Professional Documents
Culture Documents
Fundamentals of Chemistry
Fundamentals of Chemistry
Uploaded by
Junelle AlderiteCopyright:
Available Formats
You might also like
- Chemistry Form 4 Definition ListDocument14 pagesChemistry Form 4 Definition Listnnur_aimaniah75% (4)
- Ecosorb SugarDocument4 pagesEcosorb SugarReyna OrihuelaNo ratings yet
- Titian ChemistryDocument97 pagesTitian Chemistryadanielasante72No ratings yet
- FUNCHEM.2 2021 Slides 2021 2Document27 pagesFUNCHEM.2 2021 Slides 2021 2shabanaNo ratings yet
- Lecture 1. Basic Concepts of ChemistryDocument22 pagesLecture 1. Basic Concepts of ChemistryВалентина ЮзьковаNo ratings yet
- Topic 1 Quantitative Chemistry (New)Document42 pagesTopic 1 Quantitative Chemistry (New)ma hiuming100% (1)
- Part 1Document25 pagesPart 1Criselda CarinoNo ratings yet
- Atomos Moleculas IonesDocument46 pagesAtomos Moleculas IonesMarina SánchezNo ratings yet
- Chemistry: Is A Whole Branch of Science About Matter, Which Is Anything ThatDocument11 pagesChemistry: Is A Whole Branch of Science About Matter, Which Is Anything Thatmalzaben001No ratings yet
- BAB 1. Chapter - 02 - Modern - ChemistryDocument27 pagesBAB 1. Chapter - 02 - Modern - ChemistryEvi NadilahNo ratings yet
- Atoms and MoleculesDocument10 pagesAtoms and MoleculesUtsav Kumar MathurNo ratings yet
- Atomsmolecules and Ions Ppt. FinalDocument44 pagesAtomsmolecules and Ions Ppt. Finalmain.20002245No ratings yet
- Modern Chemistry: Helda Wika Amini, S.Si., M.Si., M.SCDocument27 pagesModern Chemistry: Helda Wika Amini, S.Si., M.Si., M.SCMalik KarimNo ratings yet
- Basic Composition and Organization of The Living World Living WorldDocument65 pagesBasic Composition and Organization of The Living World Living WorldanaNo ratings yet
- ChemistryDocument16 pagesChemistryCyrus Apollo Gomera TompongNo ratings yet
- Diapositivas Química Gar VII (II)Document56 pagesDiapositivas Química Gar VII (II)JairoJacobNo ratings yet
- GED Preparation Lecture 2 (28.7.2023)Document38 pagesGED Preparation Lecture 2 (28.7.2023)WilliamNo ratings yet
- Atom and MoleculesDocument76 pagesAtom and MoleculesNur Athirah Hashim0% (1)
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Chemical CompoundsDocument37 pagesChemical Compoundsyawev55732No ratings yet
- 9 Chapter-3Document13 pages9 Chapter-3dl9s6547No ratings yet
- CHM 092 CHAPTER 1 - Matter &stoichiometryDocument128 pagesCHM 092 CHAPTER 1 - Matter &stoichiometryAisyah NadhirahNo ratings yet
- 2.1 - Atoms and Reactions: 2.1.1 - Atomic Structure and IsotopesDocument13 pages2.1 - Atoms and Reactions: 2.1.1 - Atomic Structure and IsotopesArshad KhanNo ratings yet
- General Chemistry: Atoms First: The Structure and Stability of AtomsDocument44 pagesGeneral Chemistry: Atoms First: The Structure and Stability of AtomsMinh PhamNo ratings yet
- CHM012 - Module 4Document16 pagesCHM012 - Module 4haibaalisa00No ratings yet
- ChemistryDocument11 pagesChemistrydeguzmancarmenfeNo ratings yet
- CHEMISTRYII Module1Document29 pagesCHEMISTRYII Module1Cedric Omar Hernández RiescoNo ratings yet
- PERTEMUAN 4-1 Atom, Molekul IonDocument46 pagesPERTEMUAN 4-1 Atom, Molekul IonVemas SatriaNo ratings yet
- As Chemistry Note1 FinalDocument56 pagesAs Chemistry Note1 Finaltej786No ratings yet
- MATTER KMTPHDocument206 pagesMATTER KMTPHEng LuhanNo ratings yet
- MATTER - KMTPHDocument206 pagesMATTER - KMTPHMohamad Firdaus HarunNo ratings yet
- G2 ChemDocument44 pagesG2 ChemAJ Angelo De GuzmanNo ratings yet
