Professional Documents
Culture Documents
McMurry9e PPT CH16
McMurry9e PPT CH16
Uploaded by
Abdel Rawashdeh0 ratings0% found this document useful (0 votes)
10 views86 pagesOrg
Chem
16
Original Title
McMurry9e_PPT_CH16
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentOrg
Chem
16
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
10 views86 pagesMcMurry9e PPT CH16
McMurry9e PPT CH16
Uploaded by
Abdel RawashdehOrg
Chem
16
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 86
John E.
McMurry
www.cengage.com/chemistry/mcmurry
Chapter 16
Chemistry of Benzene:
Electrophilic Aromatic Substitution
© 2016 Cengage Learning. All Rights Reserved.
Learning Objectives
(16.1)
▪ Electrophilic aromatic substitution reactions:
Bromination
(16.2)
▪ Other aromatic substitutions
(16.3)
▪ Alkylation and acylation of aromatic rings: The
Friedel-Crafts reaction
(16.4)
▪ Substituent effects in electrophilic substitutions
© 2016 Cengage Learning. All Rights Reserved.
(16.5)
▪ Trisubstituted benzenes: Additivity of effects
(16.6)
▪ Nucleophilic aromatic substitution
(16.7)
▪ Benzyne
(16.8)
▪ Oxidation of aromatic compounds
© 2016 Cengage Learning. All Rights Reserved.
(16.9)
▪ Reduction of aromatic compounds
(16.10)
▪ Synthesis of polysubstituted benzenes
© 2016 Cengage Learning. All Rights Reserved.
Electrophilic Aromatic Substitution
Reactions: Bromination
▪ An electrophile reacts with an aromatic ring to
substitute a hydrogen on the ring
▪ The beginning of the reaction is similar to that of
electrophilic alkene reactions
▪ The difference is that alkenes react more readily
with electrophiles than aromatic rings
▪ In the bromination of benzene, a catalyst such as
FeBr3 is used
© 2016 Cengage Learning. All Rights Reserved.
Electrophilic Aromatic Substitution
Reactions: Bromination
▪ Stability of the intermediate in electrophilic
aromatic substitution is lesser than that of the
starting benzene ring
▪ Reaction of an electrophile is endergonic,
possesses substantial activation energy, and
comparatively slow
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.1 - Electrophile Reactions With
an Alkene and With Benzene
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.2 - Electrophilic
Bromination of Benzene
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Monobromination of toluene gives a mixture of
three bromotoluene products
▪ Draw and name them
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Other Aromatic Substitutions
▪ Chlorine and iodine (but not fluorine, which is
too reactive) can produce aromatic substitution
with the addition of other reagents to promote
the reaction
© 2016 Cengage Learning. All Rights Reserved.
Other Aromatic Substitutions
▪ Aromatic rings produce chlorobenzenes when
they react with Cl2 with FeCl3 as a catalyst
▪ Pharmaceutical agents such as Claritin are
manufactured by a similar reaction
© 2016 Cengage Learning. All Rights Reserved.
Other Aromatic Substitutions
▪ Since iodine is unreactive toward aromatic rings,
oxidizing agents such as CuCl2 are used as a
catalyst
▪ CuCl2 oxidizes I2 resulting in the production of a
substitute
© 2016 Cengage Learning. All Rights Reserved.
Natural Electrophilic Aromatic
Halogenations
▪ Widely found in marine organisms
▪ Occurs in the biosynthesis of thyroxine in
humans
© 2016 Cengage Learning. All Rights Reserved.
Aromatic Nitration
▪ Combination of concentrated nitric acid and
sulfuric acid results in NO2+ (nitronium ion)
▪ Reaction with benzene produces nitrobenzene
© 2016 Cengage Learning. All Rights Reserved.
Aromatic Sulfonation
▪ Occurs by a reaction with fuming sulphuric acid,
a mixture of H2SO4, and SO3
▪ The reactive electrophile is either HSO3+ or
neutral SO3
© 2016 Cengage Learning. All Rights Reserved.
