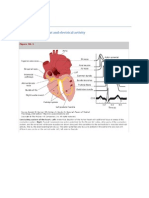
PROPAGATION
Cardiac excitation involves generation of the action potential by
individual
cells, followed by propagation of the electrical impulse along the
cardiac muscle fiber and rapidly from cell to cell throughout the
cardiac
tissue. Conduction velocity refers to the speed of propagation of the
action potential through cardiac tissue. The conduction velocity
varies
in cardiac tissues, ranging from 0.05 m/s in the atrioventricular node
(AVN), to 0.5 m/s in atrial and ventricular working myocardium, 2
m/s
in the bundle branches, and up to 4 m/s in Purkinje fibers.20 In most
regions of the heart, conduction does not occur as a continuous
process;
rather, the propagating electrical wavefronts interact with structural
boundaries that exist at the cellular level (cell membranes,
intercellular
gap junctions, the three-dimensional [3-D] arrangement of
cardiomyocytes),
as well as at the more macroscopic level (microvasculature,
connective
tissue barriers, trabeculation).21,22
Intracellular Propagation
Once initiated, the action potential propagates along the cell
membrane
until the entire cardiomyocyte is depolarized. The velocity of
propagation
increases with increasing cell diameter, action potential amplitude,
and the initial rate of the rise of the action potential.
An action potential traveling along a cardiac muscle fiber is
propagated
by local circuit currents, much as it does in nerve and skeletal
muscle. Conduction velocity along the cardiac fiber is directly
related
to the action potential amplitude (i.e., the voltage difference between
the fully depolarized and the fully polarized regions) and the rate of
change of potential (i.e., the rate of rise of phase 0 of the action
potential
You might also like
- MCQ OrthoDocument45 pagesMCQ OrthodentistsurorNo ratings yet
- Distillation ConvergenceDocument4 pagesDistillation ConvergenceSai Pavan100% (1)
- Learning Objectives: Chapter 4: Electrophysiology of The HeartDocument28 pagesLearning Objectives: Chapter 4: Electrophysiology of The HeartLaura PucheNo ratings yet
- Surgical PhsyiologyDocument170 pagesSurgical PhsyiologyTamjid Khan100% (2)
- Cardiac Ion Channels and Transporters: Abstract. Cardiac Electrophysiology Has Followed A Long WayDocument48 pagesCardiac Ion Channels and Transporters: Abstract. Cardiac Electrophysiology Has Followed A Long WayGiulia AndreeaNo ratings yet
- Grupo 1 Practica 14Document12 pagesGrupo 1 Practica 14Sadit Chauca VelaNo ratings yet
- Axon DiameterDocument2 pagesAxon Diameterolraccarmen14No ratings yet
- Skeletal Muscle Physiology PDFDocument6 pagesSkeletal Muscle Physiology PDFAstri Ggamjong Xiao LuNo ratings yet
- Functions of Nerve CellsDocument3 pagesFunctions of Nerve CellsSucheta Ghosh ChowdhuriNo ratings yet
- Describe How An Action Potential Is Propagated Along A Nerve FibreDocument4 pagesDescribe How An Action Potential Is Propagated Along A Nerve FibreDhruvalNo ratings yet
- Synapses HistologyDocument6 pagesSynapses HistologySatwant SinghNo ratings yet
- Systemic PhysiologyDocument7 pagesSystemic Physiologymichael777No ratings yet
- Neural Spiking and Synaptic Transmission: Action PotentialDocument6 pagesNeural Spiking and Synaptic Transmission: Action PotentialLoc HuynhNo ratings yet
- Heart PhysiolDocument84 pagesHeart PhysiolChintya Ayu ChampakaNo ratings yet
- Action Potential - The Resting Membrane Potential - Generation of Action Potentials - TeachMePhysiologAyDocument4 pagesAction Potential - The Resting Membrane Potential - Generation of Action Potentials - TeachMePhysiologAymohammed awolNo ratings yet
