Professional Documents
Culture Documents
Reducedformtonidisedfoomofo - TT: Redox Reaction
Reducedformtonidisedfoomofo - TT: Redox Reaction
Uploaded by
Hems MadaviCopyright:
Available Formats
You might also like
- Imagining the Nation in Nature: Landscape Preservation and German Identity, 1885–1945From EverandImagining the Nation in Nature: Landscape Preservation and German Identity, 1885–1945No ratings yet
- Module 6.9 TLEDocument115 pagesModule 6.9 TLEroseavy90% (10)
- Star Wars Republic Holonet NewsDocument13 pagesStar Wars Republic Holonet NewsJessie Abraham LaidlawNo ratings yet
- UCS Lectures PDFDocument100 pagesUCS Lectures PDFgoeffNo ratings yet
- Macro Economic - LectureDocument7 pagesMacro Economic - LectureANYA AKBARNo ratings yet
- Eco 3Document6 pagesEco 3Pannathorn SiriNo ratings yet
- Upd C11 Phy EngDocument23 pagesUpd C11 Phy EngArinjoy Mervyn GomesNo ratings yet
- Math Dance SymbolsDocument1 pageMath Dance SymbolsJerry Bhoy ReniedoNo ratings yet
- States of MatterDocument1 pageStates of MattergnanavishaljonnalagaddaNo ratings yet
- A5. States of MatterDocument1 pageA5. States of MatterHitesh KumarNo ratings yet
- If Absolutely Conditionally: ComparisonDocument3 pagesIf Absolutely Conditionally: Comparisonlimyh123abcNo ratings yet
- U&D To NLM (RY)Document277 pagesU&D To NLM (RY)Chashan DeepNo ratings yet
- @physics - 11class Vectors and ForcesDocument69 pages@physics - 11class Vectors and ForcesPranav RoyNo ratings yet
- Cheat SheetDocument3 pagesCheat Sheetjohnson2004412No ratings yet
- Rate Expression - FactRecallDocument1 pageRate Expression - FactRecallAhana KatyalNo ratings yet
- Midterm SummaryDocument10 pagesMidterm SummaryDarkos134No ratings yet
- Tema 2 - LaplaceDocument9 pagesTema 2 - LaplaceLuyi WangNo ratings yet
- CH 14 - IRDocument5 pagesCH 14 - IRElle QuizonNo ratings yet
- Physics Y1 ReviewDocument5 pagesPhysics Y1 ReviewRakeem McFarlaneNo ratings yet
- Vectors Live Class 1 NotesDocument40 pagesVectors Live Class 1 NotesKushagra TiwariNo ratings yet
- Atomic Structure Mind MapDocument2 pagesAtomic Structure Mind Maplakshminivas PingaliNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureHems MadaviNo ratings yet
- Atomic Structure Mind MapDocument2 pagesAtomic Structure Mind Mapcric.indian.womenNo ratings yet
- Summary of Tests For ConvergenceDocument1 pageSummary of Tests For ConvergenceAurelio ReyesNo ratings yet
- Solutions - Class Notes - Lakshya NEET 2025Document24 pagesSolutions - Class Notes - Lakshya NEET 2025Dipanshu MundNo ratings yet
- Trade TheoriesDocument7 pagesTrade TheoriesWajiha RajaniNo ratings yet
- P3 Motion in A Straight LineDocument1 pageP3 Motion in A Straight LineberburnleyNo ratings yet
- Gibbs Adsorption Isotherm NotesDocument3 pagesGibbs Adsorption Isotherm NotesAshish ChauhanNo ratings yet
- Clase 20220526 FQDocument10 pagesClase 20220526 FQJeshNo ratings yet
- Lecture 4Document4 pagesLecture 4Ghostrocket 017No ratings yet
- Maths For Economics-1Document20 pagesMaths For Economics-1Genshin ImpactNo ratings yet
- Unit 3Document10 pagesUnit 3Leen Al-FouzanNo ratings yet
