Professional Documents
Culture Documents
Chemistry AQA Inorganic-And-Organic Chemistry 2 Workbook Answers
Chemistry AQA Inorganic-And-Organic Chemistry 2 Workbook Answers
Uploaded by
Aman HameedCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry AQA Inorganic-And-Organic Chemistry 2 Workbook Answers
Chemistry AQA Inorganic-And-Organic Chemistry 2 Workbook Answers
Uploaded by
Aman HameedCopyright:
Available Formats
WORKBOOK ANSWERS
AQA A-level Chemistry
Inorganic and organic chemistry 2
This Answers document provides suggestions for some of the possible answers that might be
given for the questions asked in the workbook. They are not exhaustive and other answers
may be acceptable, but they are intended as a guide to give teachers and students feedback.
Inorganic chemistry
Topic 4 Properties of period 3 elements
and their oxides
1a 4Na + O2 → 2Na2O
2Mg + O2 → 2MgO
4Al + 3O2 → 2Al2O3
Si + O2 → SiO2
P4 + 5O2 → P4O10
S + O2 → SO2
Remember that it is the oxide formed on reaction of the element with oxygen. Phosphorus and
sulfur form two oxides. It is P4O10 and SO2 that form on reaction with oxygen.
1b SO2 / sulfur dioxide
The ionic (Na2O, MgO, Al2O3) oxides are solids as is the giant covalent oxide SiO2. P4O10 is a solid
as well due to the van der Waals forces between the molecules, but SO2 is a gas as it is a small
molecule with weak intermolecular forces between the molecules.
2a Basic
2b Bonding: ionic
Structure: ionic lattice
2c Na2O + H2O → 2NaOH
2d Na2O + H2SO4 → Na2SO4 + H2O
Sodium, magnesium and aluminium form ionic compounds with oxygen. The oxides of sodium
and magnesium are basic oxides. Aluminium oxide is amphoteric.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 1
3a MgO + H2O → Mg(OH)2
3b
i MgO + 2HCl → MgCl2 + H2O
ii MgO + H2SO4 → MgSO4 + H2O
4a Al2O3 + 6H+ → 2Al3+ + 3H2O
4b Al2O3 + 2OH− + 3H2O → 2Al(OH)4−
4c Aluminate(III) or aluminate ion
5a Bonding: covalent
Structure: giant/macromolecular
5b 2NaOH + SiO2 → Na2SiO3 + H2O
Sodium silicate
6a Bonding: covalent
Structure: simple/molecular
6b P4O10 + 6H2O → 4H3PO4
Phosphoric(v) acid
6c P4O10 + 12NaOH → 4Na3PO4 + 6H2O
Sodium phosphate(V)
6d P4O10 + 6MgO → 2Mg3(PO4)2
The oxidation state is important in the IUPAC name of an acid or its salt at this level. Remember
to include it when needed and also to recognise it.
7a SO2 + H2O → H2SO3
Sulfuric(IV) acid
SO3 + H2O → H2SO4
Sulfuric(VI) acid
7b SO2 + CaO → CaSO3
7c SO3 + 2NaOH → Na2SO4 + H2O
The difference between sulfuric(IV) acid and sulfuric(VI) acid is important, as is the difference
between sulfate(IV) and sulfate(VI) ions. Make sure you look for the oxidation state as this is
important.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 2
Topic 5 and 6 Transition metals and
reactions of ions in aqueous solution
1 Answer is C
The coordination number is the number of coordinate bonds between the ligand(s) and the
central metal atom or ion. EDTA4− is a hexadentate ligand, so one ion forms six coordinate
bonds. The other ligands are monodentate so each forms a single coordinate bond with the
central metal atom or ion.
2 Answer is A
All contain 24 electrons as iron has an atomic number of 26, so the number of electrons in a 2+
ion should be 24. This is an important way to check this. Add up the superscript numbers.
Transition metal atoms lose their 4s electrons first. The most obvious distractor here is C as this
would imply that iron atoms would lose their 3d electrons to form the 2+ ion.
3a Answer is A
3b Answer is D
3c Answer is E
3d Answer is E
3e Answer is C
The shapes and coordination number of complexes are important. Create a list of complexes
with shape, coordination number, oxidation state of the transition metal atom or ion and colour
of any complex you are supposed to remember.