- Neurons and The Action PotentialDocument3 pagesNeurons and The Action PotentialnesumaNo ratings yet
- Surgical PhsyiologyDocument170 pagesSurgical PhsyiologySteve FerisNo ratings yet
- Characteristics and Plasticity of Electrical SynapDocument16 pagesCharacteristics and Plasticity of Electrical SynapjaneNo ratings yet
- Physiology Unit 4 - 231102 - 212048Document23 pagesPhysiology Unit 4 - 231102 - 212048SanchezNo ratings yet
- 1.0 The Origin of Biopotentials (New)Document62 pages1.0 The Origin of Biopotentials (New)Hanif HussinNo ratings yet
- Fisiologi NerveDocument5 pagesFisiologi Nervenurul armaliaNo ratings yet
- Channelopathies: Ion Channel Defects Linked To Heritable Clinical DisordersDocument12 pagesChannelopathies: Ion Channel Defects Linked To Heritable Clinical Disorderscote_cote6951No ratings yet
- Neurons - SkinDocument7 pagesNeurons - Skindegele18No ratings yet
- CH 5 ElectricityDocument39 pagesCH 5 ElectricityAbdullahi Mohamed IsakNo ratings yet
- Group 2 Azucena Bacud Baquiran Bautista - Neurophsyiology of Nerve Impulses Part 6-9Document7 pagesGroup 2 Azucena Bacud Baquiran Bautista - Neurophsyiology of Nerve Impulses Part 6-9Timothy John BautistaNo ratings yet
- 05 Biomedical EngineeringDocument357 pages05 Biomedical EngineeringAngel Celestial100% (1)
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Document18 pagesP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)hendra2darmawanNo ratings yet
- Electricity and Nerve Cells (Part I)Document3 pagesElectricity and Nerve Cells (Part I)mcspt117No ratings yet
- Nagaba Ritah Acktone 2020Document2 pagesNagaba Ritah Acktone 2020Ritah ACKTONE NAGABANo ratings yet
- Conduction System of HeartDocument7 pagesConduction System of HeartThakur KanchanNo ratings yet
- Introduction To NeurophysiologyDocument13 pagesIntroduction To Neurophysiologyvenkata ramakrishnaiahNo ratings yet
- NROSCI 1012 - Lecture 22Document4 pagesNROSCI 1012 - Lecture 22HonzaNo ratings yet
- 38.17 Cardiac Pacing: Basic Concepts: Renato Pietro RicciDocument16 pages38.17 Cardiac Pacing: Basic Concepts: Renato Pietro RicciurtikikeNo ratings yet
- Unit 4 - Cardiovascular Physiology HandoutDocument7 pagesUnit 4 - Cardiovascular Physiology Handouttilahun aligazNo ratings yet
- Cardio Physiology FullDocument11 pagesCardio Physiology FullSara Lee Wei LiNo ratings yet
- Exam Prep - Lec 3 - KEYDocument6 pagesExam Prep - Lec 3 - KEYAndrew ShiahNo ratings yet
- Nervous System 2Document4 pagesNervous System 2P KNo ratings yet
- Fizobioqimia FinalDocument12 pagesFizobioqimia FinalTamar PertiaNo ratings yet
- Nerve and Muscle PhysiologyDocument82 pagesNerve and Muscle PhysiologychandsriNo ratings yet
- Nervous SystemDocument6 pagesNervous SystemarellanokristelleNo ratings yet
- 3 The Electrochemical Basis of Nerve FunctionDocument33 pages3 The Electrochemical Basis of Nerve FunctionEvets JarusNo ratings yet
- Voltage-Gated Ion Channels Plasma Membrane Resting Potential SodiumDocument34 pagesVoltage-Gated Ion Channels Plasma Membrane Resting Potential SodiumnvbondNo ratings yet
- Beebe-Diverse Effects Wideband Non Ionizing Radiation Cells Tissues-04216264Document4 pagesBeebe-Diverse Effects Wideband Non Ionizing Radiation Cells Tissues-04216264searchtheNo ratings yet
- Cardiac Electrophysiology Using Ls Dyna R PDFDocument15 pagesCardiac Electrophysiology Using Ls Dyna R PDFЮрий НовожиловNo ratings yet
- 2.2 Nerve Cell 2.2.1 The Main Parts of The Nerve CellDocument4 pages2.2 Nerve Cell 2.2.1 The Main Parts of The Nerve CellRoberta GabrielaNo ratings yet
- CH 4 - CoordinationDocument23 pagesCH 4 - CoordinationnawarakanNo ratings yet