- SAT Grammar One Point Question PackDocument10 pagesSAT Grammar One Point Question PackKevin LimNo ratings yet
- Defleksi Pada Rangka BatangDocument6 pagesDefleksi Pada Rangka BatangAyunda ElvandariNo ratings yet
- Formula Cheat Sheet 2023Document3 pagesFormula Cheat Sheet 2023Hardik NarangNo ratings yet
- IB Chemistry (Chapter 11)Document5 pagesIB Chemistry (Chapter 11)hyunjinp0107No ratings yet
- PDF Updated Class 11 Physics Formula Sheet CompressDocument22 pagesPDF Updated Class 11 Physics Formula Sheet CompressdrjbjpNo ratings yet
- สูตรฟิโม2Document1 pageสูตรฟิโม2Supawadee YodsuwanNo ratings yet
- Vfi F.li: UpsibeDocument5 pagesVfi F.li: UpsibeYusheng LaiNo ratings yet
- Chemical Kinetics Mind MapDocument1 pageChemical Kinetics Mind MapSamridhi MoudgilNo ratings yet
- Harlem by Langston Hughes - Poetry FoundationDocument1 pageHarlem by Langston Hughes - Poetry Foundations026103No ratings yet
- FluidsDocument15 pagesFluidsskumar123846No ratings yet
- 2.8 Linear Approximations and DifferentialsDocument7 pages2.8 Linear Approximations and Differentialskhyang0510No ratings yet
- Maths Important PointsDocument146 pagesMaths Important Pointsgspareek1111No ratings yet
- Lim Lim: Hint Apply Integration by Parts To The Integral ForDocument1 pageLim Lim: Hint Apply Integration by Parts To The Integral ForHyunSung KimNo ratings yet
- IB Chemistry (Periodic Table)Document1 pageIB Chemistry (Periodic Table)hyunjinp0107No ratings yet
- Ligand Sub Factreycall ChemistryDocument2 pagesLigand Sub Factreycall Chemistryosahai17No ratings yet
- Periodic Motion NotesDocument9 pagesPeriodic Motion Noteschlorine169No ratings yet
- Types of Quadrilateral Name Shape PropertiesDocument13 pagesTypes of Quadrilateral Name Shape Propertiesanon_64795088No ratings yet
- Ln3.Fm# - Fcoulomb 4Tfeo&Z: BalmerDocument3 pagesLn3.Fm# - Fcoulomb 4Tfeo&Z: BalmerJayNo ratings yet
- Organic ChemistryDocument5 pagesOrganic ChemistryNumpxNump 465No ratings yet
- Ifsvdociiy: Motion in 1-DDocument1 pageIfsvdociiy: Motion in 1-DHems MadaviNo ratings yet
- Ill I: SlopeDocument10 pagesIll I: SlopeMohit MehtaNo ratings yet
- Electrochemistry Mind MapDocument2 pagesElectrochemistry Mind MapBhavna BeniwalNo ratings yet
- Escavadeira Hyundai r220lc 9Document120 pagesEscavadeira Hyundai r220lc 9Wolf ChakkalNo ratings yet
- Magnetism 1 2023 by Shyam Mohan BhaiyaDocument22 pagesMagnetism 1 2023 by Shyam Mohan BhaiyaPranav KumarNo ratings yet
- Formula Sheet Class 12Document3 pagesFormula Sheet Class 12SurajNo ratings yet
- Successione GeometricaDocument1 pageSuccessione Geometricaloparco.gianfrancoNo ratings yet
- Unit Dimensions and MeasurementsDocument7 pagesUnit Dimensions and MeasurementsAarush Ram AnandhNo ratings yet
- Music NoteDocument2 pagesMusic Noteseung won OhNo ratings yet
- Convergence TestsDocument1 pageConvergence TestspgokoolNo ratings yet
- Solid Mensuration 10072023Document6 pagesSolid Mensuration 10072023Nathan AducaNo ratings yet
- Ifsvdociiy: Motion in 1-DDocument1 pageIfsvdociiy: Motion in 1-DHems MadaviNo ratings yet
- GM Mý M¡ (Õh$ Úm VM: Gamd ÀízDocument30 pagesGM Mý M¡ (Õh$ Úm VM: Gamd ÀízHems MadaviNo ratings yet
