Professional Documents
Culture Documents
Pha052 TG 8
Pha052 TG 8
Uploaded by
Alcea InguilloOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pha052 TG 8
Pha052 TG 8
Uploaded by
Alcea InguilloCopyright:
Available Formats
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
Lesson title: Molecular Emission Spectroscopy Materials: Book, pen and notebook,
Learning Targets: index card/class list
At the end of the module, students will be able to:
1. Demonstrate competence in the analysis of References:
pharmaceuticals using MES; Watson, David G. (2017).
2. Identify components of Fluorescence Pharmaceuticals analysis: a textbook for
Spectrophotometer; pharmacy students and pharmaceutical
3. Enumerate and define three types of scatter. chemists, 4th ed. Singapore: Elsevier
A. LESSON PREVIEW/REVIEW
Activity 1
1-2. Give 2 factors interfering with fluorescence intensity
● CONCENTRATION
● WEIGHT OF ATOMS
● COMPLEXITY OF FORMULATION
● TEMPERATURE
3-5. True or False
True 3. A chromophore is a part of a molecule responsible for the pigmentation.
True 4. Emission is the light being emitted observed at right angles to the light being used to excite the
sample.
False 5. In Raman Scatter, an exciting radiation is scattered by colloidal molecules [Tyndall]
B. MAIN LESSON
I. MOLECULAR EMISSION SPECTROSCOPY (Fluorescence Spectrophotometry)
IA. Introduction
Molecules with chromophores and a rigid structure can be excited by UV/VIS radiation. The molecules will
then emit the radiation absorbed at a lower wavelength. The emitted radiation will then be absorbed and
measured.
i. Applications
1. Determination of low dose formulation of fluorescent compounds in the presence of non-fluorescent
excipients.
2. In performing limit tests where the impurity is fluorescent or can be rendered as fluorescent.
3. Useful for studying the binding of drugs to components in complex formulations.
4. Bioanalysis for measuring small amounts of drugs for studying drug protein binding.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
ii. Limitations
Fluorescence is subject to interference by UV absorbing species and heavy ions in solution, and is affected
by temperature. Therefore, it is dependent on UV/VIS radiation.
➔ The transition producing the fluorescence spectrum is always from the first excited state to the ground
state. Fluorescence spectrum is independent of the wavelength used for excitation.
➔ Fig 1. shows the energy changes upon absorption of UV/VIS radiations resulting in Fluorescence.
Figure 1. Energy changes upon absorption of UV/VIS radiation resulting in fluorescence
iii. Instrumentation
Figure 2. Schematic diagram of fluorescence spectrophotometer
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
IB. Principle
Emission is the light being emitted observed at right angles to the light being used to excite the sample.
The instrument has two monochromators: one to select the wavelength to be used for excitation of the sample
and the other to scan the wavelength range of the light emitted by the sample.
Quartz halogen lamp or xenon is the lamp used that produces radiation of high intensity to take
advantage of the fact that the strength of the fluorescence is related to the number of photons absorbed
multiplied by the fluorescence quantum yield (φ).
Strongly fluorescent compounds, φ is close to 1
Non-fluorescent compounds, φ = 0
The wavelength which gives maximum excitation is not necessarily exactly the same as the longest
wavelength absorbance maximum in the compound since the intensity of light emitted by the quartz halogen
lamp varies markedly with wavelength, unlike the deuterium and tungsten lamps used in UV/visible
spectrophotometers. The lamp gives radiation of maximum intensity between 300 and 400 nm.
Although the radiation emitted is observed at right angles to the exciting radiation, some of the exciting
radiation can be detected by the emission detector, because:
❖ Types of Scatter
● Rayleigh Scatter - exciting radiation is scattered by solvent molecules
● Tyndall Scatter- exciting radiation is scattered by colloidal molecules
● Raman Scatter - exciting radiation is scattered depending on the solvent used.
The presence of this scatter makes the use of the second monochromator necessary and also means
that, for fluorescence measurements to be made without interference, the fluorescence band has to be shifted
by at least 20 nm beyond the excitation band.
In Raman scatter, which is solvent dependent, the wavelength of the incident radiation is shifted to a
longer wavelength by about 30 nm when methanol is used as a solvent and about 10 nm when chloroform is
used as a solvent. Raman scatter is discussed in more detail later in this chapter.