4a A = [Al(H2O)6]3+
B = [Cu(H2O)6]2+
C = [Fe(H2O)6]2+
4b [Al(H2O)6]3+ + 3OH− → Al(OH)3(H2O)3 + 3H2O
4c [Cu(NH3)4(H2O)2]2+
4d [Fe(H2O)6]2+ + CO32− → FeCO3 + 6H2O
4e Carbon dioxide
The reactions of ions in aqueous solution can be used to identify the complex. Make sure you
know the identity of any precipitates and be able to write equations for the reactions.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 3
Exam-style question
1a Oxidation state is +3
Coordination number is 6
Shape is octahedral
1b Brown precipitate
Does not redissolve in excess sodium hydroxide solution
1c [Fe(H2O)6]3+ + 3OH− → Fe(OH)3(H2O)3 + 3H2O
1d Brown precipitate
Bubbles of gas
1e 2[Fe(H2O)6]3+ + 3CO32− → 2Fe(OH)3(H2O)3 + 3CO2 + 3H2O
1f
i = 410 × 10−9 m
ΔE = = = = 4.85 × 10−19 J
ii For yellow solution it is bigger
Smaller means larger ν and larger ΔE
iii Any two from the list below:
identity of the metal/type of ligands/coordination number/oxidation state of the metal
or charge on the ion/shape of the complex
This type of question on one particular complex tests your knowledge of many different parts of
this section. The observations and equations for the reaction of [Fe(H 2O)6]2+, [Fe(H2O)6]3+,
[Cu(H2O)]2+ and [Al(H2O)6]3+ with sodium hydroxide solution, ammonia solution and sodium
carbonate solution are important. Remember that 3+ ions form the hydroxide precipitates with
sodium carbonate solution. The answer to (f)(i) using the other wavelength given (480 nm) would
be 4.14 × 10−19 J. Try the calculation backwards as well to make sure you can work out the
wavelength from the given value of ΔE.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 4
Variable oxidation states and catalysts
1 Answer is A
The substance that could carry out a reduction of vanadium must be on the right-hand side of
the half-equations, so it cannot be iodine.
Sulfur dioxide being oxidised to sulfate(VI) ions has a potential of −0.17 V. Adding this to each of
the potentials for the vanadium half-equations gives +0.83 V (so +5 to +4 is feasible), +0.17 V (so
+4 to +3 is feasible) and −0.42 V (so +3 to +2 is not feasible), so sulfur dioxide reduces vanadium
from +5 to +3 but not to +2.
Zinc being oxidised from Zn to Zn2+ has a potential of +0.76 V and this means that zinc will
reduce vanadium from +5 to +2 as all of the reactions are feasible.
Iodide ions being oxidised to iodine has a potential of −0.54 V, so adding to each of the
potentials for the vanadium half-equations gives +0.46 V (so +5 to +4 is feasible), 0.20 V (so +4 to
+3 is not feasible) and −0.80 V, so +3 to +2 is also not feasible. Iodide ions would reduce
vanadium from +5 to +4 but no further.
2a Colourless to pink
This titration is self-indicating, so the last drop of the purple potassium manganate( VII) solution
added does not decolourise but changes the colourless solution to pink. Mn 2+, Fe2+ and Fe3+ ions
have a very pale colour in solution compared to the intense colour of manganate( VII).
2b 5Fe2+ + MnO4− + 8H+ → 5Fe3+ + Mn2+ + 4H2O
ratio of Fe2+ : MnO4− = 5:1
2c Moles of MnO4− 3.534 × 10−4 mol
Moles of Fe2+ in 25 cm3 = 3.534 × 10−4 × 5 = 1.767 × 10−3 mol
Moles of Fe2+ in 1 dm3 = 1.767 × 10−3 × 40 = 0.0707 mol
Mr of FeSO4.7H2O = 277.9
Mass of FeSO4.7H2O = 0.0707 × 277.9 = 19.6 g
Using given ratio of 4:1 answers are 3.534 × 10−4 mol , 1.414 × 10−3 , 0.0565 mol ,
277.9 and 15.7 g
Often a ratio or numerical answer will be given to enable you to carry on with the rest of the
question and make sure you do this. The ×5 is from the ratio and ×40 is to multiply from 25 cm 3
to 1 dm3. Remember to give your answer to three significant figures. This question could have
asked for the degree of hydration of hydrated iron(II) sulfate using the initial mass (19.6 g) and
the final titre. Check that you would get 7 (or 6.96 which is what is obtained using the 19.6 g). It is
only the last couple of steps in the calculation that are different. Check it out below.
Moles of MnO4− = = 3.534 × 10−4 mol
Moles of Fe2+ in 25 cm3 = 3.534 × 10−4 × 5 = 1.767 × 10−3 mol
Moles of Fe2+ in 1 dm3 = 1.767 × 10−3 × 40 = 0.0707 mol
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 5
Mr of FeSO4.xH2O = = 277.2
Mr of xH2O = 277.2 − (55.8 + 32.1 + 4 × 16.0) = 125.3
x = 125.3 / 18.0 = 6.96 (i.e. 7)
3a S2O82− + 2I− → 2SO42− + I2
3b 2Fe2+ + S2O82− → 2Fe3+ + 2SO42−
2I− + 2Fe3+ → I2 + 2Fe2+
Reactions can occur in either order
3c Homogeneous catalysts are in the same state as the reactants
All ions here in solution/aqueous
This reaction is commonly used as an example of homogeneous catalysis. Make sure you know
the ionic equations for the main reaction and the catalysed reactions.