- CHAPTER 2 Elements, Compounds, Chem Equations and CalculationsDocument80 pagesCHAPTER 2 Elements, Compounds, Chem Equations and CalculationsNurhayati HasanahNo ratings yet
- Chapter 2 LectureDocument64 pagesChapter 2 LectureArshia HematpoorNo ratings yet
- CHEM111E (Chemistry For Engineers) : UNIT 1 - Fundamentals of Chemistry Module 1 - 1Document58 pagesCHEM111E (Chemistry For Engineers) : UNIT 1 - Fundamentals of Chemistry Module 1 - 1Julian CasibangNo ratings yet
- Lesson 1 - IntroductionDocument10 pagesLesson 1 - IntroductionMelvyn DarauayNo ratings yet
- Atoms and MoleculesDocument11 pagesAtoms and Moleculesprakul varshneyNo ratings yet
- Class Ix Chapter 3Q QND Answer With NumericalsDocument17 pagesClass Ix Chapter 3Q QND Answer With NumericalsABHAY PRATAP SINGH TOMARNo ratings yet
- CHEMISTRYDocument48 pagesCHEMISTRYTresha Fate Dorado DarrocaNo ratings yet
- Atoms and MoleculesDocument8 pagesAtoms and MoleculesNANDITA NAYAK BNo ratings yet
- Mole Concept & StoichiometryDocument58 pagesMole Concept & StoichiometryMuhammad Sahil KhanNo ratings yet
- Mole Concept & StoichiometryDocument58 pagesMole Concept & StoichiometryMuhammad Sahil KhanNo ratings yet
- CHEMISTRYDocument3 pagesCHEMISTRYSAN JOSE, KRIZZIA FAYE U.No ratings yet
- ChemDocument8 pagesChemtherealmaddiieNo ratings yet
- Basic Concepts of ChemistryDocument13 pagesBasic Concepts of Chemistryashi917363No ratings yet
- STPM Chem Chp1 NotesDocument29 pagesSTPM Chem Chp1 Noteskpew100% (4)
- Class 11 Chemistry Support MaterialDocument182 pagesClass 11 Chemistry Support Materialmauryadc.15No ratings yet
- Chapter1 (Lecture Note)Document53 pagesChapter1 (Lecture Note)taechimNo ratings yet
- Lessons 5 6Document22 pagesLessons 5 6Datuesmail Ala AliNo ratings yet
- 普化2Document60 pages普化2zhooongNo ratings yet
- Applications of Isotopes C11!3!01&C11!3!02Document12 pagesApplications of Isotopes C11!3!01&C11!3!02Olivia M OliverNo ratings yet
- General Chemistry 11Document9 pagesGeneral Chemistry 11Rowelyn BakekeNo ratings yet
- Chemistry ReviewerDocument17 pagesChemistry ReviewerRalph Castillo100% (3)
- Chemistry Form 4 Definition ListDocument5 pagesChemistry Form 4 Definition ListyeeteinNo ratings yet
- Ch2 Atoms Molecules IonsDocument46 pagesCh2 Atoms Molecules IonsCalonanak Sithr2020No ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Determination of Dissolve Oxygen by Winkler MethodDocument7 pagesDetermination of Dissolve Oxygen by Winkler MethodDani MughalNo ratings yet
- Kat Level - Ii Syllabus19-20Document1 pageKat Level - Ii Syllabus19-20Bhavin PanchalNo ratings yet
- Spectra and Dynamics of Small Molecules Alexander Von Humboldt Lectures (Robert W. Field (Auth.) )Document162 pagesSpectra and Dynamics of Small Molecules Alexander Von Humboldt Lectures (Robert W. Field (Auth.) )Sathapana CHAWANANONNo ratings yet
- QweenbeeDocument43 pagesQweenbeeAkinrinade Abisola OmopelolaNo ratings yet
- Ideal Gas Mixture and Moist Air: (H N H P Khí Lý Tư NG)Document9 pagesIdeal Gas Mixture and Moist Air: (H N H P Khí Lý Tư NG)Nguyễn VinhNo ratings yet
- EPF4802 - Chapter 5 (Part 1) Utilities and Energy Efficient Design - Video - NotesDocument14 pagesEPF4802 - Chapter 5 (Part 1) Utilities and Energy Efficient Design - Video - NoteshidayantiemNo ratings yet
- Dokumen - Tips - SSPC Pa 1 PDF Wordpresscom A SSPC Pa 1 PDF SSPC Pa 1 Shop Field and PDFDocument2 pagesDokumen - Tips - SSPC Pa 1 PDF Wordpresscom A SSPC Pa 1 PDF SSPC Pa 1 Shop Field and PDFAbhilashNo ratings yet