❖ What is a chromophore?
● part of a molecule responsible for the pigmentation
● molecules or unsaturated group that absorb light and reflects it at specific angle to give hue
● part of the molecule where absorption proceeds and where main change of the geometry or electron
density
● it appears after the excitation process
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
IC. Molecules which Exhibit Fluorescence
It is not entirely possible to predict how strongly fluorescent a molecule will be. For example, adrenaline
and noradrenaline differ in their structures by only a single methyl group but noradrenaline exhibits
fluorescence nearly 20 times more intensely than adrenaline.
Generally, fluorescence is associated with an extended chromophore/auxochrome system and a rigid
structure.
Quinine (Fig. 3a) is an example of a strongly fluorescent molecule, as might be expected from its extended
chromophore and rigid structure.
The chromophore in (Fig. 3b) ethinylestradiol is just an aromatic ring but the presence of a phenolic
hydroxyl group in combination with a rigid ring structure in the rest of the molecule renders it fluorescent.
Figure 3. Examples of fluorescent compounds a) Quinine b) Ethinylestradiol
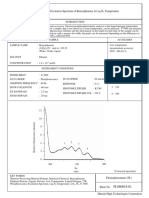
Figure 3b shows the fluorescence spectrum of ethinylestradiol. When the fluorescence spectrum of the
molecule is scanned with a wavelength of 285 nm being used for excitation, two maxima are seen.
The maxima at 285 nm is due to scatter of the exciting radiation and the second, more intense, maximum
at 310 nm is due to fluorescence. The separation of the exciting radiation and emitted radiation is not great in
this example, but this is partly because excitation is taking place at a relatively short wavelength, where the
displacement of wavelength with energy is lower.
For example, the difference between 285 and 310 nm is 0.35 eV, whereas with an excitation wavelength at
385 nm, an energy displacement of 0.35 eV would give an emission wavelength at 443 nm.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
Figure 4. Fluorescence spectrum of a 20 μg/ml solution of ethinylestradiol
➔ Like ethinylestradiol, many other phenols exhibit fluorescence and, as is the case for ethinylestradiol,
this fluorescence is pH dependent and does not occur under alkaline conditions, when the phenolic
group becomes ionised. Table 1 shows some examples of fluorescent drug and vitamin molecules.
Table 1. Examples of drugs which yield fluorescence spectra
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
ID. Factors Interfering with Fluorescence Intensity
1. CONCENTRATION. If the concentration of a solution prepared for fluorescence measurement is too
high, some of the light emitted by the sample as fluorescence will be reabsorbed by other unexcited
molecules in solution. For this reason, fluorescence measurements are best made on solutions with an
absorbance of less than 0.02 at their maximum, i.e. solutions of a sample 10–100 weaker than those which
would be used for measurement by UV spectrophotometry.
2. WEIGHT OF ATOMS. Heavy atoms in solution quench fluorescence by colliding with excited molecules
so that their energy is dissipated, e.g. chloride or bromide ions in solution cause collisional quenching.
3. COMPLEXITY OF FORMULATION. Formation of a chemical complex with other molecules in solution
can change fluorescence behaviour, e.g. the presence of caffeine in solution reduces the fluorescence of
riboflavin. This alteration of fluorescence upon binding is used to advantage when examining binding of
fluorescent molecules to proteins or other constituents of cells.
4. TEMPERATURE.
High temperature = loss of excitation by collision and bond vibration
Low temperature = ↑ Fluorescence
↑ Viscosity = ↑ Fluorescence
II. RAMAN SPECTROSCOPY
IIA. Introduction
The Raman effect is analogous to fluorescence except that it is not wavelength dependent and does
not require the molecule to have a chromophore. The energy shift in cm -1 due to inelastic scattering of laser
radiation is measured, rather than wavelength. The shifts measured correspond to the wavenumbers of the
bands present in the middle-infrared (IR) spectrum of the molecule.
i. Applications
1. Can potentially identify complex samples, e.g. drugs in formulation and in pack
2. Samples such as peptide pharmaceuticals can be analysed for changes in their three-dimensional
structure.
3. Provides additional fingerprint identity information complementary to middle-IR spectroscopy.
ii. Limitations:
1. Not yet fully established as a quantitative technique.
2. The solvent may interfere if samples are run in solution.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
➔ Wave number of the displacement of radiation by a particular group = Wave number of the radiation
absorbed by a particular group in middle IR Spectroscopy
Figure 5. Raman Scatter
➢ All molecules can be polarized so that the electrons within them are displaced slightly in the direction of
the applied field.
