Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
13 viewsReaction Types Table
Reaction Types Table
Uploaded by
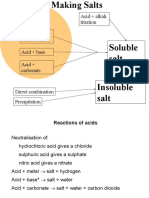
superintendentThe document describes 9 common chemical reaction types including neutralization, acid-metal, acid-carbonate, precipitation, decomposition, respiration, digestion, corrosion, and combustion. For each reaction type it provides the general chemical equation and an example reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Chapter 01 - Chemistry and MeasurementDocument56 pagesChapter 01 - Chemistry and MeasurementJamie B100% (1)
- Aluminium and Its Compound Lab ReportDocument7 pagesAluminium and Its Compound Lab ReportLevina Arastika100% (1)
- Latihan Mip Kimia 2021 Form 4 & 5Document81 pagesLatihan Mip Kimia 2021 Form 4 & 5unknown :)100% (1)
- Elements and Compounds g8Document5 pagesElements and Compounds g8Fahad RashidNo ratings yet
- Equations Worksheet 1Document2 pagesEquations Worksheet 1jaikovskyNo ratings yet
- PP Acid ReactionsDocument14 pagesPP Acid Reactionsapi-3696266No ratings yet
- Science 1.5 Answers Worksheet Five Acids: Acid + Base Salt + WaterDocument2 pagesScience 1.5 Answers Worksheet Five Acids: Acid + Base Salt + Watermintesinot esayasNo ratings yet
- NeutralizationDocument13 pagesNeutralizationbarakahmuluziNo ratings yet
- Arlan Neutralization WorksheetDocument3 pagesArlan Neutralization WorksheetHEY ERLNo ratings yet
- Chemistry F4 SaltsDocument13 pagesChemistry F4 Saltscivichitam18No ratings yet
- BalanceDocument2 pagesBalancebratajeet.ghosh.14No ratings yet
- Chemical ReactionsDocument2 pagesChemical ReactionsKrydztom UyNo ratings yet
- Acids, Bases & Salts: IndicatorsDocument7 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Topic7-Oxides and Salts-L2Document43 pagesTopic7-Oxides and Salts-L2haotongxu14No ratings yet
- Cramming NotesDocument7 pagesCramming NotesMuhammad ali WasimNo ratings yet
- Worksheet 1 Reactions of Acids and Bases: Danielle AkinlaluDocument3 pagesWorksheet 1 Reactions of Acids and Bases: Danielle AkinlaludanielleNo ratings yet
- Salts and AcidsDocument3 pagesSalts and AcidskmbgtssnbmNo ratings yet
- Preparing Common Salts G8Document20 pagesPreparing Common Salts G8diamehta1512No ratings yet
- Acid and Base ReactionsDocument15 pagesAcid and Base ReactionsNubar MammadovaNo ratings yet
- 04 Acid Reaction DrillsDocument2 pages04 Acid Reaction Drillsfuzzyatom12345No ratings yet
- Acids, Bases & Salts: IndicatorsDocument4 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Cha 11Document11 pagesCha 11Tun Lin AungNo ratings yet
- Acids, Bases and SaltsDocument4 pagesAcids, Bases and Saltsbubutrain2003No ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Chemical Changes Mastery Part 4: AcidsDocument2 pagesChemical Changes Mastery Part 4: AcidsJoeNo ratings yet
- CA DEM Y: Chapter - 2 - NOTESDocument2 pagesCA DEM Y: Chapter - 2 - NOTESvarun puriNo ratings yet
- Prep3 Final Revision..Document26 pagesPrep3 Final Revision..Amira Hekal0% (1)
- Common Names of CompoundsDocument1 pageCommon Names of CompoundsRiannNo ratings yet
- EquationsWorksheet3 PDFDocument2 pagesEquationsWorksheet3 PDFNarci ssusNo ratings yet
- !chemistry Review ANSDocument3 pages!chemistry Review ANSAngel LiNo ratings yet