- Chapter-50 Applications of Isotopes in Medicine: January 2017Document8 pagesChapter-50 Applications of Isotopes in Medicine: January 2017Saikumar SobaradNo ratings yet
- Texturized Vegetable Protein (TVP) : Dr. Zanwar S.R. Principal, SCFT, MaldadDocument14 pagesTexturized Vegetable Protein (TVP) : Dr. Zanwar S.R. Principal, SCFT, MaldadTejas KshirsagarNo ratings yet
- Ductile Detailing & Its Important CLPIU EduDocument73 pagesDuctile Detailing & Its Important CLPIU EduSankalp LamaNo ratings yet
- Safety Questions and AnswersDocument30 pagesSafety Questions and AnswersSubash RockNo ratings yet
- Sri CatalogDocument9 pagesSri Catalog梁铭斌No ratings yet
- 5.PCB Manufacturing ProcessDocument19 pages5.PCB Manufacturing ProcessAshutosh PradhanNo ratings yet
- Astm A101Document3 pagesAstm A101kashif ehsanNo ratings yet
- The Encyclopedia of Explosives and Related Items PATR 2700 VOLUME 5Document785 pagesThe Encyclopedia of Explosives and Related Items PATR 2700 VOLUME 5Bloc22100% (1)
- Nutrition in PlantsDocument44 pagesNutrition in Plantsgeorgy shibuNo ratings yet
- Chemistry Lesson 2.2 (Transcribed)Document6 pagesChemistry Lesson 2.2 (Transcribed)chem recordingsNo ratings yet
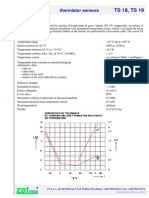
- Zat - M TS 18, TS 19: Thermistor SensorsDocument4 pagesZat - M TS 18, TS 19: Thermistor SensorsHusejin MehmedovićNo ratings yet
- Prestressed RectDocument1 pagePrestressed Rectmahmoud IbrahemNo ratings yet
- Us03cicv21 Unit3Document28 pagesUs03cicv21 Unit3ashokNo ratings yet
- Fluidized-Bed Bioreactor Applications For Biological Wastewater Treatment - A Review of Research and DevelopmentsDocument13 pagesFluidized-Bed Bioreactor Applications For Biological Wastewater Treatment - A Review of Research and DevelopmentsAgeng RizkyNo ratings yet
- Tylok Pipe FittingsDocument12 pagesTylok Pipe FittingsLutfi IsmailNo ratings yet
- Removal of Heavy Metals Using Waste EggshellDocument6 pagesRemoval of Heavy Metals Using Waste EggshellangelbroderNo ratings yet
- My Husband Is Mafia Boss Season 3Document6 pagesMy Husband Is Mafia Boss Season 3قى قىNo ratings yet
- Experiment 3Document3 pagesExperiment 3R100% (1)
- Chemgene CleanerDocument5 pagesChemgene CleanerEduardo GomesNo ratings yet
- Mechanical Properties 301015Document47 pagesMechanical Properties 301015Eugene Daga-angNo ratings yet
- Civil Engineering JournalDocument14 pagesCivil Engineering Journaldave tafadzwa kuyeriNo ratings yet
- Ductility Characteristics of Partially Restrained Beam-to-Column Composite Connections in Concrete Filled Square TubesDocument11 pagesDuctility Characteristics of Partially Restrained Beam-to-Column Composite Connections in Concrete Filled Square TubesPrapa KaranNo ratings yet
Fundamentals of Chemistry
Fundamentals of Chemistry
Uploaded by
Junelle AlderiteOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fundamentals of Chemistry
Fundamentals of Chemistry
Uploaded by
Junelle AlderiteCopyright:
Available Formats
TECHNOLOGICAL UNIVERSITY OF THE PHILIPPINES – TAGUIG
Chemistry Section
CHEMBT
CHEM ENGG T General Chemistry
Chemistry for Engineers
Module 01 Fundamentals of Chemistry for Engineers
Prepared by Jonathan Daved D. Dela Cruz
Chemistry: A Science For The 21st Century
Health and Medicine Energy and the
Sanitation systems Environment
Surgery with anesthesia Fossil fuels
Vaccines and Solar energy
antibiotics Nuclear energy
Gene therapyF
Materials and
Technology Food and Agriculture
Polymers, ceramics, Genetically modified
liquid crystals crops
Room-temperature “Natural” pesticides
superconductors? Specialized fertilizers
Molecular computing?