➢ This effect is not subject exactly to the laws of quantum mechanics, but the wavenumber of the
displacement radiation by a particular group is the same as the wavenumber of the radiation absorbed
by that particular group in middle-IR spectroscopy.
➢ In fact, the Raman effect is encountered when making fluorescence measurements in the UV/VIS
region, although it is usually weak in comparison with Rayleigh and Tyndall scatter.
➢ Fig 5 illustrates the Raman effect; the radiation can be shifted to either slightly higher energy
(anti-Stokes shift) or slightly lower energy (Stokes shift). The Stokes shift is usually determined in
Raman spectroscopy.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
iii. Instrumentation
Figure 6. Schematic diagram of a Raman spectrometer
The geometry of a Raman spectrometer is analogous to that for a fluorescence instrument. Since the
Raman effect is weak but proportional to the intensity of energy applied, lasers are used to provide
high-intensity radiation in the visible region, generally somewhere between 450 and 800 nm.
Lasers provide several emission lines, and in the case of a fluorescent molecule, a line may be selected
that gives Raman scatter where fluorescence does not interfere with the measurement. In recent years, NIR
lasers in conjunction with Fourier transform instruments have become available. The use of NIR radiation has
two advantages:
✔ Unlike UV/VIS radiation, it does not excite fluorescence in molecules, which can result in interference in
measurements.
✔ It has good penetration properties, so a sample in the solid phase can be examined without any sample
preparation.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
C. CHECK FOR UNDERSTANDING
The instructor will prepare 10-15 questions that can enhance critical thinking skills. Students will work by
themselves to answer these questions and write the rationale for each question.
Multiple Choice
(For 1-10 items, please refer to the questions in the Rationalization Activity)
RATIONALIZATION ACTIVITY (DURING THE FACE TO FACE INTERACTION WITH THE STUDENTS)
The instructor will now rationalize the answers to the students and will encourage them to ask questions and to
discuss among their classmates for 20 minutes.
1. Which of the following factors does NOT interfere with fluorescence intensity:
a. Temperature
b. Weight of solvent
c. Concentration of solution
d. Weight of atoms
e. Complex formulations
Answer: B
Rationale: Weight of solvent. Collisional quenching is caused by the weight of heavy atoms through collision
with other excited molecules in the solution so that energy can be dissipated.
2. What is the lamp used in producing radiation in fluorescence spectrophotometer?
a. Fluoride Lamp
b. Tungsten Lamp
c. Quartz Halogen Lamp/Xenon
d. UV light
Answer: C
Rationale: Quartz Halogen Lamp/Xenon is the lamp used in producing high intensity radiation in order to take
advantage of the strength of the fluorescence that is related to the number of photons absorbed and multiplied
by the fluorescence quantum yield.
3. What is the type of scatter that is being produced by emission radiation that is scattered by solvent
molecules?
a. Rayleigh Scatter
b. Tyndall Scatter
c. Raman Scatter
d. None of the above
Answer: A
Rationale: Rayleigh scattering is the type of scattering produced when emission radiation is spread by solvent
molecules. It is also known as elastic scattering.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
4. Just like UV/VIS absorption spectroscopy, radiation is used to excite the sample. Since fluorescence
involves an excitation and emission process, and the wavelengths that these two processes occur at will
almost always be the same.
a. First statement is correct
b. Second statement is correct
c. Both statements are correct
d. Both statements are incorrect
Answer: A
Rationale: Wavelength that absorption spectroscopy and emission spectroscopy processes occur at will, will
almost always be different. Fluorescence spectrophotometer always requires an excitation and emission
monochromator.
5. It is when light energy, or photons stimulate the emission of a photon.
a. Photoluminescence
b. Chemiluminescence
c. ElectroluminescencE
d. Fluorescence
Answer: A
Rationale: Chemiluminescence is defined when chemical energy stimulates the emission of a photon.
Electroluminescence is when electrical energy or strong electric field stimulates the emission of a photon.
Fluorescence is a type of photoluminescence where light raises an electron to an excited state.