4 moles of MnO4− = = 1.98 × 10–3 mol
Ratio of FeC2O4 : MnO4− = 5:3
moles of FeC2O4 in 25 cm3 = × 5 = 3.3 × 10-3
moles of FeC2O4 in 250 cm3 = 3.3 × 10-3 × 10 = 0.033 mol
Mr of FeC2O4.xH2O = = 179.7
Mr of xH2O = 179.7 – (55.8 + (2 × 12.0) + (4 × 16.0)) = 35.9
x = 35.9/18.0 = 1.99 so x = 2
The most difficult part of this calculation is the ratio of FeC 2O4 : MnO4– = 5:3. It is important you
remember this or can work it out and sometimes the question will take you through the half-
equations and the ionic equations and working out the ratio. If you remember the overall ratio
then you know where you are going with the question. Both the Fe 2+ and C2O42– ions react with
MnO4−. Try repeating the calculation with an average titre of 8.40 cm3. This should give x = 3. This
is not a true value but allows you another chance to do the calculation.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 6
Exam-style questions
1a
i 5C2O42− + 2MnO4− + 16H+ → 10CO2 + 2Mn2+ + 8H2O
This equation can be worked out from the two half-equations by multiplying the top one by 2 and
the bottom one by 5 to get 10e− in each. The equations when added together form the ionic
equation.
ii C oxidation state changes from + 3 to + 4
C is oxidised
Mn oxidation state changes from + 7 to +2
Mn is reduced
Make sure you can clearly identify the element being oxidised and the element being reduced
using oxidation states. This was first met at AS but often forms parts of the A-level papers.
iii Bubbles of gas
Purple solution
Changes to colourless
You can use an equation to determine the observations expected during a reaction even if you
have never seen the reaction. In this case carbon dioxide is produced so bubbles should be
seen in the solution. Also manganate(VII) ions are purple in solution and Mn2+ ions are virtually
colourless. You should know this from manganate(VII) titrations.
1b
i Two negative ions repel each other/high activation energy
ii Production of Mn2+
Autocatalysis by Mn2+
4Mn2+ + MnO4− + 8H+ → 5Mn3+ + 4H2O
2Mn3+ + C2O42− → 2Mn2+ + 2CO2
iii Manganate(VII) ions are coloured/purple
Colorimetry
Using solutions of known concentration/calibration curve
iv Ions are used up
This reaction is often used as an example of autocatalysis, as it shows the Mn2+ ions being
formed that catalyse the reaction. This is why the reaction is slow initially as it has no catalyst
present. As the Mn2+ ions are formed in the reaction the reaction rate increases. You may be
asked to sketch this shape of graph based on your knowledge of the reaction.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 7
Organic chemistry
Topic 7 Optical isomerism
1 Answer is C
Optical isomers have a chiral centre which is a carbon with four different atoms or groups
attached.
2 Answer is C
It its good practice to quickly sketch out the isomers of C4H10O butan-1-ol, butan-2-ol, 2-
methylpropan-1-ol and 2-methylpropan-2-ol. Butan-2-ol has a chiral centre, and therefore
stereoisomers, hence the total is 5.
3 a, c, d and e have optical isomers
b and f do not
First decide if the structures have chiral centres. You may need to draw the structure out.
4a Rotates the plane of plane polarised light
4b
Remember to find the chiral centre, with four different groups attached, then draw two three-
dimensional structures, arrange the four groups on the structure and draw a mirror image.
4c Mixing equal amounts of the same concentration of two enantiomers
No effect on plane polarised light
One isomer rotates plane polarised light in one direction, the other rotates plane
polarised light in the opposite direction and the effect cancels
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 8
Topic 8 Aldehydes and ketones
1a Propanone
1b Ethanal
1c Pentan-3-one
1d Butanal
1e 3-methylpentanal
1f Pentan-2-one
1g Propanal
1h 2-methylpropanal
1i 4-methylpentanal
1j Methanal
1k 2-bromo-4-methylhexanal
1l Hexan-2-one
2a (a) and (g) (e) and (l)/(i) (c) and (f)
2b C7H13OBr
3 Answer is C
4a CH3CH2CHO + 2[H] → CH3CH2CH2OH
Reduction of an aldehyde produces a primary alcohol, in this case propan-1-ol
4b Heat under reflux propan-1-ol
4c Hydride ion
4d
4e CH3COCH3 or
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 9
An isomer has the same molecular formula (C3H6O) and a different structure hence propanone is
the isomer.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 10
Topic 9 Carboxylic acids and derivatives
Carboxylic acids and esters
1a Ethyl ethanoate
1b Pentanoic acid
1c Butanoic acid
1d 3-hydroxypropanoic acid
1e Methyl butanoate
1f Propanoic acid
1g Propyl butanoate
1h Propyl methanoate
2 Answer is D
There cannot be a straight-chain ketone with two carbon atoms as the C=O has to be in the
middle of a molecule, so the simplest straight-chain ketone is propanone C 3H6O.