- An Ultrasensitive Vibrating Probe For Measuring Steady Extracellular CurrentsDocument16 pagesAn Ultrasensitive Vibrating Probe For Measuring Steady Extracellular CurrentsgghgfhNo ratings yet
- 10.3 Muscle Fiber Excitation, Contraction, and Relaxation - Anatomy & PhysiologyDocument1 page10.3 Muscle Fiber Excitation, Contraction, and Relaxation - Anatomy & Physiologyjszkutak24No ratings yet
- Keener 1999Document17 pagesKeener 1999nmmMJKJNo ratings yet
- Adn EscalarDocument5 pagesAdn Escalarmedellincolombia100% (2)
- Neurochem FinalDocument20 pagesNeurochem Finalelifulku.a1No ratings yet
- Matchkov, Jacob Freiberg and Niels-Henrik Holstein-Rathlou Jens Christian Brings Jacobsen, Christian Aalkjær, Holger Nilsson, Vladimir VDocument10 pagesMatchkov, Jacob Freiberg and Niels-Henrik Holstein-Rathlou Jens Christian Brings Jacobsen, Christian Aalkjær, Holger Nilsson, Vladimir VHDYangNo ratings yet
- Action PotentialDocument24 pagesAction PotentialJohn MarcelNo ratings yet
- KIN 270 Unit 2 Neural Physiology Part 1Document4 pagesKIN 270 Unit 2 Neural Physiology Part 1Armando AlehandroNo ratings yet
- Treating Cardiac Diseas 2Document20 pagesTreating Cardiac Diseas 2Muhammed Luthufi E100% (1)
- Cell To Cell ContactDocument6 pagesCell To Cell ContactHarini BalasubramanianNo ratings yet
- Action Potential ConductionDocument9 pagesAction Potential ConductiondvoyevodNo ratings yet
- Neurophysiology: Central Nervous System Peripheral Nervous SystemDocument8 pagesNeurophysiology: Central Nervous System Peripheral Nervous SystemJeevikaGoyalNo ratings yet
- CH 04 DDocument34 pagesCH 04 DVijayRajNo ratings yet
- A M I E: Dvanced Ethods N LectrophysiologyDocument43 pagesA M I E: Dvanced Ethods N LectrophysiologyArtur LiberatoNo ratings yet
- Neural CommunicationDocument28 pagesNeural CommunicationIkponmwosa EseosaNo ratings yet
- EKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXFrom EverandEKG/ECG Interpretation Made Easy: A Practical Approach to Passing the ECG/EKG Portion of NCLEXRating: 5 out of 5 stars5/5 (2)
- DecritirDocument3 pagesDecritirJOSE HERNANDO JOYA HERERANo ratings yet
- BetameroDocument3 pagesBetameroJOSE HERNANDO JOYA HERERANo ratings yet
- AlfacentauroDocument2 pagesAlfacentauroJOSE HERNANDO JOYA HERERANo ratings yet
- Do Des TerosDocument3 pagesDo Des TerosJOSE HERNANDO JOYA HERERANo ratings yet
- Cer Con ErosDocument2 pagesCer Con ErosJOSE HERNANDO JOYA HERERANo ratings yet
- DesdalerDocument3 pagesDesdalerJOSE HERNANDO JOYA HERERANo ratings yet
- A Priori Evident Judgements". If He Is Guilty of This Charge, at Least He May BeDocument2 pagesA Priori Evident Judgements". If He Is Guilty of This Charge, at Least He May BeJOSE HERNANDO JOYA HERERANo ratings yet
- Des DalerDocument2 pagesDes DalerJOSE HERNANDO JOYA HERERANo ratings yet
- Memorizing Chess: Methods and Material: 3220 Pieces-848.html#comment-1618Document16 pagesMemorizing Chess: Methods and Material: 3220 Pieces-848.html#comment-1618JOSE HERNANDO JOYA HERERANo ratings yet
- Ursprung Sittlicher Erkenntnis (1889) With Certain Characteristic Statements From TheDocument2 pagesUrsprung Sittlicher Erkenntnis (1889) With Certain Characteristic Statements From TheJOSE HERNANDO JOYA HERERANo ratings yet
- Irrealia. Strictly Speaking, The Lecture On Truth Contains The Key To TheDocument2 pagesIrrealia. Strictly Speaking, The Lecture On Truth Contains The Key To TheJOSE HERNANDO JOYA HERERANo ratings yet
- Ideas 25Document3 pagesIdeas 25JOSE HERNANDO JOYA HERERANo ratings yet
- FlareTot - Total Flare AnalysisDocument8 pagesFlareTot - Total Flare AnalysisArjun KapoorNo ratings yet