- Acid, Bases and Salts Class 10Document7 pagesAcid, Bases and Salts Class 10Gowtham LNo ratings yet
- Reactions of Metals With Dilute AcidsDocument3 pagesReactions of Metals With Dilute AcidsDarshanaK 728714No ratings yet
- Cha 12Document9 pagesCha 12Tun Lin AungNo ratings yet
- Chemistry Worksheet 5Document4 pagesChemistry Worksheet 5Deandra AliciaNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- Summary of ReactionsDocument1 pageSummary of ReactionsBubbleNo ratings yet
- Acids, Bases and SaltsDocument8 pagesAcids, Bases and Saltsaakashb1918No ratings yet
- Acids Bases and SaltsDocument45 pagesAcids Bases and SaltsTejas PagarNo ratings yet
- 15281456Document15 pages15281456Manar KamelNo ratings yet
- OXIDES (Metals & Non-Metals)Document4 pagesOXIDES (Metals & Non-Metals)gauri guptaNo ratings yet
- VIII Chemistry PWS 2Document2 pagesVIII Chemistry PWS 2Ishama ZarintaNo ratings yet
- Evolution of Gas:: Other Endothermic ReactionsDocument7 pagesEvolution of Gas:: Other Endothermic Reactionssara parkNo ratings yet
- Common Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLDocument3 pagesCommon Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLFrederick FranciscoNo ratings yet
- Final - Naming of Compounds PDFDocument7 pagesFinal - Naming of Compounds PDFSnorlax Magno100% (1)
- Danielle N. - Making-Salts-differentiated-worksheetDocument2 pagesDanielle N. - Making-Salts-differentiated-worksheetdanielle njorogeNo ratings yet
- Equations Involving AcidsDocument1 pageEquations Involving AcidsYoussef AmrNo ratings yet
- Week 9 l1Document1 pageWeek 9 l1JennieNo ratings yet
- Carbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDocument3 pagesCarbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDSE No WorriesNo ratings yet
- Worksheet 9.6B: Word EquationsDocument1 pageWorksheet 9.6B: Word Equationsshaha waseemNo ratings yet
- 1.1 Types of ReactionsDocument10 pages1.1 Types of ReactionstangwindsonNo ratings yet
- Acids and Bases SummaryDocument2 pagesAcids and Bases SummaryTan Yong KhaiNo ratings yet
- Acid Bases SummaryDocument8 pagesAcid Bases Summaryibraheemgamer786No ratings yet
- Lesson Element Making Salts: Instructions and Answers For TeachersDocument17 pagesLesson Element Making Salts: Instructions and Answers For TeachersGracey- Ann JohnsonNo ratings yet
- SLOP - Writing Word and Symbol Equations For Reactions of MetalsDocument2 pagesSLOP - Writing Word and Symbol Equations For Reactions of Metalsmarcos.vaqueNo ratings yet
- ABS Complete - ChemisteryDocument71 pagesABS Complete - ChemisterymitaNo ratings yet
- Acid Base and Salt Revision NoteDocument7 pagesAcid Base and Salt Revision NoteHassan mahmud50% (2)
- Oxides, Acids, Bases and SaltsDocument8 pagesOxides, Acids, Bases and Salts12&13 SciencesNo ratings yet
- Acids + CarbonatesDocument13 pagesAcids + CarbonatesVithuNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Preparing Common Salts G8Document21 pagesPreparing Common Salts G8shanaayaa kunder100% (1)
- Chemistry Notes Acids Bases and SaltsDocument7 pagesChemistry Notes Acids Bases and SaltsGouri RajNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- EXTQSORW3Document1 pageEXTQSORW3superintendentNo ratings yet
- 10.5 Legal Studies MC QuizDocument4 pages10.5 Legal Studies MC QuizsuperintendentNo ratings yet
- PLKDocument7 pagesPLKsuperintendentNo ratings yet