- VH©$ D Azw MZ: Gamd ÀízDocument8 pagesVH©$ D Azw MZ: Gamd ÀízHems MadaviNo ratings yet
- Idioms and PhrasalDocument17 pagesIdioms and PhrasalHems MadaviNo ratings yet
- Bagal ClassesDocument44 pagesBagal ClassesHems MadaviNo ratings yet
- 5 6174888431512979210Document34 pages5 6174888431512979210Hems MadaviNo ratings yet
- UnemploymentDocument2 pagesUnemploymentHems MadaviNo ratings yet
- Carbon Use in IndustryDocument1 pageCarbon Use in IndustryHems MadaviNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureHems MadaviNo ratings yet
- Verbal ReasoningDocument39 pagesVerbal ReasoningHems MadaviNo ratings yet
- Organic ChemistryDocument3 pagesOrganic ChemistryHems MadaviNo ratings yet
- Liquid SolutionDocument2 pagesLiquid SolutionHems MadaviNo ratings yet
- Redor ReactionDocument1 pageRedor ReactionHems MadaviNo ratings yet
- EcoDocument2 pagesEcoHems MadaviNo ratings yet
- Mba 2 Sem Financial Management p2 5685 Summer 2019Document4 pagesMba 2 Sem Financial Management p2 5685 Summer 2019Hems MadaviNo ratings yet
- Braintree 15Document6 pagesBraintree 15paypaltrexNo ratings yet
- 322 Dynamic Demographic Characteristic Slum Population in Nashik City With Special Reference From 2011Document6 pages322 Dynamic Demographic Characteristic Slum Population in Nashik City With Special Reference From 2011B-15 Keyur BhanushaliNo ratings yet
- Briggs-Myers Personality Test and Careers: MindDocument2 pagesBriggs-Myers Personality Test and Careers: Mindapi-378982090No ratings yet
- Hotels in MandalayDocument149 pagesHotels in Mandalayzaw khaingNo ratings yet
- Sunil Panda Commerce Classes: Before Exam Practice Questions For Term 2 Boards Accounts-Not For Profit OrganisationDocument3 pagesSunil Panda Commerce Classes: Before Exam Practice Questions For Term 2 Boards Accounts-Not For Profit OrganisationHigi SNo ratings yet
- Solar Power PlantDocument23 pagesSolar Power Plantmadhu_bedi12No ratings yet
- Plug Valves To API 599Document11 pagesPlug Valves To API 599Marten HaneNo ratings yet
- International Dairy Journal: D.K. Hickey, T.P. Guinee, J. Hou, M.G. WilkinsonDocument6 pagesInternational Dairy Journal: D.K. Hickey, T.P. Guinee, J. Hou, M.G. WilkinsonBianca AndreeaNo ratings yet
- ISC 2016 Chemistry Paper 2 Practical SolvedDocument12 pagesISC 2016 Chemistry Paper 2 Practical SolvedMritunjay Kumar SharmaNo ratings yet
- Toad For OracleDocument1,157 pagesToad For OraclesatsriniNo ratings yet
- Castel Airco 2014-15Document68 pagesCastel Airco 2014-15Anderson Giovanny Herrera DelgadoNo ratings yet
- PSS5000-USGU LicenseKey Installation 80558701Document10 pagesPSS5000-USGU LicenseKey Installation 80558701LongNo ratings yet
- Pawan's ResumeDocument1 pagePawan's ResumePAWAN YADAVNo ratings yet
- BIRD Internet Routing DaemonDocument4 pagesBIRD Internet Routing DaemonAleksandra FeyNo ratings yet
- Financial Statement Analysis of Automobile Sector: Tata Motors Mahindra and MahindraDocument25 pagesFinancial Statement Analysis of Automobile Sector: Tata Motors Mahindra and MahindraAshwini MahaleNo ratings yet
