Professional Documents
Culture Documents
Nphoton 2017 55-3
Nphoton 2017 55-3
Uploaded by
billyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nphoton 2017 55-3
Nphoton 2017 55-3
Uploaded by
billyCopyright:
Available Formats
ARTICLES NATURE PHOTONICS DOI: 10.1038/NPHOTON.2017.
55
a b 47 nm e
Intensity (norm.)
Diffraction dSTORM
limited ×60 NA 1.2 1.0 50 µm
0.5
0.0
0 50 100 150
2 µm Position (nm)
1 µm
f dSTORM
c Objective-based Waveguide chip- ×20 NA 0.45
TIRF dSTORM based dSTORM
5 µm Diffraction
50 nm 50 nm limited
Intensity (norm.)
Intensity (norm.)
d 49 nm g 138 nm
1.0 1.0
0.5 0.5
0.0 0.0 Diffraction dSTORM
−50 0 50 100 0 200 400 limited ×20 NA 0.45
Position (nm) Position (nm)
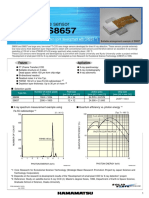
Figure 2 | Demonstration of chip-based dSTORM. a, Diffraction-limited and dSTORM imaging of immunostained tubulin in liver sinusoidal endothelial cells
(LSECs). b, Measuring a lateral profile of 540 nm width along a straight microtubule (magenta marking in the inset of the dSTORM image in a) reveals its
hollow structure. c, The resolution capability is further investigated by imaging DNA-origami nanorulers of (50 ± 5) nm specified length, which can be clearly
resolved with waveguide-based dSTORM, similar to objective-based TIRF dSTORM. d, Analysing their line profiles, a mean nanoruler length of 49 nm is
found in both cases, confirming that the chip-based implementation shows comparable performance to a conventional inverted dSTORM set-up.
e, Waveguide chip-based illumination also allows for using a low-magnification/low-NA (×20/NA 0.45) objective lens for dSTORM imaging over a
FOV of 0.5 mm × 0.5 mm. f, A detail from the white box in e. g, The profile over adjacent tubulin filaments reveals their separation by 138 nm.
this problem, a piezo stage is used to oscillate the coupling objective in contrast to the dSTORM acquisition procedure where single-
lens or fibre back and forth along the input facet of the waveguide molecule switching is required. While continuously changing the
during the measurement. This maintains continuous coupling but excitation pattern in this manner, about 200 frames are recorded.
shifts the mode pattern to obtain an average distribution that The acquired data are used as the input for the ESI reconstruction
shows significantly less modulation over larger length scales than algorithm to generate a super-resolved image (Fig. 3a), which we
in conventional TIRF set-ups (Supplementary Figs 8 and 9 and demonstrate by imaging tubulin in LSECs (Fig. 3b,c).
Supplementary Movie 2). Consequently, the distinct modes are Following the ESI acquisition, the same sample is again imaged
not visible in the reconstructed dSTORM images. using dSTORM by increasing the illumination intensity. This
allows for a direct comparison of the ESI image with the
Chip-based fluctuation imaging. In contrast, fluctuations induced dSTORM image of higher resolution but requiring a much higher
by the shifted mode pattern are desired in the case of chip-based number of input frames (Supplementary Table 1). Hence,
ESI. Although original implementations of the fluctuation-based dSTORM serves as a control reference for assessing the performance
approaches SOFI and ESI use intrinsic quantum dot or of chip-based ESI. This verifies that the resolution of the ESI image
fluorophore temporal intensity fluctuations (for example, due to is on the order of 110 nm, as adjacent microtubules at 106 nm
blinking and bleaching), it has recently been shown that speckle distance are still resolved and simultaneously observed in both
pattern illumination38 can also invoke temporal emission super-resolved images (Fig. 3c,d). This resolution is also confirmed
fluctuations, allowing for super-resolved fluctuation imaging39. by a FWHM of 104 nm of a single tubule in the ESI image
Intrinsic intensity fluctuations originate from single emitters and (Supplementary Fig. 12). Thus, chip-based ESI using spatial
therefore spatially tightly confined sources. In contrast, the spatial excitation pattern fluctuations readily achieves a resolution
frequencies of the waveguide illumination pattern define the enhancement of about a factor of 2, using an NA 1.2 objective
length scales on which the fluctuations occur and, hence, the lens for fluorescence detection (Supplementary Note 1).
obtainable resolution (Supplementary Fig. 10 and Supplementary Chip-based dSTORM pushes the resolution even further, admittedly
Note 1). Using dSTORM, we have measured fringes in the at the cost of longer acquisition times.
multimode interference pattern of a waveguide. The FWHM of
the structure sizes is as small as 140 nm for a vacuum laser Scalable super-resolution imaging. As the evanescent field generation
excitation wavelength of 660 nm (Supplementary Fig. 11). in waveguide chip-based nanoscopy does not depend on the objective
To use the illumination intensity fluctuations induced by the lens used for fluorescence detection, the presented approaches can be
waveguide for ESI analysis, we oscillate the coupling along the applied for successive image acquisitions at different magnifications,
input facet such that the mode distribution changes from image allowing for scalable FOV imaging. To obtain an overview image
to image. As random intensity fluctuations are desired, there is no with a large FOV, low-magnification/low-NA lenses can be used.
need to further control the illumination pattern besides changing If higher resolution is desired, specific regions of interest (ROIs)
it from frame to frame. Low input power is used, keeping the inten- can be imaged at superior resolution afterwards by switching to a
sity under the threshold of undesired single-molecule switching, high-magnification/high-NA objective lens.