The Study of Chemistry
Chemistry is the central science,
encompassing all other fields of
sciences.
It must be viewed Macroscopically,
Microscopically, and Symbolically
Chemistry is the study of matter and the changes it undergoes
Matter is anything that occupies space and has mass.
liquid nitrogen gold ingots silicon crystals
TUP-T |CHEM ENGG T | JDDELACRUZ 1
Classifications of Matter by Composition
A substance is a form of matter that has a definite composition and distinct properties.
A compound is a substance composed of atoms of two or more elements chemically united in fixed
proportions.
Compounds can only be separated into their pure components (elements) by chemical means.
Classifications of Matter by State
Types of Changes
A physical change does not alter the composition or identity of a substance.
A chemical change alters the composition or identity of the substance(s) involved.
Atoms, Molecules and Ions
Dalton’s Atomic Theory (1808)
1. Elements are composed of extremely small particles called atoms.
2. All atoms of a given element are identical, having the same size, mass and chemical properties. The
atoms of one element are different from the atoms of all other elements.
3. Compounds are composed of atoms of more than one element. In any compound, the ratio of the
numbers of atoms of any two of the elements present is either an integer or a simple fraction.
4. A chemical reaction involves only the separation, combination, or rearrangement of atoms; it does not
result in their creation or destruction.
TUP-T |CHEM ENGG T | JDDELACRUZ 2
Law of Multiple Proportions
Law of Conservation of Mass
Atomic number, Mass number and Isotopes
Atomic number (Z) = number of protons in nucleus
Mass number (A) = number of protons + number of neutrons
= atomic number (Z) + number of neutrons
Isotopes are atoms of the same element (X) with different numbers of neutrons in their nuclei
Periodic Table (s,p,d,f block completed periodic table as of 2019)
TUP-T |CHEM ENGG T | JDDELACRUZ 3
An ion is an atom, or group of atoms, that has a net positive or negative charge.
A molecular formula shows the exact number of atoms of each element in the smallest unit of a
substance
An empirical formula shows the simplest
whole-number ratio of the atoms in a substance
ionic compounds consist of a combination of cations and an anions
• The formula is usually the same as the empirical formula
• The sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero
The ionic compound NaCl
TUP-T |CHEM ENGG T | JDDELACRUZ 4
Molecular compounds
Nonmetals or nonmetals + metalloids
Common names
H2O, NH3, CH4,
Element furthest to the left in a period and closest to
the bottom of a group on periodic table is placed
first in formula
If more than one compound can be formed from the
same elements, use prefixes to indicate number of
each kind of atom
Last element name ends in ide
HI hydrogen iodide
NF3 nitrogen trifluoride
SO2 sulfur dioxide
N2Cl4 dinitrogen tetrachloride
NO2 nitrogen dioxide
N2O dinitrogen monoxide
TUP-T |CHEM ENGG T | JDDELACRUZ 5
An acid can be defined as a substance that yields
hydrogen ions (H+ ) when dissolved in water.
For example: HCl gas and HCl in water
• Pure substance, hydrogen
chloride
• Dissolved in water (H3O+ and Cl−),
hydrochloric acid
An oxoacid is an acid that contains hydrogen, oxygen, and another element.