- W1LEGALANSDocument1 pageW1LEGALANSsuperintendentNo ratings yet
- Year10 Insight Chapter 3 Linear RelationshipsDocument35 pagesYear10 Insight Chapter 3 Linear RelationshipssuperintendentNo ratings yet
- Ch9 Signpost 10Document77 pagesCh9 Signpost 10superintendentNo ratings yet
- AKDocument25 pagesAKsuperintendentNo ratings yet
- Data Analysis & Evaluation ANSWERSDocument7 pagesData Analysis & Evaluation ANSWERSsuperintendentNo ratings yet
- Gaveigggueman: EnglishDocument4 pagesGaveigggueman: EnglishsuperintendentNo ratings yet
- Jesus's Miracles & ParablesDocument7 pagesJesus's Miracles & Parablessuperintendent100% (1)
- 4) Chemical ReactionsDocument13 pages4) Chemical ReactionsEricka Jane Roga PalenciaNo ratings yet
- Form 1 Endterm 3 ExamsDocument74 pagesForm 1 Endterm 3 ExamsStephen RatumoNo ratings yet
- Discovering Metals - A Historical OverviewDocument12 pagesDiscovering Metals - A Historical OverviewMonisha SharmaNo ratings yet
- Lecture 2Document10 pagesLecture 2roonyrania715No ratings yet
- General Knowledge Notes For Competitive ExamsDocument90 pagesGeneral Knowledge Notes For Competitive ExamsBibi FaridaNo ratings yet
- MalleabilityDocument10 pagesMalleabilityChristine Jane RodriguezNo ratings yet
- Aeration and Water SofteningDocument8 pagesAeration and Water SofteningKarl TimtimNo ratings yet
- Chemistry: Metall RgyDocument136 pagesChemistry: Metall Rgymukesh kannaNo ratings yet
- Chapter 10 - Reversible Reactions & Equilibrium: 10.1 Reversible Reaction and Chemical EquilibriaDocument16 pagesChapter 10 - Reversible Reactions & Equilibrium: 10.1 Reversible Reaction and Chemical EquilibriaEunice YeohNo ratings yet
- Ores and Metallurgy PDFDocument38 pagesOres and Metallurgy PDFAniruddha KawadeNo ratings yet
- Sharobem Columbia 0054D 13547Document161 pagesSharobem Columbia 0054D 13547serleb44No ratings yet
- Pt. Triyasa Pirsa Utama: Certificate OF Draught SurveyDocument5 pagesPt. Triyasa Pirsa Utama: Certificate OF Draught SurveyRonaldNo ratings yet
- Science 2019 Sa2 SolutionsDocument14 pagesScience 2019 Sa2 SolutionsNeha malavNo ratings yet
- (Brady Fire) Eugene Meyer - Chemistry of Hazardous Materials - Pearson (2010)Document888 pages(Brady Fire) Eugene Meyer - Chemistry of Hazardous Materials - Pearson (2010)Simon ThaonNo ratings yet
- Baum 1971Document14 pagesBaum 1971Sama AljohaniNo ratings yet
- F23 Topic 3 Periodicity QuizCDocument6 pagesF23 Topic 3 Periodicity QuizCNiambi WillsNo ratings yet
- Fisa Tehnica Alcool Etilic 2019-EnglezaDocument7 pagesFisa Tehnica Alcool Etilic 2019-EnglezaMADALINA LAZARNo ratings yet
- Summative-Sci7-1st QuarterDocument3 pagesSummative-Sci7-1st QuarterRav De Venecia100% (1)
- Weathering, Erosion, Landforms and Regolith: Teacher Notes and Student ActivitiesDocument79 pagesWeathering, Erosion, Landforms and Regolith: Teacher Notes and Student ActivitiesArijit KhanNo ratings yet
- ChemistryDocument11 pagesChemistryVon A. DamirezNo ratings yet
- Chemistry (XII) MCQ - S PDFDocument153 pagesChemistry (XII) MCQ - S PDFDawood AhmadNo ratings yet
- Chemistry Notes For Class 12 Chapter 6 General Principles and Processes of Isolation of Elements PDFDocument14 pagesChemistry Notes For Class 12 Chapter 6 General Principles and Processes of Isolation of Elements PDFbharatarora0106No ratings yet