324 NATURE PHOTONICS | VOL 11 | MAY 2017 | www.nature.com/naturephotonics
© 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved.
You might also like
- 2019 NatureElec M. Holler Et Al. - Three-Dimensional Imaging of Integrated CircuitsDocument7 pages2019 NatureElec M. Holler Et Al. - Three-Dimensional Imaging of Integrated CircuitsMaximeNo ratings yet
- FIRAT: A Fast and Sensitive Probe Structure For SPMDocument38 pagesFIRAT: A Fast and Sensitive Probe Structure For SPMLIAKMANNo ratings yet
- Advantages of The Microscope For Raman SamplingDocument11 pagesAdvantages of The Microscope For Raman SamplingJESUS RAUL BELTRAN RAMIREZNo ratings yet
- Validasi Uv-Vis Spektrofotometer: Solution For ScienceDocument13 pagesValidasi Uv-Vis Spektrofotometer: Solution For ScienceadelasyafiraNo ratings yet
- Specification Mechelle 7500 Integrated With The DiCAM-PRO ICCD ...Document4 pagesSpecification Mechelle 7500 Integrated With The DiCAM-PRO ICCD ...Saranya VsNo ratings yet
- Determination of The Diffraction Intensity at Slit and Double Slit SystemsDocument5 pagesDetermination of The Diffraction Intensity at Slit and Double Slit SystemsJose Galvan100% (1)
- Product-Specification-EAGLE-Raman-SDocument6 pagesProduct-Specification-EAGLE-Raman-SHasan KurtNo ratings yet
- Basico - Chapter 1 PDA Overview Rev 32512Document22 pagesBasico - Chapter 1 PDA Overview Rev 32512MarlonNo ratings yet
- Interactions of 3D Mask Effects and NA in EUV LithographyDocument12 pagesInteractions of 3D Mask Effects and NA in EUV LithographyGary Ryan DonovanNo ratings yet
- Sky Radiance and ConversionDocument10 pagesSky Radiance and Conversioncours2thomasNo ratings yet
- Design of Piezoresistive Microcantilever For Mass Sensing: V. Jyothi, B. Rajesh Kumar, V. Suresh, Ch. IndraniDocument7 pagesDesign of Piezoresistive Microcantilever For Mass Sensing: V. Jyothi, B. Rajesh Kumar, V. Suresh, Ch. Indraniijieee ijieeeNo ratings yet
- Diffraction Intensity of Multiple Slits and GridsDocument5 pagesDiffraction Intensity of Multiple Slits and GridsJose Galvan0% (1)
- Lab Manuel Michelson InterformerDocument9 pagesLab Manuel Michelson InterformerAryan VaidNo ratings yet
- Sentaurus Technology Template - Light-Triggered Thyristor - SynopsysDocument5 pagesSentaurus Technology Template - Light-Triggered Thyristor - SynopsysShyam TrivediNo ratings yet
- Diffraction IntensityDocument3 pagesDiffraction IntensityOktafiani Nurita SariNo ratings yet
- Laser Raman SpectrometerDocument8 pagesLaser Raman SpectrometerMai PhuNo ratings yet
- InterformatersDocument3 pagesInterformatersRadha BagriNo ratings yet
- Field Guide To Digital Micro-OpticsDocument5 pagesField Guide To Digital Micro-OpticslantordoNo ratings yet
- Konica Centuria Super 1600 Film: Technical Data SheetDocument3 pagesKonica Centuria Super 1600 Film: Technical Data SheetRichard KingstonNo ratings yet
- InfiniteFocusSL - AliconaDocument4 pagesInfiniteFocusSL - AliconaGian RemundiniNo ratings yet
- Veraview X800 F150 Brochure L-1349 0418 v6 BDocument18 pagesVeraview X800 F150 Brochure L-1349 0418 v6 Bمصعب بابكرNo ratings yet
- Holographic Optical Tweezers With Real-Time Hologram Calculation Using A Phase-Only Modulating LCOS-based SLM at 1064 NMDocument10 pagesHolographic Optical Tweezers With Real-Time Hologram Calculation Using A Phase-Only Modulating LCOS-based SLM at 1064 NMcarlosj114No ratings yet
- Effect of Deposition Time On The Properties of Al Doped Zno Films Prepared by DC Magnetron SputteringDocument4 pagesEffect of Deposition Time On The Properties of Al Doped Zno Films Prepared by DC Magnetron SputteringLoubna MentarNo ratings yet
- Schatz MinnesotaDocument46 pagesSchatz MinnesotaAnunnaki SumerianNo ratings yet
- 3-High Resolution Transmission Electron MicrosDocument47 pages3-High Resolution Transmission Electron Microskmesjimmy58745No ratings yet