6. In Beer’s Law equation An = l x c, l in the light path in m. While c is the concentration.
a. The first statement is correct. The second statement is wrong.
b. The first statement is wrong. The second statement is correct.
c. Both of the statements are correct.
d. Both of the statements are wrong.
Answer: B
Rationale: A is the absorbance at n nm, l is the light path in cm, and c is the concentration.
7. The range over which absorbance is proportional to concentration varies according to the analyte and the
wavelength of light used. To ensure that there is a direct relationship between absorbance and
concentration, we must prepare a standard curve.
a. The first statement is correct. The second statement is wrong.
b. The first statement is wrong. The second statement is correct.
c. Both of the statements are correct.
d. Both of the statements are wrong.
Answer: C
Rationale: The part of the standard curve that gives a proportional relationship is a straight line.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
8. Spectrophotometry is a measurement of how much a chemical substance absorbs or transmits.
Spectrophotometry is widely used for quantitative analysis in various areas (e.g., chemistry, physics,
biology, biochemistry, material and chemical engineering, clinical applications, industrial applications, etc).
a. The first statement is correct. The second statement is wrong.
b. The first statement is wrong. The second statement is correct.
c. Both of the statements are correct.
d. Both of the statements are wrong.
Answer: C
Rationale: Every chemical compound absorbs, transmits, or reflects light (electromagnetic radiation) over a
certain range of wavelengths.
9. A spectroscope is an instrument that measures the amount of photons (the intensity of light) absorbed after
it passes through sample solution. With the spectrophotometer, the amount of a known chemical substance
(concentrations) can also be determined by measuring the intensity of light detected.
a. The first statement is correct. The second statement is wrong.
b. The first statement is wrong. The second statement is correct.
c. Both of the statements are correct.
d. Both of the statements are wrong.
Answer: C
Spectrophotometer. It is an analytical instrument used to quantitatively measure the transmission or reflection
of visible light, UV light or infrared light.
10. Transmittance is the fraction of light that passes through the sample. In this equation, It is the light intensity
before the beam of light passes through the cuvette.
a. The first statement is correct. The second statement is wrong.
b. The first statement is wrong. The second statement is correct.
c. Both of the statements are correct.
d. Both of the statements are wrong.
Answer: A
Rationale: It is the light intensity after the beam of light passes through the cuvette and Io is the light intensity
before the beam of light passes through the cuvette. Whereby it is related to absorption by the absorbance
formula.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 8 Teacher’s Guide
D. LESSON WRAP-UP
Teacher directs the student to mark (encircle) their place in the work tracker which is simply a visual to help
students track how much work they have accomplished and how much work there is left to do. This tracker will
be part of the student activity sheet.
PERIOD 1 PERIOD 2 PERIOD 3
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
AL Strategy: MINUTE PAPER
After the instructor collects all papers, he/she will now summarize the topic. Towards the end of the class, ask
the students to bring out and write on a half sheet of paper written feedback to the following questions: Firstly,
what was the most meaningful or important thing they learned during the class. Secondly, the important
question remains unanswered. Make sure to position yourself at the door. Conversely, instruct the students to
file out towards the exit door and collect the “minute papers” as students depart from the room. Respond to
students’ feedback during the next class meeting or as soon as possible
Most meaningful or important thing they learned from this session: (Why did they find it meaningful or
important?)
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
Question which remains unanswered: (What will they do to find the answer?)