3a 2CH3CH2COOH + Mg → (CH3CH2COO)2Mg + H2
3b 2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
3c CH3CH2CH2COOH + NaOH → CH3CH2COONa + H2O
3d CH3COOH + CH3CH2CH2OH ⇌ CH3COO CH2CH2CH3 H2O
3e CH3CH2COOH + CH3OH ⇌ CH3CH2COOCH3 + H2O
Remember that acid + metal gives salt and hydrogen, acid and base gives salt and water and
that acid and carbonate gives salt and water and carbon dioxide. The salts are -anoates.
Carboxylic acids react with alcohols to give esters. Esters are named as alkyl carboxylates
where the alkyl name comes from the alcohol and the carboxylate name comes from the
carboxylic acid from which they are formed.
4a Name: propane-1, 2, 3-triol
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 11
4b
4c
4d Heat with methanol
Acid catalyst
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 12
Acylation
1 Answer is D
An acid chloride reacts with an alcohol at room temperature. In this case the ester formed is
propyl ethanoate. It is not a reversible reaction.
2a Nucleophilic addition–elimination
2b N-methylpropanamide
Exam-style questions
1 Answer is A
Always make sure you number from one side; this eliminates answers B and C. The smallest
combination of numbers is chosen, hence D is incorrect.
2 Answer is C
An optically active compound has a chiral centre; a carbon with four different atoms or groups
attached. A and D do not have chiral centres. Tollens’ reagent is only reduced by aldehydes. C is
an aldehyde with a chiral centre.
3 Answer is C
The structure is an acyl chloride and it reacts with alcohols to form esters, in a reaction where
HCl is removed.
4a CH2O
The empirical formula is the simplest ratio. C16H32O2 is the molecular formula so the empirical
formula is C8H16O.
4b
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 13
5a
5b CH3CHO + HCN → CH3CHOHCN
2-hydroxypropanenitrile
5c Nucleophilic addition
5d
ii CH3CH=CHCHO
Remember that en means that a double bond is present and that it is on position 2
6a CH3OH + CH3CH2COOH ⇌ CH3CH2COOCH3 + H2O
6b Catalyst
6c H bonds between molecules of propanoic acid
These are stronger than the dipole–dipole attractions between methyl propanoate
molecules
When explaining the difference in boiling points in molecular covalent substances, always refer
to intermolecular forces.
6d Goes to completion
No heat or catalyst needed
6e C2H5COONH3 C2H5CH2OH C2H5COONa
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 14
Topic 10 Aromatic chemistry
1a C6H5COOC2H5 + HNO3 → C6H4NO2COOC2H5 + H2O
1b Conc sulfuric acid
Conc nitric acid
1c Moles of ethyl 3-nitrobenzoate = 5.85/195.0 = 0.03
For 70% yield this (0.03 × 100)/70.0 = 0.043 moles
Ratio 1:1
0.043 moles ethyl benzoate = 0.043 × 150.0 = 6.45 g
1d Substitution of NO2 at different positions forms different isomers / multiple substitution
or nitration can also occur
1e To remove impurities
Dissolve in minimum amount of hot ethanol / methanol
Filter while hot and allow (filtrate) to cool and crystals to form
Suction filtration to separate crystals
1f Between filter papers / cool oven / desiccator
1g Sharp m.pt compare to 42°C
2a Benzene is more stable than cyclohexatriene
Expected enthalpy of hydrogenation of C 6H6 is 3(−120) = −360 kJ mol−1
Actual value is less because of delocalisation, which makes benzene more stable
2b Cyclohexatriene orange → colourless
Benzene stays orange
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 15
Topic 11 Amines
1a Ethylamine/aminoethane
1b N-methylpropylamine
The prefix N is used to show that the alkyl groups are attached to the main chain via the nitrogen
atom.
1c Phenylamine
1d N-methylethylamine
1e 2-aminopropane
1f 2,4-diaminopentane
The prefix diamino is used if a compound contains two amino groups.
1g 1,3-diaminopropane
1h Diethylamine
1i 2-aminopropanoic acid
1j N,N-diethylpropylamine
2a
i CH3CH2NH2 + HCl → CH3CH2NH3Cl
ii 2CH3NH2 + H2SO4 → (CH3NH3)2SO4
iii C6H5NH2 + HNO3 → C6H5NH3NO3
iv CH3CH2NH2 + H2O → CH3CH2NH3+ + OH−
v CH3CH2NH3Cl + NaOH → CH3CH2NH2 + NaCl + H2O
2b
i Ethylamine as the electron-donating alkyl group releases electrons to the N,
meaning the lone pair is more able to accept a proton
ii Ammonia as in phenylamine the lone pair on N becomes delocalised into the pi
system so the electron density on the N is less and the lone pair is less available to
accept a proton
iii Butylamine as it has a larger electron-donating group
Remember that aliphatic amines are more basic than ammonia, which in turn is more basic than
aromatic amines.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 16
Exam-style questions
1 Answer is A
First calculate the number of moles of 4-hydroxyphenylamine (10.9/109.0 = 0.1).