HNO3 nitric acid
H2CO3
carbonic acid
H3PO4 phosphoric acid
TUP-T |CHEM ENGG T | JDDELACRUZ 6
Naming Oxoacids and Oxoanions
The rules for naming oxoanions, anions of
oxoacids, are as follows:
1. When all the H ions are removed from the “-ic” acid, the anion’s name ends with “-ate.”
2. When all the H ions are removed from the “-ous” acid, the anion’s name ends with “-ite.”
3. The names of anions in which one or more but not all the hydrogen ions have been removed must
indicate the number of H ions present.
For example:
• H2PO4- dihydrogen phosphate
• HPO4 2- hydrogen phosphate
• PO43- phosphate
A base can be defined as a substance that yields
hydroxide ions (OH-) when dissolved in water.
NaOH sodium hydroxide
KOH potassium hydroxide
Ba(OH)2 barium hydroxide
TUP-T |CHEM ENGG T | JDDELACRUZ 7
Mass Relationships in Chemical Reactions
Atomic mass is the mass of an atom in atomic mass units (amu)
The average atomic mass is the weighted
average of all of the naturally occurring
isotopes of the element.
The mole (mol) is the amount of a substance that
contains as many elementary entities as there
are atoms in exactly 12.00 grams of 12C
Avogadro’s number (NA)
1 mol = NA = 6.0221367 x 1023
1 mole 12C atoms = 6.022 x 1023 atoms = 12.00 g
1 12C atom = 12.00 amu
1 mole 12C atoms = 12.00 g 12C
1 mole lithium atoms = 6.941 g of Li
For any element
atomic mass (amu) = molar mass (grams)
TUP-T |CHEM ENGG T | JDDELACRUZ 8
A process in which one or more substances is changed into one or more new substances is a chemical
reaction
A chemical equation uses chemical symbols to show what happens during a chemical reaction
TUP-T |CHEM ENGG T | JDDELACRUZ 9
Balancing Chemical Equations
1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the
product(s) on the right side of the equation.
2. Change the numbers in front of the formulas (coefficients) to make the number of atoms of each
element the same on both sides of the equation. Do not change the subscripts.
3. Start by balancing those elements that appear in only one reactant and one product.
4. Balance those elements that appear in two or more reactants or products.
5. Check to make sure that you have the same number of each type of atom on both sides of the
equation.
TUP-T |CHEM ENGG T | JDDELACRUZ 10
Amounts of Reactants and Products
1. Write balanced chemical equation
2. Convert quantities of known substances into moles
3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity
4. Convert moles of sought quantity into desired units
Fundamentals of Stoichiometry
• Stoichiometry is a term used to describe quantitative relationships in chemistry.
o “How much?” of a product is produced or reactant is consumed.
o Balanced chemical equation needed.
o Conversion between mass or volume to number of moles frequently needed.
• This flow diagram illustrates the various steps involved in solving a typical reaction stoichiometry
problem
• No different than unit conversion
• Usually more than one conversion is necessary
• Write all quantities with their complete units
TUP-T |CHEM ENGG T | JDDELACRUZ 11
Limiting Reactants
• In many chemical reactions, one reactant is often exhausted before the other reactants.
This reactant is the limiting reactant.
• Limiting reactant is determined using stoichiometry.
• The limiting reactant limits the quantity of product produced.
• In many cases, we manipulate the amounts of reactants to ensure that one specific compound is
the limiting reactant.
• For example, a more expensive or scarce reagent is usually chosen to be the limiting
reagent.
• Other times, it is best to have a stoichiometric mixture (equal ratio of moles) to prevent
waste.
• For example, rocket fuel is designed so that no mass is left over, which would add
unnecessary weight to the rocket.
Reaction Yield
Theoretical Yield is the amount of product that would
result if all the limiting reagent reacted.
Actual Yield is the amount of product actually obtained
from a reaction.
• The maximum mass of a product that can be obtained in a reaction is determined by the limiting
reactant.
• Determine which reactant is the limiting reactant.
• Calculate the mass of product that can be made from the limiting reactant. This mass is
the theoretical yield.
• In stoichiometric mixtures, however, both reactants are consumed completely, so either
could be considered the limiting reactant.
• Many factors determine the amount of desired product actually produced in a reaction.