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
This document is the property of PHINMA EDUCATION
You might also like
- Experiment 34: Excited-State Properties of 2-Naphthol Part II: Deprotonation and Protonation Rate Constants ObjectiveDocument6 pagesExperiment 34: Excited-State Properties of 2-Naphthol Part II: Deprotonation and Protonation Rate Constants Objectivediego prado100% (1)
- Handbook of Fluorescent ProbesDocument950 pagesHandbook of Fluorescent ProbesYenverve KilegNo ratings yet
- Photocolorimetry and SpectrophotometryDocument11 pagesPhotocolorimetry and SpectrophotometryRay Mondy100% (1)
- Lecture 5spectro 2021 ClinicDocument10 pagesLecture 5spectro 2021 Clinicayaessam392002No ratings yet
- FluorimetryDocument24 pagesFluorimetryAhmed ZaghloulNo ratings yet
- 4 FluorescenceDocument14 pages4 FluorescenceAnushri VaidyaNo ratings yet
- IMA Lecture 11Document4 pagesIMA Lecture 11Shahrukh SindhiNo ratings yet
- Pha052 TG 5Document7 pagesPha052 TG 5Alcea InguilloNo ratings yet
- Fluorescence Spectroscopy of 5thDocument22 pagesFluorescence Spectroscopy of 5thimam mahdi tv officialNo ratings yet
- Molecular FluorescenceDocument3 pagesMolecular FluorescenceHafiz Muhammad YousafNo ratings yet
- Fluorescence SpectrosDocument2 pagesFluorescence SpectrosRishi SinghNo ratings yet
- Uv Vis & FtirDocument15 pagesUv Vis & FtirVannessa Shallomy100% (2)
- Spectro 6241 Et 6241 Et EtDocument15 pagesSpectro 6241 Et 6241 Et Etanshuman0001No ratings yet
- Uv SPDocument17 pagesUv SPछेरबहादुर लेउवाNo ratings yet
- Experiment 4Document9 pagesExperiment 4CarlosLorenzoSaninNo ratings yet
- VSI Week7 Lecture7 InstAnal 4th Stage Theory 2022Document43 pagesVSI Week7 Lecture7 InstAnal 4th Stage Theory 2022Sozdar ArgoshiNo ratings yet
- Pa A EditorialDocument4 pagesPa A EditorialChNo ratings yet
- Ultraviolet-Visible SpectroscopyDocument12 pagesUltraviolet-Visible SpectroscopySoumya Ranjan SahooNo ratings yet
- 5 FluorescenceDocument10 pages5 FluorescenceKrithika ReddyNo ratings yet
- Application in SepraratingDocument13 pagesApplication in SepraratingAta Al Subhan 355No ratings yet
- 1336336587.0185labeled Immunoassays Part 4Document7 pages1336336587.0185labeled Immunoassays Part 4Khyati B. ShahNo ratings yet
- FluorometryDocument23 pagesFluorometryFitsum DemissieNo ratings yet
- Fb5e 0507 FileDocument9 pagesFb5e 0507 FileMiyyada AichaouiNo ratings yet
- Comparison Between Spectrophotometry and Spectrofluorimetry, Its Application in Agriculture and Medicine.Document8 pagesComparison Between Spectrophotometry and Spectrofluorimetry, Its Application in Agriculture and Medicine.Ayolotu Muyiwa100% (2)
- FluorescenceDocument16 pagesFluorescenceAnkit TiwariNo ratings yet
- Flu Rome TryDocument44 pagesFlu Rome TryDanish KhanNo ratings yet
- FluorophoreDocument17 pagesFluorophoreBasab BijayeeNo ratings yet
- FAQ ColourDocument16 pagesFAQ Colourtpa141a06No ratings yet
- Spectrophotometry Guided Questions 1 PDFDocument1 pageSpectrophotometry Guided Questions 1 PDFLuci FernNo ratings yet
- BOE201 Principle Techniques of Basic Spectrophotometer AAS (1) LEE CHI YIEN 151877Document14 pagesBOE201 Principle Techniques of Basic Spectrophotometer AAS (1) LEE CHI YIEN 151877Chiyien LeeNo ratings yet
- Fluorescence Spectroscopy RDocument18 pagesFluorescence Spectroscopy RPratik KulkarniNo ratings yet
- Spectrophotometry and ColorimetryDocument5 pagesSpectrophotometry and ColorimetryHarish.UNo ratings yet
- AP Lab 4 PhotosynthesisDocument6 pagesAP Lab 4 PhotosynthesisJack LiuNo ratings yet
- Analytical MethodsDocument9 pagesAnalytical Methodspiscestrading0324No ratings yet
- IJCRT2306255Document13 pagesIJCRT2306255Ankit Kumar VermaNo ratings yet