Moles of paracetamol produced = 0.1. Multiply this by the formula mass, 151.0, to get 15.1 g if the
yield is 100%. The yield is 80% so the answer is 15.1 × 0.8 = 12.1 g.
2a 1,3-dinitrobenzene
The NO2 group is nitro. There are two of them, so dinitro is used in the name. The position of the
nitro groups must be given; remember to place commas between numbers and a hyphen
between a number and word.
2b 1,2-dinitrobenzene or 1,4-dinitrobenzene
The position of the NO2 group can change to give an isomer, a different structure with the same
molecular formula.
2c
i NO2+
HNO3 + 2H2SO4 → NO2+ + 2HSO4− + H3O+
ii Electrophilic substitution
Be careful that the + is on the N and that the arrow comes from the ring. The horseshoe must not
extend beyond C2 to C6.
iii Manufacture of explosives / formation of amines / organic synthesis
2d H2/Ni or H2/Pt or Sn/HCl or Fe/HCl
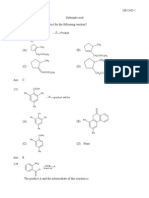
3a
Phenylethanone
3b
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 17
3c
4a A nucleophile is a lone pair donor.
Amines have a lone pair on the nitrogen and so can act as nucleophiles.
4b CH3CH2CH2Br + 2NH3 → CH3CH2CH2NH2 + NH4Br
4c Quaternary ammonium salt
Topic 12 Polymers
1 Answer is C
A and B would both form condensation polymers; A would form a polyamide polymer and B
would form a polyester polymer. D would form an addition polymer. The alcohol groups in
HO(CH2)2OH and the amino groups in H2N(CH2)4NH2 cannot react to form a polymer.
2a
or other appropriate
Any proper repeating unit is correct such as all the ones shown below. They are shown in
different colours to show you that the repeating unit can start from any part in the polymer
structure.
There are six possible repeating groups based on where you start from in the structure of the
polymer.
2b Benzene-1,4-dicarboxylic acid
Benzene-1,4-diamine
Kevlar
Clothing / bullet-proof vests
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 18
Topic 13 Amino acids, proteins and DNA
1a 2-amino-4-methylpentanoic acid
1b 2,6-diaminohexanoic acid
1c
1d
At low pH the NH2 groups and COOH groups are protonated. All NH2 groups are protonated.
2a
i
ii Adenine
iii Thymine
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 19
iv Condensation
2b Cisplatin is [Pt(NH3)2Cl2]
Coordinate bonds between nitrogen atom in guanine and cisplatin
DNA cannot be replicated/copied
Cells which are replicating are killed
Topic 14 Organic synthesis
1 Route A uses KCN
In ethanol (or aqueous)
Intermediate is butanenitrile or CH3CH2CH2CN
Lithal/LiAlH4 or H2
In dry ether for lithal or Ni/Pt/Pd for H2
Route B uses NH3
Excess NH3
It is important to be able to process the organic reactions you have met. Make sure you think
through the reactions. In Route A the carbon chain is lengthened so the reaction of the
halogenoalkane with KCN provides an extra carbon atom in the chain. The nitrile produced is
then reduced using either lithal or hydrogen to form the amine. The longer chain halogenoalkane
in Route B reacts directly with ammonia to form the amine. The conditions required in each step
are necessary. These questions are a great way to revise your organic chemistry.
Topic 15 Nuclear magnetic resonance
spectroscopy
1 Answer is C
As the molecule is symmetrical, the 1H nuclei in the two CH3 groups are chemically equivalent
and so occur at the same chemical shift. As there are no 1H nuclei bonded to the middle carbon
atom, there is no spin‒spin splitting, so there is only one singlet in the spectrum.
2 Answer is C
The first thing to consider here is the number of chemical environments. A and B have four
chemical environments whereas the spectrum shows only three sets of peaks which would
indicate three environments. C and D have three environments of 1H nuclei. In order of
increasing chemical shift, C would produce a triplet, a singlet and then a quartet as shown in the
spectrum. D would produce a triplet, a quartet and the singlet would be at the highest value.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 20
3 L is 2-methylbutanal
3-methylbutanal is not optically active and the only one which has four different groups bonded
to the same carbon atom is 2-methylbutanal. Be able to work out that C2H5, CH3 CHO and H are
the different groups.
M is 2-methylbut-2-en-1-ol or 2-methylbut-1-en-1-ol or 3-methylbut-1-en-1-ol.