- Answers Assignment4Document7 pagesAnswers Assignment4MuhammadHazran100% (1)
- M08CDocument16 pagesM08CMiriam LópezNo ratings yet
- Principles of Organic Synthesis 2th EditionDocument643 pagesPrinciples of Organic Synthesis 2th Editionminh leNo ratings yet
- GC Section 6 MasterDocument24 pagesGC Section 6 Masterapi-246009015No ratings yet
- Corken Gas CompressorsDocument16 pagesCorken Gas CompressorssamvendanNo ratings yet
Reaction Types Table
Reaction Types Table
Uploaded by
superintendent0 ratings0% found this document useful (0 votes)
13 views1 pageThe document describes 9 common chemical reaction types including neutralization, acid-metal, acid-carbonate, precipitation, decomposition, respiration, digestion, corrosion, and combustion. For each reaction type it provides the general chemical equation and an example reaction.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document describes 9 common chemical reaction types including neutralization, acid-metal, acid-carbonate, precipitation, decomposition, respiration, digestion, corrosion, and combustion. For each reaction type it provides the general chemical equation and an example reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
13 views1 pageReaction Types Table
Reaction Types Table
Uploaded by
superintendentThe document describes 9 common chemical reaction types including neutralization, acid-metal, acid-carbonate, precipitation, decomposition, respiration, digestion, corrosion, and combustion. For each reaction type it provides the general chemical equation and an example reaction.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Reaction Type General Equation Example
Neutralisation Acid + Base Salt + Water NaOH + HCl NaCl + H2O
Acid/Metal Acid + Metal Salt + Hydrogen Gas Magnesium + HCl Magnesium chloride
+ H2O
Acid/Carbonate Acid + Carbon Salt + Water + Carbon Dioxide Calcium Carbonate + HCl Calcium
Chloride + Water + CO2
Precipitation Soluble Salt A + Soluble Salt B Insoluble Salt Sodium Carbonate + Calcium Nitrate
+ Soluble Salt Calcium Carbonate
Copper nitrate + Sodium Hydroxide
Copper hydroxide
Lead nitrate + sodium iodide
Decomposition AB A + B Water splitting – H2O H2 + O2
Respiration Glucose + Oxygen Water + Carbon Dioxide + C6H12O6 + O2 H2O + CO2 + ATP (energy)
Energy
Digestion Protein + Stomach Acid Amino Acids N/a
Corrosion Metal + Oxygen Metal Oxide Rusting – Fe + O2 FeO
Combustion Fuel + Oxygen Carbon Dioxide + Water Burning Coal/Fuel
Reaction Type General Equation Example
Neutralisation Acid + Base Salt + Water NaOH + HCl NaCl + H2O
Acid/Metal Acid + Metal Salt + Hydrogen Gas Magnesium + HCl Magnesium chloride
+ H2O
Acid/Carbonate Acid + Carbonate Salt + Water + Carbon Calcium Carbonate + HCl Calcium
Dioxide Chloride + Water + CO2
Precipitation Soluble Salt A + Soluble Salt B Insoluble Salt Sodium Carbonate + Calcium Nitrate
+ Soluble Salt Calcium Carbonate
Copper nitrate + Sodium Hydroxide
Copper hydroxide
Lead nitrate + sodium iodide
Decomposition AB A + B Water splitting – H2O H2 + O2
Respiration Glucose + Oxygen Water + Carbon Dioxide + C6H12O6 + O2 H2O + CO2 + ATP (energy)
Energy
Digestion Protein + Stomach Acid Amino Acids N/a
Corrosion Metal + Oxygen Metal Oxide Rusting – Fe + O2 FeO
Combustion Fuel + Oxygen Carbon Dioxide + Water Burning Coal/Fuel