- Overlapped Chromosome Segmentation and Separation of Touching Chromosome For Automated Chromosome ClassificationDocument4 pagesOverlapped Chromosome Segmentation and Separation of Touching Chromosome For Automated Chromosome ClassificationĐặng Thanh HuyềnNo ratings yet
- B. TECH 2nd Semester June 2019-1 PDFDocument5 pagesB. TECH 2nd Semester June 2019-1 PDFHarshitNo ratings yet
- Film Formation in Coatings - Properties, Mechanisms, and ApplicationsDocument10 pagesFilm Formation in Coatings - Properties, Mechanisms, and ApplicationsAadhi InnovativesNo ratings yet
- 7sd522 SiemensDocument17 pages7sd522 SiemensSanthosh Kumar VinayagamNo ratings yet
- (23 176 of Polycrystalline Nickel: On The Mechanism of Low-Temperature OxidationDocument5 pages(23 176 of Polycrystalline Nickel: On The Mechanism of Low-Temperature OxidationPaty ChiluisaNo ratings yet
- Small Bore Fitting (SBF) Vibration Fatigue CalculationDocument26 pagesSmall Bore Fitting (SBF) Vibration Fatigue CalculationgopaltryNo ratings yet
- Latest 2020 Microsoft AZ-400 Dumps Question & Answers - Microsoft AZ-400Document13 pagesLatest 2020 Microsoft AZ-400 Dumps Question & Answers - Microsoft AZ-400noranayelNo ratings yet
- 3.5 ArchimedesDocument13 pages3.5 ArchimedesNURUL FAEZAH BINTI MUSTAPA MoeNo ratings yet
- Stiffness at Small Strain-Research and PracticeDocument33 pagesStiffness at Small Strain-Research and PracticeebalicNo ratings yet
- Service ManualDocument429 pagesService ManualChris CoulsonNo ratings yet
- YuiDocument1 pageYuireddy pNo ratings yet
- DataDog EBookMonitoringModernInfrastructureDocument86 pagesDataDog EBookMonitoringModernInfrastructurezangui mehdiNo ratings yet
- In Asset AccountingDocument5 pagesIn Asset AccountingVishal YadavNo ratings yet
- Research TerminologiesDocument42 pagesResearch TerminologiesPriya bhattiNo ratings yet
- Fdocuments - in Chapter 1 Introduction Utoledo Wevanschap1spdfthe Machine Tool and AutomotiveDocument27 pagesFdocuments - in Chapter 1 Introduction Utoledo Wevanschap1spdfthe Machine Tool and AutomotiveFoo BrandonNo ratings yet
- Activity 2: Mathematical Modeling of Physical SystemsDocument10 pagesActivity 2: Mathematical Modeling of Physical SystemsNico SilorioNo ratings yet
- Picture Group Name/Command Description ClipboardDocument13 pagesPicture Group Name/Command Description ClipboardAnn JimenezNo ratings yet
- Questions For JavaScriptDocument4 pagesQuestions For JavaScriptNikolaiNo ratings yet
- Install WinQSB For Window 7 PDFDocument4 pagesInstall WinQSB For Window 7 PDFHẬU Nguyễn NhưNo ratings yet
- Intro To Data Science SummaryDocument17 pagesIntro To Data Science SummaryHussein ElGhoulNo ratings yet
- Different Approaches To Frame Interpolation and Motion InterpolationDocument13 pagesDifferent Approaches To Frame Interpolation and Motion InterpolationJuan David Rengifo CastroNo ratings yet
- Performance Study of Virtual Fence Unit Using Wireless Sensor NetworkDocument4 pagesPerformance Study of Virtual Fence Unit Using Wireless Sensor NetworkMuhtar IzuNo ratings yet
- bullion-HT003 PCIe Adapter Firmware Updatev2Document14 pagesbullion-HT003 PCIe Adapter Firmware Updatev2Fabrice PLATELNo ratings yet
- How FACTS Controllers Function in An AC Transmission System: Series Controllers TopicsDocument16 pagesHow FACTS Controllers Function in An AC Transmission System: Series Controllers TopicsAwesomegalNo ratings yet
- Photovoltaic Systems Engineering 4th Messenger Solution ManualDocument12 pagesPhotovoltaic Systems Engineering 4th Messenger Solution ManualJesus Carter100% (41)
- Thermal Conductivity Gauges: Pirani GaugeDocument9 pagesThermal Conductivity Gauges: Pirani GaugeSanket GogteNo ratings yet
- 6104 BSF SM EstufaDocument20 pages6104 BSF SM EstufadieguineoNo ratings yet