Or any other appropriate structure
Any correct E‒Z isomer would be accepted but these are the answers accepted.
N is pentan-3-one.
This is the only ketone that has three peaks in its 13C NMR spectrum.
Topic 16 Chromatography
1a For spot 1 Rf = 2.5/8 = 0.3125
For spot 2 Rf = 5/8 = 0.625
For spot 3 Rf = 6.1/8 = 0.7625
Rf values are calculated by dividing the distance moved by the spot by the distance moved by
the solvent.
1b The most polar substance is 1
Slowest movement in non-polar solvent and held by polar stationery phase
The silica gel support is the stationary phase and is polar and so polar substances are held by
the stationary phase.
© Alyn G. McFarland and Nora Henry 2016 Hodder Education 21
You might also like
- Marshall Clinical Chemistry 8E (2017) PDFDocument383 pagesMarshall Clinical Chemistry 8E (2017) PDFafifah zabidi100% (3)
- M2: Exam-Requires Respondus Lockdown Browser: Not Yet Graded / 10 PtsDocument10 pagesM2: Exam-Requires Respondus Lockdown Browser: Not Yet Graded / 10 Ptssophia onuNo ratings yet
- To Synthesize Potassium Tri Oxalato Ferr PDFDocument5 pagesTo Synthesize Potassium Tri Oxalato Ferr PDFNur Aim100% (1)
- Midterm 2 - Key PDFDocument8 pagesMidterm 2 - Key PDFMichael NguyenNo ratings yet
- Redox ReactionsDocument37 pagesRedox ReactionsJack Lupino85% (13)
- Thermax ION Exchange Resins SDS BrochureDocument8 pagesThermax ION Exchange Resins SDS Brochureparthibanemails5779No ratings yet
- Prodew 400: Amino Acid Based Humectant For Skin CareDocument26 pagesProdew 400: Amino Acid Based Humectant For Skin CareKlara Miliktrisa KirbitievnaNo ratings yet
- 3A and 3BDocument13 pages3A and 3BashNo ratings yet
- Mod 5 Revision Guide 4. Transition MetalsDocument10 pagesMod 5 Revision Guide 4. Transition MetalsrammiejimjamsNo ratings yet
- Yr 10 Chem Summer NoteDocument22 pagesYr 10 Chem Summer NoteTokoni DanielNo ratings yet
- Redox Reactions II2023Document92 pagesRedox Reactions II2023nadeemyakubu47No ratings yet
- Topic 2.3: Redox: 1. Concept of Oxidation NumberDocument12 pagesTopic 2.3: Redox: 1. Concept of Oxidation NumberUmarNo ratings yet
- SELINA Solutions For Class 9 Chemistry Chapter 1Document53 pagesSELINA Solutions For Class 9 Chemistry Chapter 1thakurrmcplNo ratings yet
- REDOX REACTIONS - Practice Sheet - PACE CLASS - 11TH ONE SHOT SERIESDocument7 pagesREDOX REACTIONS - Practice Sheet - PACE CLASS - 11TH ONE SHOT SERIESpriya anbuNo ratings yet
- JEE Main Chemistry Previous Year Questions With Solutions On P BlockDocument5 pagesJEE Main Chemistry Previous Year Questions With Solutions On P BlockAshwinNo ratings yet
- Oxidation States PDFDocument5 pagesOxidation States PDFWeb BooksNo ratings yet
- What Is A Transition Metal?: 1s 2s 2p 3s 3p 4s 3d 4pDocument13 pagesWhat Is A Transition Metal?: 1s 2s 2p 3s 3p 4s 3d 4pAya MahmoudNo ratings yet
- B Masia c315 Exp 3Document6 pagesB Masia c315 Exp 3MphoNo ratings yet
- EAMCET PB Chemistry JR Inter Chem 6.hydrogen Its Comopunds 119-152Document6 pagesEAMCET PB Chemistry JR Inter Chem 6.hydrogen Its Comopunds 119-152yeateshwarriorNo ratings yet
- 15.d and F Block ElementsExerciseDocument25 pages15.d and F Block ElementsExerciseDevansh ParasharNo ratings yet
- Redox ReactionsDocument8 pagesRedox ReactionsHadia RehmanNo ratings yet
- Kmno4 and K2cr2o7 PDFDocument6 pagesKmno4 and K2cr2o7 PDFBrown BoyNo ratings yet
- Ib Chemistry: Higher LevelDocument64 pagesIb Chemistry: Higher LevelLouis RahardjaNo ratings yet
- A TransironDocument2 pagesA TransironManoj oliNo ratings yet
- Chem (Soln) CH 8Document27 pagesChem (Soln) CH 8RahulMittalNo ratings yet
- CLS Aipmt 16 17 XI Che Study Package 2 SET 1 Chapter 8Document24 pagesCLS Aipmt 16 17 XI Che Study Package 2 SET 1 Chapter 8Aakash PatilNo ratings yet