• Temperature of the reaction
• The possibility of side reactions
• Further reaction of the product
• Time
TUP-T |CHEM ENGG T | JDDELACRUZ 12
You might also like
- Chemistry Form 4 Definition ListDocument14 pagesChemistry Form 4 Definition Listnnur_aimaniah75% (4)
- Ecosorb SugarDocument4 pagesEcosorb SugarReyna OrihuelaNo ratings yet
- Titian ChemistryDocument97 pagesTitian Chemistryadanielasante72No ratings yet
- FUNCHEM.2 2021 Slides 2021 2Document27 pagesFUNCHEM.2 2021 Slides 2021 2shabanaNo ratings yet
- Lecture 1. Basic Concepts of ChemistryDocument22 pagesLecture 1. Basic Concepts of ChemistryВалентина ЮзьковаNo ratings yet
- Topic 1 Quantitative Chemistry (New)Document42 pagesTopic 1 Quantitative Chemistry (New)ma hiuming100% (1)
- Part 1Document25 pagesPart 1Criselda CarinoNo ratings yet
- Atomos Moleculas IonesDocument46 pagesAtomos Moleculas IonesMarina SánchezNo ratings yet
- Chemistry: Is A Whole Branch of Science About Matter, Which Is Anything ThatDocument11 pagesChemistry: Is A Whole Branch of Science About Matter, Which Is Anything Thatmalzaben001No ratings yet
- BAB 1. Chapter - 02 - Modern - ChemistryDocument27 pagesBAB 1. Chapter - 02 - Modern - ChemistryEvi NadilahNo ratings yet
- Atoms and MoleculesDocument10 pagesAtoms and MoleculesUtsav Kumar MathurNo ratings yet
- Atomsmolecules and Ions Ppt. FinalDocument44 pagesAtomsmolecules and Ions Ppt. Finalmain.20002245No ratings yet
- Modern Chemistry: Helda Wika Amini, S.Si., M.Si., M.SCDocument27 pagesModern Chemistry: Helda Wika Amini, S.Si., M.Si., M.SCMalik KarimNo ratings yet
- Basic Composition and Organization of The Living World Living WorldDocument65 pagesBasic Composition and Organization of The Living World Living WorldanaNo ratings yet
- ChemistryDocument16 pagesChemistryCyrus Apollo Gomera TompongNo ratings yet
- Diapositivas Química Gar VII (II)Document56 pagesDiapositivas Química Gar VII (II)JairoJacobNo ratings yet
- GED Preparation Lecture 2 (28.7.2023)Document38 pagesGED Preparation Lecture 2 (28.7.2023)WilliamNo ratings yet
- Atom and MoleculesDocument76 pagesAtom and MoleculesNur Athirah Hashim0% (1)
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Applied Chemistry Chem 101: Instructor: Dr. Mehr NigarDocument14 pagesApplied Chemistry Chem 101: Instructor: Dr. Mehr NigarẄâQâŗÂlïNo ratings yet
- Chemical CompoundsDocument37 pagesChemical Compoundsyawev55732No ratings yet
- 9 Chapter-3Document13 pages9 Chapter-3dl9s6547No ratings yet
- CHM 092 CHAPTER 1 - Matter &stoichiometryDocument128 pagesCHM 092 CHAPTER 1 - Matter &stoichiometryAisyah NadhirahNo ratings yet
- 2.1 - Atoms and Reactions: 2.1.1 - Atomic Structure and IsotopesDocument13 pages2.1 - Atoms and Reactions: 2.1.1 - Atomic Structure and IsotopesArshad KhanNo ratings yet
- General Chemistry: Atoms First: The Structure and Stability of AtomsDocument44 pagesGeneral Chemistry: Atoms First: The Structure and Stability of AtomsMinh PhamNo ratings yet
- CHM012 - Module 4Document16 pagesCHM012 - Module 4haibaalisa00No ratings yet
- ChemistryDocument11 pagesChemistrydeguzmancarmenfeNo ratings yet
- CHEMISTRYII Module1Document29 pagesCHEMISTRYII Module1Cedric Omar Hernández RiescoNo ratings yet
- PERTEMUAN 4-1 Atom, Molekul IonDocument46 pagesPERTEMUAN 4-1 Atom, Molekul IonVemas SatriaNo ratings yet