- UV SpectrosDocument4 pagesUV SpectrosCarlton GrantNo ratings yet
- Chapter - 9 Fluorescence SpectrosDocument12 pagesChapter - 9 Fluorescence SpectrosMadhur ShrivastavaNo ratings yet
- 7483 Et EtDocument11 pages7483 Et Etrudalgupt88No ratings yet
- UV Vis DNUDocument41 pagesUV Vis DNUNasima akterNo ratings yet
- HJS Theory and Applications of Fluorescence Spectros PDFDocument37 pagesHJS Theory and Applications of Fluorescence Spectros PDFHirakjyoti SarkarNo ratings yet
- FluroscenceDocument5 pagesFluroscencePranay ChandrikapureNo ratings yet
- Fluorescence SpectrosDocument5 pagesFluorescence SpectrosrutwickNo ratings yet
- Project ReporyDocument46 pagesProject ReporyPULKIT ASATINo ratings yet
- Phychem 2 - Lab Report 2Document9 pagesPhychem 2 - Lab Report 2Ralph EvidenteNo ratings yet
- 05 FluorometerDocument26 pages05 FluorometerHassan GillNo ratings yet
- SeminarDocument23 pagesSeminarbaroque.nacNo ratings yet
- Matrix-Assisted Laser Desorption/ionization (MALDI-TOF) : MD Exam (2011) NotesDocument3 pagesMatrix-Assisted Laser Desorption/ionization (MALDI-TOF) : MD Exam (2011) Notesdr_dev09No ratings yet
- 3 UvDocument15 pages3 UvAnushri VaidyaNo ratings yet
- Indo Kiman 2Document8 pagesIndo Kiman 2HasdiNo ratings yet
- Term Photoluninescence.: Fluorescence Occurs in Complex Gaseous, Liquid, and Solid Chemical SystemsDocument8 pagesTerm Photoluninescence.: Fluorescence Occurs in Complex Gaseous, Liquid, and Solid Chemical SystemsSnape the PrinceNo ratings yet
- Limitations of LambertDocument8 pagesLimitations of Lambertmmak946.lkiNo ratings yet
- BHN Pak Adek STLH HPLCDocument175 pagesBHN Pak Adek STLH HPLCAci Lusiana100% (2)
- Spectrophotometer: - Class: 1ADocument12 pagesSpectrophotometer: - Class: 1Aniken larasatiNo ratings yet
- Ei 6501 Analytical Instruments Unit-I Colorimetry and SpectrophotometryDocument24 pagesEi 6501 Analytical Instruments Unit-I Colorimetry and SpectrophotometryBarani DharanNo ratings yet
- Himanshu Shara 2010B2A2196P: Prepared byDocument24 pagesHimanshu Shara 2010B2A2196P: Prepared byHimanshu SharaNo ratings yet
- ASSIGNMENT On FLUOROMETRY AND POLARIMETRY Pha 305 Sec-1 Group-08Document30 pagesASSIGNMENT On FLUOROMETRY AND POLARIMETRY Pha 305 Sec-1 Group-08Mahadi Hasan KhanNo ratings yet
- Molecules 17 04047 v2Document86 pagesMolecules 17 04047 v2asfsdgsadghadfhNo ratings yet
- Experiment 5 - : Quantitative Analysis by Spectrophotometric MethodsDocument7 pagesExperiment 5 - : Quantitative Analysis by Spectrophotometric MethodsBryanNo ratings yet
- 16527Document22 pages16527Jaikrishna SukumarNo ratings yet
- UV Spectroscopy - Principle, Instrumentation, Applications - Instrumentation - Microbe NotesDocument5 pagesUV Spectroscopy - Principle, Instrumentation, Applications - Instrumentation - Microbe NotesIJAJ-PHARMA TUTOR100% (2)
- Application of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionFrom EverandApplication of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionNo ratings yet
- Pha052 TG 9Document14 pagesPha052 TG 9Alcea InguilloNo ratings yet
- Pha052 TG 7Document7 pagesPha052 TG 7Alcea InguilloNo ratings yet
- Pha052 TG 5Document7 pagesPha052 TG 5Alcea InguilloNo ratings yet
- Pha052 TG 1Document7 pagesPha052 TG 1Alcea InguilloNo ratings yet
- Fluorescence SpectrosDocument31 pagesFluorescence SpectrosApurba Sarker Apu100% (2)
- Fluorescence Spectroscopy and Its Applications: A Review: International Journal of Advances in Pharmaceutical AnalysisDocument8 pagesFluorescence Spectroscopy and Its Applications: A Review: International Journal of Advances in Pharmaceutical AnalysisPriyanka KasturiaNo ratings yet