- Egger Nursery's in KeralaDocument6 pagesEgger Nursery's in KeralanidhinpillaiNo ratings yet
- Dubai Workshop RegistrationDocument2 pagesDubai Workshop RegistrationmfkmughalNo ratings yet
- Erba BilubrinDocument2 pagesErba BilubrinOjie SarojieNo ratings yet
- Blake Problem ComputationDocument3 pagesBlake Problem ComputationNiño del Mundo75% (4)
- Top 5 Strumming Patterns OK PDFDocument6 pagesTop 5 Strumming Patterns OK PDFjumpin_around100% (1)
- Pre Board POL - SCI Paper 2Document6 pagesPre Board POL - SCI Paper 2harsh jatNo ratings yet
- Guide Raotm2014 JakartaDocument23 pagesGuide Raotm2014 JakartarajavinugmailcomNo ratings yet
- Powermax45 Despiece AntorchaDocument5 pagesPowermax45 Despiece AntorchaWall OmarNo ratings yet
- Expt. 8 Salivary DigestionDocument25 pagesExpt. 8 Salivary DigestionLESLIE JANE BALUYOS JALANo ratings yet
- 1700 N Manhattan Ave - Royal Towers - Notice of CondemnationDocument14 pages1700 N Manhattan Ave - Royal Towers - Notice of CondemnationMatthew SelfNo ratings yet
- Sabam Sariaman CVDocument1 pageSabam Sariaman CVsabamsiregarNo ratings yet
- PRojectDocument61 pagesPRojectAditya SalunkheNo ratings yet
- Golden Gate PDFDocument42 pagesGolden Gate PDFJose David Vivas AbrilNo ratings yet
Reducedformtonidisedfoomofo - TT: Redox Reaction
Reducedformtonidisedfoomofo - TT: Redox Reaction
Uploaded by
Hems MadaviOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reducedformtonidisedfoomofo - TT: Redox Reaction
Reducedformtonidisedfoomofo - TT: Redox Reaction
Uploaded by
Hems MadaviCopyright:
Available Formats
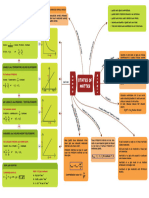
Redox reaction
No.of equivalents,equivalent mass,normality Redox reaction
Equivalent concept → reaction oidependent it is
basically transfer
↳ reaction doesn't need to be of e-
balanced A B
mole d d
Balanced - use
concept
reaction / at B-
↳ Unbalanced → Balance it → Mole concept
onidised reduced
↳ calculate
ng→eg concept
.
[ R.A. ] [ 8. A ] .
No .
of equivalent → no .
ofmolesx n -
factor
e- Molal mass
nf
normality Molarity ✗ nf
__
Law of equivalence
'
Molar is no
.ofe equivalent equivalent
-
E = mass
=
calculated
Total no .ofµYoñ] for amore
transferred
permoye of acid of base
s
"
repeatable or to be only Mn
replaced N=no.of equivalent/v mnoy
gMnOÉ
Calculation of n-factor for different
¥→Mn0z
type of redox reaction-
↳ SIMPLE REDOX Balancing of
chemical reaction
0 A- 1- RA
reducedformtonidisedfoomofo.tt
- . →
.
of RA .
→ Calculate n -
factor of
↳ COMBINED REDOX onidisingagentavreduuiy
Mitt fest agent .
Kmnoyt Feczoy →
+ coz cross with the
multiply
→
'
nf=5 ng =3 nf=5 nf= "
f- 1 simplest uatioofn f -
→ Balance atoms under -
↳ SELF DECOMPOSITION REACTION
going oxidation
-
oxidised
µ
In
503 > 502-1>1202 a
molecule
single one →
reduction .
reduced
7 , To Balance 0 & H
element will
✓\
nf=n Reduction undergo coxing Acidic Basic
other one is reduced
medium medium
↳ DISPROPORTIONATION REACTION ☐→ comes 0 → comes
from
-
on
element from Hzo
Hiii → Hit -11202°
same
onidised from nt us from 40
gets as n →
well reduced
nf=no×nR as
not nR
"+mn4+ ream in
3mn05 > 2mn
only for
which same element
total 24-27+14-4
a- charge =
gets reduced in 2
diff .