You might also like
- Chapter 01 - Chemistry and MeasurementDocument56 pagesChapter 01 - Chemistry and MeasurementJamie B100% (1)
- Aluminium and Its Compound Lab ReportDocument7 pagesAluminium and Its Compound Lab ReportLevina Arastika100% (1)
- Latihan Mip Kimia 2021 Form 4 & 5Document81 pagesLatihan Mip Kimia 2021 Form 4 & 5unknown :)100% (1)
- Elements and Compounds g8Document5 pagesElements and Compounds g8Fahad RashidNo ratings yet
- Equations Worksheet 1Document2 pagesEquations Worksheet 1jaikovskyNo ratings yet
- PP Acid ReactionsDocument14 pagesPP Acid Reactionsapi-3696266No ratings yet
- Science 1.5 Answers Worksheet Five Acids: Acid + Base Salt + WaterDocument2 pagesScience 1.5 Answers Worksheet Five Acids: Acid + Base Salt + Watermintesinot esayasNo ratings yet
- NeutralizationDocument13 pagesNeutralizationbarakahmuluziNo ratings yet
- Arlan Neutralization WorksheetDocument3 pagesArlan Neutralization WorksheetHEY ERLNo ratings yet
- Chemistry F4 SaltsDocument13 pagesChemistry F4 Saltscivichitam18No ratings yet
- BalanceDocument2 pagesBalancebratajeet.ghosh.14No ratings yet
- Chemical ReactionsDocument2 pagesChemical ReactionsKrydztom UyNo ratings yet
- Acids, Bases & Salts: IndicatorsDocument7 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Topic7-Oxides and Salts-L2Document43 pagesTopic7-Oxides and Salts-L2haotongxu14No ratings yet
- Cramming NotesDocument7 pagesCramming NotesMuhammad ali WasimNo ratings yet
- Worksheet 1 Reactions of Acids and Bases: Danielle AkinlaluDocument3 pagesWorksheet 1 Reactions of Acids and Bases: Danielle AkinlaludanielleNo ratings yet
- Salts and AcidsDocument3 pagesSalts and AcidskmbgtssnbmNo ratings yet
- Preparing Common Salts G8Document20 pagesPreparing Common Salts G8diamehta1512No ratings yet
- Acid and Base ReactionsDocument15 pagesAcid and Base ReactionsNubar MammadovaNo ratings yet
- 04 Acid Reaction DrillsDocument2 pages04 Acid Reaction Drillsfuzzyatom12345No ratings yet
- Acids, Bases & Salts: IndicatorsDocument4 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Cha 11Document11 pagesCha 11Tun Lin AungNo ratings yet
- Acids, Bases and SaltsDocument4 pagesAcids, Bases and Saltsbubutrain2003No ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Chemical Changes Mastery Part 4: AcidsDocument2 pagesChemical Changes Mastery Part 4: AcidsJoeNo ratings yet
- CA DEM Y: Chapter - 2 - NOTESDocument2 pagesCA DEM Y: Chapter - 2 - NOTESvarun puriNo ratings yet
- Prep3 Final Revision..Document26 pagesPrep3 Final Revision..Amira Hekal0% (1)
- Common Names of CompoundsDocument1 pageCommon Names of CompoundsRiannNo ratings yet
- EquationsWorksheet3 PDFDocument2 pagesEquationsWorksheet3 PDFNarci ssusNo ratings yet
- !chemistry Review ANSDocument3 pages!chemistry Review ANSAngel LiNo ratings yet
- Acid, Bases and Salts Class 10Document7 pagesAcid, Bases and Salts Class 10Gowtham LNo ratings yet
- Reactions of Metals With Dilute AcidsDocument3 pagesReactions of Metals With Dilute AcidsDarshanaK 728714No ratings yet
- Cha 12Document9 pagesCha 12Tun Lin AungNo ratings yet
- Chemistry Worksheet 5Document4 pagesChemistry Worksheet 5Deandra AliciaNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- Summary of ReactionsDocument1 pageSummary of ReactionsBubbleNo ratings yet