- As Chemistry Note1 FinalDocument56 pagesAs Chemistry Note1 Finaltej786No ratings yet
- MATTER KMTPHDocument206 pagesMATTER KMTPHEng LuhanNo ratings yet
- MATTER - KMTPHDocument206 pagesMATTER - KMTPHMohamad Firdaus HarunNo ratings yet
- G2 ChemDocument44 pagesG2 ChemAJ Angelo De GuzmanNo ratings yet
- CHAPTER 2 Elements, Compounds, Chem Equations and CalculationsDocument80 pagesCHAPTER 2 Elements, Compounds, Chem Equations and CalculationsNurhayati HasanahNo ratings yet
- Chapter 2 LectureDocument64 pagesChapter 2 LectureArshia HematpoorNo ratings yet
- CHEM111E (Chemistry For Engineers) : UNIT 1 - Fundamentals of Chemistry Module 1 - 1Document58 pagesCHEM111E (Chemistry For Engineers) : UNIT 1 - Fundamentals of Chemistry Module 1 - 1Julian CasibangNo ratings yet
- Lesson 1 - IntroductionDocument10 pagesLesson 1 - IntroductionMelvyn DarauayNo ratings yet
- Atoms and MoleculesDocument11 pagesAtoms and Moleculesprakul varshneyNo ratings yet
- Class Ix Chapter 3Q QND Answer With NumericalsDocument17 pagesClass Ix Chapter 3Q QND Answer With NumericalsABHAY PRATAP SINGH TOMARNo ratings yet
- CHEMISTRYDocument48 pagesCHEMISTRYTresha Fate Dorado DarrocaNo ratings yet
- Atoms and MoleculesDocument8 pagesAtoms and MoleculesNANDITA NAYAK BNo ratings yet
- Mole Concept & StoichiometryDocument58 pagesMole Concept & StoichiometryMuhammad Sahil KhanNo ratings yet
- Mole Concept & StoichiometryDocument58 pagesMole Concept & StoichiometryMuhammad Sahil KhanNo ratings yet
- CHEMISTRYDocument3 pagesCHEMISTRYSAN JOSE, KRIZZIA FAYE U.No ratings yet
- ChemDocument8 pagesChemtherealmaddiieNo ratings yet
- Basic Concepts of ChemistryDocument13 pagesBasic Concepts of Chemistryashi917363No ratings yet
- STPM Chem Chp1 NotesDocument29 pagesSTPM Chem Chp1 Noteskpew100% (4)
- Class 11 Chemistry Support MaterialDocument182 pagesClass 11 Chemistry Support Materialmauryadc.15No ratings yet
- Chapter1 (Lecture Note)Document53 pagesChapter1 (Lecture Note)taechimNo ratings yet
- Lessons 5 6Document22 pagesLessons 5 6Datuesmail Ala AliNo ratings yet
- 普化2Document60 pages普化2zhooongNo ratings yet
- Applications of Isotopes C11!3!01&C11!3!02Document12 pagesApplications of Isotopes C11!3!01&C11!3!02Olivia M OliverNo ratings yet
- General Chemistry 11Document9 pagesGeneral Chemistry 11Rowelyn BakekeNo ratings yet
- Chemistry ReviewerDocument17 pagesChemistry ReviewerRalph Castillo100% (3)
- Chemistry Form 4 Definition ListDocument5 pagesChemistry Form 4 Definition ListyeeteinNo ratings yet
- Ch2 Atoms Molecules IonsDocument46 pagesCh2 Atoms Molecules IonsCalonanak Sithr2020No ratings yet
- Practice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersFrom EverandPractice Makes Perfect in Chemistry: Compounds, Reactions and Moles with AnswersRating: 3 out of 5 stars3/5 (2)
- Determination of Dissolve Oxygen by Winkler MethodDocument7 pagesDetermination of Dissolve Oxygen by Winkler MethodDani MughalNo ratings yet
- Kat Level - Ii Syllabus19-20Document1 pageKat Level - Ii Syllabus19-20Bhavin PanchalNo ratings yet
- Spectra and Dynamics of Small Molecules Alexander Von Humboldt Lectures (Robert W. Field (Auth.) )Document162 pagesSpectra and Dynamics of Small Molecules Alexander Von Humboldt Lectures (Robert W. Field (Auth.) )Sathapana CHAWANANONNo ratings yet