- NEET 2015 Question Paper With Answers (Code A) PDF DownloadDocument56 pagesNEET 2015 Question Paper With Answers (Code A) PDF Downloadharsharma5636No ratings yet
- U3 Oxidation and Reduction PPT WatermarkDocument45 pagesU3 Oxidation and Reduction PPT Watermarkapi-125934329No ratings yet
- Chem Ch04 Lecture 6eDocument85 pagesChem Ch04 Lecture 6eJF LohNo ratings yet
- 6 Redox (2) (S)Document18 pages6 Redox (2) (S)Mr TanNo ratings yet
- Redox Reactions Chemistry Unit 1Document7 pagesRedox Reactions Chemistry Unit 1mcleodtravis14No ratings yet
- D-And F-Block ElementsDocument5 pagesD-And F-Block ElementsArchanaa PadmavathiNo ratings yet
- An Introduction To The Chemistry of Transition ElementsDocument13 pagesAn Introduction To The Chemistry of Transition Elementsbubutrain2003No ratings yet
- D-F-Block IIT JEEDocument26 pagesD-F-Block IIT JEEThe Rock89% (9)
- 6.hydrogen Its Comopunds 119-152Document6 pages6.hydrogen Its Comopunds 119-152eamcetmaterialsNo ratings yet
- Stoichiometry 7Document4 pagesStoichiometry 7sophiaccharlotte876No ratings yet
- Ib PPT 9 SL PDFDocument38 pagesIb PPT 9 SL PDFzarna nirmal rawalNo ratings yet
- Chem Basic FB Answer Key CH 22 (06.14.16)Document4 pagesChem Basic FB Answer Key CH 22 (06.14.16)eman mamdohNo ratings yet
- 8 Redox Reactions: SolutionsDocument22 pages8 Redox Reactions: SolutionsAnil AggaarwalNo ratings yet
- Chapter 8 Redox-ReactionsDocument33 pagesChapter 8 Redox-ReactionsRohan PatelNo ratings yet
- Redox Titrations-Lectures 8-9Document47 pagesRedox Titrations-Lectures 8-9noor88No ratings yet
- ElectrochemistryDocument28 pagesElectrochemistryeuginemwakhaNo ratings yet
- Physical Properties and Reactions of Period 3 OxidesDocument2 pagesPhysical Properties and Reactions of Period 3 OxidesShaNthini ManohaRan100% (1)
- To Synthesize Potassium Tri Oxalato Ferr PDFDocument5 pagesTo Synthesize Potassium Tri Oxalato Ferr PDFApheleleNo ratings yet
- CLS Aipmt-18-19 XIII Che Study-Package-2 SET-1 Chapter-8 PDFDocument24 pagesCLS Aipmt-18-19 XIII Che Study-Package-2 SET-1 Chapter-8 PDFAnonymous 3BGpnwQ100% (1)
- JEE Main Chemistry Previous Year Questions With Solutions On Salt AnalysisDocument4 pagesJEE Main Chemistry Previous Year Questions With Solutions On Salt AnalysisAbhishek KumarNo ratings yet
- Ncert Redox Reaction SolutionsDocument32 pagesNcert Redox Reaction SolutionsLikhith UsurupatiNo ratings yet
- Pages From Chemical Bonding Jee MainDocument5 pagesPages From Chemical Bonding Jee MainYuvarajNo ratings yet
- +1 Chemistry - Some Important Questions & Answers PART 2Document12 pages+1 Chemistry - Some Important Questions & Answers PART 2ahammedkayangalkayangalNo ratings yet
- Naming CompoundsDocument27 pagesNaming CompoundsAtulya BharadwajNo ratings yet
- 2-Excellent Chemistry Assignment D-And F-Block ElementsDocument5 pages2-Excellent Chemistry Assignment D-And F-Block ElementsSachin B SNo ratings yet
- Chem Practice Test: 7.50 Moles 4.41 Moles 4.16 Moles 1.35 × 103 Moles 75.0 MolesDocument39 pagesChem Practice Test: 7.50 Moles 4.41 Moles 4.16 Moles 1.35 × 103 Moles 75.0 MolesMorgan BlockNo ratings yet
- C5 ElectrochemistryDocument87 pagesC5 ElectrochemistryLily Anth100% (1)
- D and F Study MaterialDocument5 pagesD and F Study MaterialxxcosmozerxxNo ratings yet
- Learner'S Activity Sheet in Grade 12 General Chemistry 1 For Quarter 1, Week 3Document7 pagesLearner'S Activity Sheet in Grade 12 General Chemistry 1 For Quarter 1, Week 3cutiepie creampieNo ratings yet
- Oxidation NumbersDocument6 pagesOxidation NumbersWeb BooksNo ratings yet
- 16Document4 pages16Shazia FarheenNo ratings yet
- Chapter 8 11th Class NewlyDocument29 pagesChapter 8 11th Class NewlyanujkhotaNo ratings yet
- Jr. INTER CHEMISTRY (E.m) PDFDocument12 pagesJr. INTER CHEMISTRY (E.m) PDFkrish100% (1)