- Acids, Bases and SaltsDocument8 pagesAcids, Bases and Saltsaakashb1918No ratings yet
- Acids Bases and SaltsDocument45 pagesAcids Bases and SaltsTejas PagarNo ratings yet
- 15281456Document15 pages15281456Manar KamelNo ratings yet
- OXIDES (Metals & Non-Metals)Document4 pagesOXIDES (Metals & Non-Metals)gauri guptaNo ratings yet
- VIII Chemistry PWS 2Document2 pagesVIII Chemistry PWS 2Ishama ZarintaNo ratings yet
- Evolution of Gas:: Other Endothermic ReactionsDocument7 pagesEvolution of Gas:: Other Endothermic Reactionssara parkNo ratings yet
- Common Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLDocument3 pagesCommon Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLFrederick FranciscoNo ratings yet
- Final - Naming of Compounds PDFDocument7 pagesFinal - Naming of Compounds PDFSnorlax Magno100% (1)
- Danielle N. - Making-Salts-differentiated-worksheetDocument2 pagesDanielle N. - Making-Salts-differentiated-worksheetdanielle njorogeNo ratings yet
- Equations Involving AcidsDocument1 pageEquations Involving AcidsYoussef AmrNo ratings yet
- Week 9 l1Document1 pageWeek 9 l1JennieNo ratings yet
- Carbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDocument3 pagesCarbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDSE No WorriesNo ratings yet
- Worksheet 9.6B: Word EquationsDocument1 pageWorksheet 9.6B: Word Equationsshaha waseemNo ratings yet
- 1.1 Types of ReactionsDocument10 pages1.1 Types of ReactionstangwindsonNo ratings yet
- Acids and Bases SummaryDocument2 pagesAcids and Bases SummaryTan Yong KhaiNo ratings yet
- Acid Bases SummaryDocument8 pagesAcid Bases Summaryibraheemgamer786No ratings yet
- Lesson Element Making Salts: Instructions and Answers For TeachersDocument17 pagesLesson Element Making Salts: Instructions and Answers For TeachersGracey- Ann JohnsonNo ratings yet
- SLOP - Writing Word and Symbol Equations For Reactions of MetalsDocument2 pagesSLOP - Writing Word and Symbol Equations For Reactions of Metalsmarcos.vaqueNo ratings yet
- ABS Complete - ChemisteryDocument71 pagesABS Complete - ChemisterymitaNo ratings yet
- Acid Base and Salt Revision NoteDocument7 pagesAcid Base and Salt Revision NoteHassan mahmud50% (2)
- Oxides, Acids, Bases and SaltsDocument8 pagesOxides, Acids, Bases and Salts12&13 SciencesNo ratings yet
- Acids + CarbonatesDocument13 pagesAcids + CarbonatesVithuNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Preparing Common Salts G8Document21 pagesPreparing Common Salts G8shanaayaa kunder100% (1)
- Chemistry Notes Acids Bases and SaltsDocument7 pagesChemistry Notes Acids Bases and SaltsGouri RajNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- EXTQSORW3Document1 pageEXTQSORW3superintendentNo ratings yet
- 10.5 Legal Studies MC QuizDocument4 pages10.5 Legal Studies MC QuizsuperintendentNo ratings yet
- PLKDocument7 pagesPLKsuperintendentNo ratings yet
- W1LEGALANSDocument1 pageW1LEGALANSsuperintendentNo ratings yet
- Year10 Insight Chapter 3 Linear RelationshipsDocument35 pagesYear10 Insight Chapter 3 Linear RelationshipssuperintendentNo ratings yet
- Ch9 Signpost 10Document77 pagesCh9 Signpost 10superintendentNo ratings yet
- AKDocument25 pagesAKsuperintendentNo ratings yet
- Data Analysis & Evaluation ANSWERSDocument7 pagesData Analysis & Evaluation ANSWERSsuperintendentNo ratings yet