- QweenbeeDocument43 pagesQweenbeeAkinrinade Abisola OmopelolaNo ratings yet
- Ideal Gas Mixture and Moist Air: (H N H P Khí Lý Tư NG)Document9 pagesIdeal Gas Mixture and Moist Air: (H N H P Khí Lý Tư NG)Nguyễn VinhNo ratings yet
- EPF4802 - Chapter 5 (Part 1) Utilities and Energy Efficient Design - Video - NotesDocument14 pagesEPF4802 - Chapter 5 (Part 1) Utilities and Energy Efficient Design - Video - NoteshidayantiemNo ratings yet
- Dokumen - Tips - SSPC Pa 1 PDF Wordpresscom A SSPC Pa 1 PDF SSPC Pa 1 Shop Field and PDFDocument2 pagesDokumen - Tips - SSPC Pa 1 PDF Wordpresscom A SSPC Pa 1 PDF SSPC Pa 1 Shop Field and PDFAbhilashNo ratings yet
- Chapter-50 Applications of Isotopes in Medicine: January 2017Document8 pagesChapter-50 Applications of Isotopes in Medicine: January 2017Saikumar SobaradNo ratings yet
- Texturized Vegetable Protein (TVP) : Dr. Zanwar S.R. Principal, SCFT, MaldadDocument14 pagesTexturized Vegetable Protein (TVP) : Dr. Zanwar S.R. Principal, SCFT, MaldadTejas KshirsagarNo ratings yet
- Ductile Detailing & Its Important CLPIU EduDocument73 pagesDuctile Detailing & Its Important CLPIU EduSankalp LamaNo ratings yet
- Safety Questions and AnswersDocument30 pagesSafety Questions and AnswersSubash RockNo ratings yet
- Sri CatalogDocument9 pagesSri Catalog梁铭斌No ratings yet
- 5.PCB Manufacturing ProcessDocument19 pages5.PCB Manufacturing ProcessAshutosh PradhanNo ratings yet
- Astm A101Document3 pagesAstm A101kashif ehsanNo ratings yet
- The Encyclopedia of Explosives and Related Items PATR 2700 VOLUME 5Document785 pagesThe Encyclopedia of Explosives and Related Items PATR 2700 VOLUME 5Bloc22100% (1)
- Nutrition in PlantsDocument44 pagesNutrition in Plantsgeorgy shibuNo ratings yet
- Chemistry Lesson 2.2 (Transcribed)Document6 pagesChemistry Lesson 2.2 (Transcribed)chem recordingsNo ratings yet
- Zat - M TS 18, TS 19: Thermistor SensorsDocument4 pagesZat - M TS 18, TS 19: Thermistor SensorsHusejin MehmedovićNo ratings yet
- Prestressed RectDocument1 pagePrestressed Rectmahmoud IbrahemNo ratings yet
- Us03cicv21 Unit3Document28 pagesUs03cicv21 Unit3ashokNo ratings yet
- Fluidized-Bed Bioreactor Applications For Biological Wastewater Treatment - A Review of Research and DevelopmentsDocument13 pagesFluidized-Bed Bioreactor Applications For Biological Wastewater Treatment - A Review of Research and DevelopmentsAgeng RizkyNo ratings yet
- Tylok Pipe FittingsDocument12 pagesTylok Pipe FittingsLutfi IsmailNo ratings yet
- Removal of Heavy Metals Using Waste EggshellDocument6 pagesRemoval of Heavy Metals Using Waste EggshellangelbroderNo ratings yet
- My Husband Is Mafia Boss Season 3Document6 pagesMy Husband Is Mafia Boss Season 3قى قىNo ratings yet
- Experiment 3Document3 pagesExperiment 3R100% (1)
- Chemgene CleanerDocument5 pagesChemgene CleanerEduardo GomesNo ratings yet
- Mechanical Properties 301015Document47 pagesMechanical Properties 301015Eugene Daga-angNo ratings yet
- Civil Engineering JournalDocument14 pagesCivil Engineering Journaldave tafadzwa kuyeriNo ratings yet
- Ductility Characteristics of Partially Restrained Beam-to-Column Composite Connections in Concrete Filled Square TubesDocument11 pagesDuctility Characteristics of Partially Restrained Beam-to-Column Composite Connections in Concrete Filled Square TubesPrapa KaranNo ratings yet