3
total item
stages
→
if oxidised → combined
redone
You might also like
- Imagining the Nation in Nature: Landscape Preservation and German Identity, 1885–1945From EverandImagining the Nation in Nature: Landscape Preservation and German Identity, 1885–1945No ratings yet
- Module 6.9 TLEDocument115 pagesModule 6.9 TLEroseavy90% (10)
- Star Wars Republic Holonet NewsDocument13 pagesStar Wars Republic Holonet NewsJessie Abraham LaidlawNo ratings yet
- UCS Lectures PDFDocument100 pagesUCS Lectures PDFgoeffNo ratings yet
- Macro Economic - LectureDocument7 pagesMacro Economic - LectureANYA AKBARNo ratings yet
- Eco 3Document6 pagesEco 3Pannathorn SiriNo ratings yet
- Upd C11 Phy EngDocument23 pagesUpd C11 Phy EngArinjoy Mervyn GomesNo ratings yet
- Math Dance SymbolsDocument1 pageMath Dance SymbolsJerry Bhoy ReniedoNo ratings yet
- States of MatterDocument1 pageStates of MattergnanavishaljonnalagaddaNo ratings yet
- A5. States of MatterDocument1 pageA5. States of MatterHitesh KumarNo ratings yet
- If Absolutely Conditionally: ComparisonDocument3 pagesIf Absolutely Conditionally: Comparisonlimyh123abcNo ratings yet
- U&D To NLM (RY)Document277 pagesU&D To NLM (RY)Chashan DeepNo ratings yet
- @physics - 11class Vectors and ForcesDocument69 pages@physics - 11class Vectors and ForcesPranav RoyNo ratings yet
- Cheat SheetDocument3 pagesCheat Sheetjohnson2004412No ratings yet
- Rate Expression - FactRecallDocument1 pageRate Expression - FactRecallAhana KatyalNo ratings yet
- Midterm SummaryDocument10 pagesMidterm SummaryDarkos134No ratings yet
- Tema 2 - LaplaceDocument9 pagesTema 2 - LaplaceLuyi WangNo ratings yet
- CH 14 - IRDocument5 pagesCH 14 - IRElle QuizonNo ratings yet
- Physics Y1 ReviewDocument5 pagesPhysics Y1 ReviewRakeem McFarlaneNo ratings yet
- Vectors Live Class 1 NotesDocument40 pagesVectors Live Class 1 NotesKushagra TiwariNo ratings yet
- Atomic Structure Mind MapDocument2 pagesAtomic Structure Mind Maplakshminivas PingaliNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureHems MadaviNo ratings yet
- Atomic Structure Mind MapDocument2 pagesAtomic Structure Mind Mapcric.indian.womenNo ratings yet
- Summary of Tests For ConvergenceDocument1 pageSummary of Tests For ConvergenceAurelio ReyesNo ratings yet
- Solutions - Class Notes - Lakshya NEET 2025Document24 pagesSolutions - Class Notes - Lakshya NEET 2025Dipanshu MundNo ratings yet
- Trade TheoriesDocument7 pagesTrade TheoriesWajiha RajaniNo ratings yet
- P3 Motion in A Straight LineDocument1 pageP3 Motion in A Straight LineberburnleyNo ratings yet
- Gibbs Adsorption Isotherm NotesDocument3 pagesGibbs Adsorption Isotherm NotesAshish ChauhanNo ratings yet
- Clase 20220526 FQDocument10 pagesClase 20220526 FQJeshNo ratings yet
- Lecture 4Document4 pagesLecture 4Ghostrocket 017No ratings yet
- Maths For Economics-1Document20 pagesMaths For Economics-1Genshin ImpactNo ratings yet
- Unit 3Document10 pagesUnit 3Leen Al-FouzanNo ratings yet
- SAT Grammar One Point Question PackDocument10 pagesSAT Grammar One Point Question PackKevin LimNo ratings yet
- Defleksi Pada Rangka BatangDocument6 pagesDefleksi Pada Rangka BatangAyunda ElvandariNo ratings yet
- Formula Cheat Sheet 2023Document3 pagesFormula Cheat Sheet 2023Hardik NarangNo ratings yet
- IB Chemistry (Chapter 11)Document5 pagesIB Chemistry (Chapter 11)hyunjinp0107No ratings yet
- PDF Updated Class 11 Physics Formula Sheet CompressDocument22 pagesPDF Updated Class 11 Physics Formula Sheet CompressdrjbjpNo ratings yet