- Gaveigggueman: EnglishDocument4 pagesGaveigggueman: EnglishsuperintendentNo ratings yet
- Jesus's Miracles & ParablesDocument7 pagesJesus's Miracles & Parablessuperintendent100% (1)
- 4) Chemical ReactionsDocument13 pages4) Chemical ReactionsEricka Jane Roga PalenciaNo ratings yet
- Form 1 Endterm 3 ExamsDocument74 pagesForm 1 Endterm 3 ExamsStephen RatumoNo ratings yet
- Discovering Metals - A Historical OverviewDocument12 pagesDiscovering Metals - A Historical OverviewMonisha SharmaNo ratings yet
- Lecture 2Document10 pagesLecture 2roonyrania715No ratings yet
- General Knowledge Notes For Competitive ExamsDocument90 pagesGeneral Knowledge Notes For Competitive ExamsBibi FaridaNo ratings yet
- MalleabilityDocument10 pagesMalleabilityChristine Jane RodriguezNo ratings yet
- Aeration and Water SofteningDocument8 pagesAeration and Water SofteningKarl TimtimNo ratings yet
- Chemistry: Metall RgyDocument136 pagesChemistry: Metall Rgymukesh kannaNo ratings yet
- Chapter 10 - Reversible Reactions & Equilibrium: 10.1 Reversible Reaction and Chemical EquilibriaDocument16 pagesChapter 10 - Reversible Reactions & Equilibrium: 10.1 Reversible Reaction and Chemical EquilibriaEunice YeohNo ratings yet
- Ores and Metallurgy PDFDocument38 pagesOres and Metallurgy PDFAniruddha KawadeNo ratings yet
- Sharobem Columbia 0054D 13547Document161 pagesSharobem Columbia 0054D 13547serleb44No ratings yet
- Pt. Triyasa Pirsa Utama: Certificate OF Draught SurveyDocument5 pagesPt. Triyasa Pirsa Utama: Certificate OF Draught SurveyRonaldNo ratings yet
- Science 2019 Sa2 SolutionsDocument14 pagesScience 2019 Sa2 SolutionsNeha malavNo ratings yet
- (Brady Fire) Eugene Meyer - Chemistry of Hazardous Materials - Pearson (2010)Document888 pages(Brady Fire) Eugene Meyer - Chemistry of Hazardous Materials - Pearson (2010)Simon ThaonNo ratings yet
- Baum 1971Document14 pagesBaum 1971Sama AljohaniNo ratings yet
- F23 Topic 3 Periodicity QuizCDocument6 pagesF23 Topic 3 Periodicity QuizCNiambi WillsNo ratings yet
- Fisa Tehnica Alcool Etilic 2019-EnglezaDocument7 pagesFisa Tehnica Alcool Etilic 2019-EnglezaMADALINA LAZARNo ratings yet
- Summative-Sci7-1st QuarterDocument3 pagesSummative-Sci7-1st QuarterRav De Venecia100% (1)
- Weathering, Erosion, Landforms and Regolith: Teacher Notes and Student ActivitiesDocument79 pagesWeathering, Erosion, Landforms and Regolith: Teacher Notes and Student ActivitiesArijit KhanNo ratings yet
- ChemistryDocument11 pagesChemistryVon A. DamirezNo ratings yet
- Chemistry (XII) MCQ - S PDFDocument153 pagesChemistry (XII) MCQ - S PDFDawood AhmadNo ratings yet
- Chemistry Notes For Class 12 Chapter 6 General Principles and Processes of Isolation of Elements PDFDocument14 pagesChemistry Notes For Class 12 Chapter 6 General Principles and Processes of Isolation of Elements PDFbharatarora0106No ratings yet
- Answers Assignment4Document7 pagesAnswers Assignment4MuhammadHazran100% (1)
- M08CDocument16 pagesM08CMiriam LópezNo ratings yet
- Principles of Organic Synthesis 2th EditionDocument643 pagesPrinciples of Organic Synthesis 2th Editionminh leNo ratings yet
- GC Section 6 MasterDocument24 pagesGC Section 6 Masterapi-246009015No ratings yet
- Corken Gas CompressorsDocument16 pagesCorken Gas CompressorssamvendanNo ratings yet