- Literature Review of Uv SpectrosDocument6 pagesLiterature Review of Uv Spectroseeyjzkwgf100% (1)
- 10.1007@978 3 030 21638 2Document214 pages10.1007@978 3 030 21638 2dortiNo ratings yet
- Algae AnalyzersDocument136 pagesAlgae Analyzerskallinganishanth3786No ratings yet
- Molecular Luminescence SpectrosDocument57 pagesMolecular Luminescence SpectrosNatasya Dwi PutriNo ratings yet
- Phosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureDocument5 pagesPhosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureNisar Ali Mphil-Chem ANo ratings yet
- Absorption and Fluoresence Spectra of Methyl Salicylate in The Vapor PhaseDocument7 pagesAbsorption and Fluoresence Spectra of Methyl Salicylate in The Vapor PhaseAriNo ratings yet
- Measurement of Fluorescence Quantum YieldsDocument4 pagesMeasurement of Fluorescence Quantum YieldsChaudhary Mandeep Singh Dalal100% (1)
- L. C. O'Brien Et Al - Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonocarboxylatesDocument5 pagesL. C. O'Brien Et Al - Gas-Phase Inorganic Chemistry: Laser Spectroscopy of Calcium and Strontium MonocarboxylatesDamxz5No ratings yet
- Summary of EXP3Document9 pagesSummary of EXP3Tany TurkiNo ratings yet
- Qubit 4 Fluorometer: Catalog Number Q33226Document72 pagesQubit 4 Fluorometer: Catalog Number Q33226bicemanNo ratings yet
- QuinineDocument9 pagesQuinineAhmad AlbabNo ratings yet
- Assignment Analysis Final2Document8 pagesAssignment Analysis Final2Mahadi Hasan KhanNo ratings yet
- Spectra, Energy Levels, and Symmetry Assignments For Stark Components of Eu3+ (4f6) in Gadolinium Gallium Garnet (Gd3Ga5O12)Document8 pagesSpectra, Energy Levels, and Symmetry Assignments For Stark Components of Eu3+ (4f6) in Gadolinium Gallium Garnet (Gd3Ga5O12)Diogo GálicoNo ratings yet
- Flu InstruDocument21 pagesFlu InstruAshok KumarNo ratings yet
- Introduction To Flow Cytometry: Principles Data Analysis Protocols TroubleshootingDocument35 pagesIntroduction To Flow Cytometry: Principles Data Analysis Protocols TroubleshootingMario LuigiNo ratings yet
- Formulation and Evaluation of Cream of AzadirachtaDocument9 pagesFormulation and Evaluation of Cream of AzadirachtashrikantmsdNo ratings yet
- Spectroscopic TechniquesDocument38 pagesSpectroscopic Techniquessamhossain1907No ratings yet
- D 3731 - 87 r98 - Rdm3mzetoddsotgDocument4 pagesD 3731 - 87 r98 - Rdm3mzetoddsotgDavid AriasNo ratings yet
- DyeLaser PDFDocument9 pagesDyeLaser PDFRavi AutiNo ratings yet
- Birks RepProgPhys 1975 38 903Document72 pagesBirks RepProgPhys 1975 38 903vanalexbluesNo ratings yet
- Chemistry 421-Lab Manual Advanced Instrumental Analysis Prof. Mike Degrandpre Spring 2012Document45 pagesChemistry 421-Lab Manual Advanced Instrumental Analysis Prof. Mike Degrandpre Spring 2012Rijjy On FreedayNo ratings yet
- FluorSpec 11Document5 pagesFluorSpec 11jtolentino88No ratings yet
- Environmental Sampling and Analysis Methods FIRST SEM 2022-2023 - SolutionsDocument12 pagesEnvironmental Sampling and Analysis Methods FIRST SEM 2022-2023 - SolutionssuryaprakashNo ratings yet
- Excitation Spectrum: If Emitting From A Single Species: Excitation Spectrum Should Match Absorption Spectrum!Document11 pagesExcitation Spectrum: If Emitting From A Single Species: Excitation Spectrum Should Match Absorption Spectrum!SidharthNo ratings yet
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDocument27 pages4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HeNo ratings yet
- Atomic Fluorescence Spectroscopy ASSESSMENTDocument4 pagesAtomic Fluorescence Spectroscopy ASSESSMENTNEIL CHRISTIAN ESTRADANo ratings yet
- Introduction To Pharmaceutical AnalysisDocument15 pagesIntroduction To Pharmaceutical AnalysisSwaroopSinghJakharNo ratings yet