- สูตรฟิโม2Document1 pageสูตรฟิโม2Supawadee YodsuwanNo ratings yet
- Vfi F.li: UpsibeDocument5 pagesVfi F.li: UpsibeYusheng LaiNo ratings yet
- Chemical Kinetics Mind MapDocument1 pageChemical Kinetics Mind MapSamridhi MoudgilNo ratings yet
- Harlem by Langston Hughes - Poetry FoundationDocument1 pageHarlem by Langston Hughes - Poetry Foundations026103No ratings yet
- FluidsDocument15 pagesFluidsskumar123846No ratings yet
- 2.8 Linear Approximations and DifferentialsDocument7 pages2.8 Linear Approximations and Differentialskhyang0510No ratings yet
- Maths Important PointsDocument146 pagesMaths Important Pointsgspareek1111No ratings yet
- Lim Lim: Hint Apply Integration by Parts To The Integral ForDocument1 pageLim Lim: Hint Apply Integration by Parts To The Integral ForHyunSung KimNo ratings yet
- IB Chemistry (Periodic Table)Document1 pageIB Chemistry (Periodic Table)hyunjinp0107No ratings yet
- Ligand Sub Factreycall ChemistryDocument2 pagesLigand Sub Factreycall Chemistryosahai17No ratings yet
- Periodic Motion NotesDocument9 pagesPeriodic Motion Noteschlorine169No ratings yet
- Types of Quadrilateral Name Shape PropertiesDocument13 pagesTypes of Quadrilateral Name Shape Propertiesanon_64795088No ratings yet
- Ln3.Fm# - Fcoulomb 4Tfeo&Z: BalmerDocument3 pagesLn3.Fm# - Fcoulomb 4Tfeo&Z: BalmerJayNo ratings yet
- Organic ChemistryDocument5 pagesOrganic ChemistryNumpxNump 465No ratings yet
- Ifsvdociiy: Motion in 1-DDocument1 pageIfsvdociiy: Motion in 1-DHems MadaviNo ratings yet
- Ill I: SlopeDocument10 pagesIll I: SlopeMohit MehtaNo ratings yet
- Electrochemistry Mind MapDocument2 pagesElectrochemistry Mind MapBhavna BeniwalNo ratings yet
- Escavadeira Hyundai r220lc 9Document120 pagesEscavadeira Hyundai r220lc 9Wolf ChakkalNo ratings yet
- Magnetism 1 2023 by Shyam Mohan BhaiyaDocument22 pagesMagnetism 1 2023 by Shyam Mohan BhaiyaPranav KumarNo ratings yet
- Formula Sheet Class 12Document3 pagesFormula Sheet Class 12SurajNo ratings yet
- Successione GeometricaDocument1 pageSuccessione Geometricaloparco.gianfrancoNo ratings yet
- Unit Dimensions and MeasurementsDocument7 pagesUnit Dimensions and MeasurementsAarush Ram AnandhNo ratings yet
- Music NoteDocument2 pagesMusic Noteseung won OhNo ratings yet
- Convergence TestsDocument1 pageConvergence TestspgokoolNo ratings yet
- Solid Mensuration 10072023Document6 pagesSolid Mensuration 10072023Nathan AducaNo ratings yet
- Ifsvdociiy: Motion in 1-DDocument1 pageIfsvdociiy: Motion in 1-DHems MadaviNo ratings yet
- GM Mý M¡ (Õh$ Úm VM: Gamd ÀízDocument30 pagesGM Mý M¡ (Õh$ Úm VM: Gamd ÀízHems MadaviNo ratings yet
- VH©$ D Azw MZ: Gamd ÀízDocument8 pagesVH©$ D Azw MZ: Gamd ÀízHems MadaviNo ratings yet
- Idioms and PhrasalDocument17 pagesIdioms and PhrasalHems MadaviNo ratings yet
- Bagal ClassesDocument44 pagesBagal ClassesHems MadaviNo ratings yet
- 5 6174888431512979210Document34 pages5 6174888431512979210Hems MadaviNo ratings yet
- UnemploymentDocument2 pagesUnemploymentHems MadaviNo ratings yet
- Carbon Use in IndustryDocument1 pageCarbon Use in IndustryHems MadaviNo ratings yet
- Atomic StructureDocument2 pagesAtomic StructureHems MadaviNo ratings yet
- Verbal ReasoningDocument39 pagesVerbal ReasoningHems MadaviNo ratings yet
- Organic ChemistryDocument3 pagesOrganic ChemistryHems MadaviNo ratings yet
- Liquid SolutionDocument2 pagesLiquid SolutionHems MadaviNo ratings yet
- Redor ReactionDocument1 pageRedor ReactionHems MadaviNo ratings yet