- Redox Practice 3Document3 pagesRedox Practice 3docmagnusNo ratings yet
- Chemistry An Introduction To The Chemistry of D-Block ElementsDocument27 pagesChemistry An Introduction To The Chemistry of D-Block ElementsSIVANESVARAN100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Practice Makes Perfect in Chemistry: Compounds, Reactions and MolesFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and MolesNo ratings yet
- Cambridge IGCSE Chemistry Paper 32 June 2014Document12 pagesCambridge IGCSE Chemistry Paper 32 June 2014FazalAmin100% (1)
- Carboxylic Acid and DerivativesDocument8 pagesCarboxylic Acid and Derivativesmark angel andayaNo ratings yet
- PDF Intermediate Organic Chemistry Third Edition Fabirkiewicz Ebook Full ChapterDocument63 pagesPDF Intermediate Organic Chemistry Third Edition Fabirkiewicz Ebook Full Chaptercalvin.stewart388100% (4)
- Hasan Sayginel: Edexcel A Level Organic ChemistryDocument41 pagesHasan Sayginel: Edexcel A Level Organic ChemistryDEEBANNo ratings yet
- Class 12 Chemistry Ch-8.Aldehydes, Ketones and Carboxylic AcidsDocument53 pagesClass 12 Chemistry Ch-8.Aldehydes, Ketones and Carboxylic Acidskingoo0f1No ratings yet
- Sma Negeri 1 Kediri: Veteran Street Number 1Document54 pagesSma Negeri 1 Kediri: Veteran Street Number 1gnuga12417No ratings yet
- Class 12 Book 5 Organic Chemistry Carbonyl CompoundDocument28 pagesClass 12 Book 5 Organic Chemistry Carbonyl CompoundHarshad SSNo ratings yet
- Carboxylic Acid Derivatives: 1. The Correct Explanation For The Below Reaction IsDocument20 pagesCarboxylic Acid Derivatives: 1. The Correct Explanation For The Below Reaction Issree anugraphicsNo ratings yet
- Classification of MatterDocument22 pagesClassification of MatterDeane Marc TorioNo ratings yet
- Synthesis of Benzyl Acetate Through Fischer Esterification ReactionDocument10 pagesSynthesis of Benzyl Acetate Through Fischer Esterification ReactionAnonymous GO6JVW9Wud100% (4)
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- HemiJske Osobine Mravlje KiselineDocument20 pagesHemiJske Osobine Mravlje KiselinemiljceNo ratings yet
- Bull'S Eye Content: C H E M I S T R YDocument3 pagesBull'S Eye Content: C H E M I S T R YHitesh KumarNo ratings yet
- Organic 2 Chapter 1&2Document94 pagesOrganic 2 Chapter 1&2bahru demekeNo ratings yet
- General Organic Chemistry: Type of Hybridisation SP SP SPDocument28 pagesGeneral Organic Chemistry: Type of Hybridisation SP SP SPjesiNo ratings yet
- Oxygen Containing Organic Compound-III - WorkbookDocument41 pagesOxygen Containing Organic Compound-III - Workbookagrimsinghal28No ratings yet
- Chem204 Lecture 1 EnglishDocument32 pagesChem204 Lecture 1 EnglishДууяа Б.No ratings yet
- Alcohols Ethers and Phenol-02 Solved ProblemsDocument13 pagesAlcohols Ethers and Phenol-02 Solved ProblemsRaju SinghNo ratings yet
- 2020 H2 MCT Section C Ans (2023 Edition) 2Document4 pages2020 H2 MCT Section C Ans (2023 Edition) 2Shereen LimNo ratings yet
- Jurnal Aldehid Dan KetonDocument170 pagesJurnal Aldehid Dan KetonMsv Desma AdriansyahNo ratings yet
- Amine PDFDocument10 pagesAmine PDFRakesh ChadhaNo ratings yet
- Carboxylic Acids: Organic ChemistryDocument39 pagesCarboxylic Acids: Organic ChemistryHezron BumbunganNo ratings yet
- Carboxylic AcidsDocument12 pagesCarboxylic Acidsxdawy2004xdNo ratings yet
- Chemical Identification Surface: GroupsDocument96 pagesChemical Identification Surface: GroupsAbdelhak BelbaliNo ratings yet
- CLS JEEAD-19-20 XII Che Target-4 Level-1 Chapter-12 PDFDocument18 pagesCLS JEEAD-19-20 XII Che Target-4 Level-1 Chapter-12 PDFDK SainiNo ratings yet
- Cita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicDocument107 pagesCita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicYushau Muhammad LawalNo ratings yet