- LUCID Time of Flight GuideDocument18 pagesLUCID Time of Flight GuideGiovaniAricettiNo ratings yet
- Fourier Transform Infrared (FT-IR) Microspectroscopy: Methods and ApplicationsDocument77 pagesFourier Transform Infrared (FT-IR) Microspectroscopy: Methods and ApplicationsmaopacificNo ratings yet
- Deep Learning-Based Image Classification Through ADocument10 pagesDeep Learning-Based Image Classification Through AJeya SionaNo ratings yet
- TMP FD52Document4 pagesTMP FD52FrontiersNo ratings yet
- 0-7803-7185-2/02/$10.00 ©2002 IeeeDocument4 pages0-7803-7185-2/02/$10.00 ©2002 IeeedenghueiNo ratings yet
- Lecture4-RadPhysNucMed PartcDocument15 pagesLecture4-RadPhysNucMed PartcMSNo ratings yet
- JBX-6300FSe2 12P 0329Document6 pagesJBX-6300FSe2 12P 0329johnNo ratings yet
- Pavement Design IRC 37Document2 pagesPavement Design IRC 37Subhransu Sekhar SwainNo ratings yet
- CA2IDocument12 pagesCA2IayjezNo ratings yet
- MOE and GDocument5 pagesMOE and GJuan Cruz RaccaNo ratings yet
- Lab Manual_CSE STREAMDocument49 pagesLab Manual_CSE STREAMImpanaNo ratings yet
- SpectralGuide - Duncan Spectral Configuration Guide For DuncanTech 3-CCD CamerasDocument7 pagesSpectralGuide - Duncan Spectral Configuration Guide For DuncanTech 3-CCD CamerascabrahaoNo ratings yet
- SESAM Saturable AbsorbersDocument51 pagesSESAM Saturable AbsorbersVineeth PLNo ratings yet
- Diffraction Gratings - HORIBADocument10 pagesDiffraction Gratings - HORIBAhasantapNo ratings yet
- 6 Ginzel PDFDocument8 pages6 Ginzel PDFChromoNo ratings yet
- DAC CurveDocument8 pagesDAC Curvemkseth20071No ratings yet
- Chapter 2 - Wave Diffraction - Part 3Document26 pagesChapter 2 - Wave Diffraction - Part 3Goh boon tongNo ratings yet
- Brosur Zeiss IntrabeamDocument24 pagesBrosur Zeiss IntrabeamRlisastNo ratings yet
- Fabry PerotDocument11 pagesFabry PerotG. P HrishikeshNo ratings yet
- Uniform Fiber Bragg Grating: PurposeDocument39 pagesUniform Fiber Bragg Grating: PurposeAnonymous gdutVXlUTNo ratings yet
- FDTD Calculations of The Divergence Angle of Multi-Mode VCSELsDocument10 pagesFDTD Calculations of The Divergence Angle of Multi-Mode VCSELsJohn pengNo ratings yet
- 2009 Tacona-2009Document1 page2009 Tacona-2009Josep Ferre-BorrullNo ratings yet
- FHDK10Document4 pagesFHDK10Nugroho EkoNo ratings yet
- New! F-2710 Fluorescence Spectrophotometer: Hitachi Product NewsDocument2 pagesNew! F-2710 Fluorescence Spectrophotometer: Hitachi Product NewsdaikizhitaNo ratings yet
- Sieving 1Document3 pagesSieving 1Deby Hajjar RakhmadumilaNo ratings yet
- CCD X-Ray Detectors8656Document2 pagesCCD X-Ray Detectors8656richartinNo ratings yet
- Supplementary Information Tinylev: A Multi-Emitter Single-Axis Acoustic LevitatorDocument8 pagesSupplementary Information Tinylev: A Multi-Emitter Single-Axis Acoustic LevitatorPaul Si Adriana VintilaNo ratings yet
- Film Negative ScanningMetricDocument13 pagesFilm Negative ScanningMetricMoaz ElgabryNo ratings yet
- Melding Two Worlds Into One Benefit.: Zeiss Intrabeam 600Document24 pagesMelding Two Worlds Into One Benefit.: Zeiss Intrabeam 600chNo ratings yet
- Nano - Micro-Scale Thermal Transport - Sept 19, 2023 - CompressedDocument88 pagesNano - Micro-Scale Thermal Transport - Sept 19, 2023 - CompressedtamernuraqNo ratings yet
- Fabry Perot Interferometer GovindDocument3 pagesFabry Perot Interferometer Govinddevanarayan2000No ratings yet
- Impact of Apodisation Slope Asymmetry in Linearly Chirped Dispersion Compensating Fiber Bragg GratingDocument4 pagesImpact of Apodisation Slope Asymmetry in Linearly Chirped Dispersion Compensating Fiber Bragg Gratingakashbhargava1No ratings yet
- The Performance of The Model 7400 VSM SensitivityDocument4 pagesThe Performance of The Model 7400 VSM Sensitivityamr khaledNo ratings yet
- Budinger 1983Document6 pagesBudinger 1983Merve CinoğluNo ratings yet