- EcoDocument2 pagesEcoHems MadaviNo ratings yet
- Mba 2 Sem Financial Management p2 5685 Summer 2019Document4 pagesMba 2 Sem Financial Management p2 5685 Summer 2019Hems MadaviNo ratings yet
- Braintree 15Document6 pagesBraintree 15paypaltrexNo ratings yet
- 322 Dynamic Demographic Characteristic Slum Population in Nashik City With Special Reference From 2011Document6 pages322 Dynamic Demographic Characteristic Slum Population in Nashik City With Special Reference From 2011B-15 Keyur BhanushaliNo ratings yet
- Briggs-Myers Personality Test and Careers: MindDocument2 pagesBriggs-Myers Personality Test and Careers: Mindapi-378982090No ratings yet
- Hotels in MandalayDocument149 pagesHotels in Mandalayzaw khaingNo ratings yet
- Sunil Panda Commerce Classes: Before Exam Practice Questions For Term 2 Boards Accounts-Not For Profit OrganisationDocument3 pagesSunil Panda Commerce Classes: Before Exam Practice Questions For Term 2 Boards Accounts-Not For Profit OrganisationHigi SNo ratings yet
- Solar Power PlantDocument23 pagesSolar Power Plantmadhu_bedi12No ratings yet
- Plug Valves To API 599Document11 pagesPlug Valves To API 599Marten HaneNo ratings yet
- International Dairy Journal: D.K. Hickey, T.P. Guinee, J. Hou, M.G. WilkinsonDocument6 pagesInternational Dairy Journal: D.K. Hickey, T.P. Guinee, J. Hou, M.G. WilkinsonBianca AndreeaNo ratings yet
- ISC 2016 Chemistry Paper 2 Practical SolvedDocument12 pagesISC 2016 Chemistry Paper 2 Practical SolvedMritunjay Kumar SharmaNo ratings yet
- Toad For OracleDocument1,157 pagesToad For OraclesatsriniNo ratings yet
- Castel Airco 2014-15Document68 pagesCastel Airco 2014-15Anderson Giovanny Herrera DelgadoNo ratings yet
- PSS5000-USGU LicenseKey Installation 80558701Document10 pagesPSS5000-USGU LicenseKey Installation 80558701LongNo ratings yet
- Pawan's ResumeDocument1 pagePawan's ResumePAWAN YADAVNo ratings yet
- BIRD Internet Routing DaemonDocument4 pagesBIRD Internet Routing DaemonAleksandra FeyNo ratings yet
- Financial Statement Analysis of Automobile Sector: Tata Motors Mahindra and MahindraDocument25 pagesFinancial Statement Analysis of Automobile Sector: Tata Motors Mahindra and MahindraAshwini MahaleNo ratings yet
- Egger Nursery's in KeralaDocument6 pagesEgger Nursery's in KeralanidhinpillaiNo ratings yet
- Dubai Workshop RegistrationDocument2 pagesDubai Workshop RegistrationmfkmughalNo ratings yet
- Erba BilubrinDocument2 pagesErba BilubrinOjie SarojieNo ratings yet
- Blake Problem ComputationDocument3 pagesBlake Problem ComputationNiño del Mundo75% (4)
- Top 5 Strumming Patterns OK PDFDocument6 pagesTop 5 Strumming Patterns OK PDFjumpin_around100% (1)
- Pre Board POL - SCI Paper 2Document6 pagesPre Board POL - SCI Paper 2harsh jatNo ratings yet
- Guide Raotm2014 JakartaDocument23 pagesGuide Raotm2014 JakartarajavinugmailcomNo ratings yet
- Powermax45 Despiece AntorchaDocument5 pagesPowermax45 Despiece AntorchaWall OmarNo ratings yet
- Expt. 8 Salivary DigestionDocument25 pagesExpt. 8 Salivary DigestionLESLIE JANE BALUYOS JALANo ratings yet
- 1700 N Manhattan Ave - Royal Towers - Notice of CondemnationDocument14 pages1700 N Manhattan Ave - Royal Towers - Notice of CondemnationMatthew SelfNo ratings yet
- Sabam Sariaman CVDocument1 pageSabam Sariaman CVsabamsiregarNo ratings yet
- PRojectDocument61 pagesPRojectAditya SalunkheNo ratings yet
- Golden Gate PDFDocument42 pagesGolden Gate PDFJose David Vivas AbrilNo ratings yet