Aromatic Hydroxylation
▪ Direct hydroxylation of an aromatic ring is
difficult in the laboratory
▪ Usually occurs in biological pathways
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.6 - Mechanism for the Electrophilic
Hydroxylation of p-hydroxyphenylacetate
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Propose a mechanism for the electrophilic
fluorination of benzene with F-TEDA-BF4
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ The pi electrons of benzene attack the fluorine of
F-TEDA-BF4
▪ The nonaromatic intermediate loses –H to give the
fluorinated product
© 2016 Cengage Learning. All Rights Reserved.
Alkylation of Aromatic Rings:
The Friedel-Crafts Reaction
▪ Alkylation: Introducing an alkyl group onto the
benzene ring
▪ Also called the Friedel-Crafts reaction
▪ Involves treatment of an aromatic compound with
an alkyl chloride to yield a carbocation
electrophile
▪ Aluminium chloride used as a catalyst which causes
dissociation of the alkyl halide
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.7 - Mechanism of the
Friedel-Crafts Reaction
© 2016 Cengage Learning. All Rights Reserved.
Limitations of the Friedel-Crafts
Reaction
▪ Only alkyl halides can be used (F, Cl, I, Br)
▪ High energy levels of aromatic and vinylic halides
are not suitable to Friedel-Crafts requirements
▪ Not feasible on rings containing an amino group
substituent or a strong electron-withdrawing
group
© 2016 Cengage Learning. All Rights Reserved.
Limitations of the Friedel-Crafts
Reaction
▪ Termination of the reaction allowing a single
substitution is difficult
▪ Polyalkylation occurs
© 2016 Cengage Learning. All Rights Reserved.
Limitations of the Friedel-Crafts
Reaction
▪ Occasional skeletal rearrangement of the alkyl
carbocation electrophile
▪ Occurs more often with the use of a primary alkyl
halide
© 2016 Cengage Learning. All Rights Reserved.
Acylation of Aromatic Rings
▪ Acylation: Reaction of an aromatic ring with a
carboxylic acid chloride in the presence of AlCl3
resulting in an acyl group substitution
© 2016 Cengage Learning. All Rights Reserved.
Mechanism of Friedel-Crafts
Acylation
▪ Similar to Freidel-Crafts alkylation and also
possesses the same limitations on the aromatic
substrate
© 2016 Cengage Learning. All Rights Reserved.
Alkylation of Aromatic Rings:
The Friedel-Crafts Reaction
▪ Natural aromatic alkylations are a part of many
biological pathways
▪ Catalyzing effect of AlCl3 is replaced by
organidiphosphate dissociation
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.10 - Biosynthesis of
Phylloquinone from 1,4-
dihydroxynaphthoic Acid
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Identify the carboxylic acid that might be used in
a Friedel-Crafts acylation to prepare the
following acylbenzene
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
▪ Identification of the carboxylic acid chloride used
in the Friedel-Crafts acylation of benzene
involves:
▪ Breaking the bond between benzene and the ketone
carbon
▪ Using a –Cl replacement
© 2016 Cengage Learning. All Rights Reserved.
Substituent Effects in
Substituted Aromatic Rings
▪ Reactivity of the aromatic ring is affected
▪ Substitution can result in an aromatic ring with a
higher or a lower reactivity than benzene
© 2016 Cengage Learning. All Rights Reserved.
Table 16.1 - Orientation of Nitration
in Substituted Bezenes
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.11 - Classification of
Substituent Effects in Electrophilic
Aromatic Substitution
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Predict the major product in the nitration of
bromobenzene
▪ Solution:
▪ Even though bromine is a deactivator, it is used
as an ortho-para director
© 2016 Cengage Learning. All Rights Reserved.
Activating or Deactivating
Effects
▪ Activating groups contribute electrons to the
aromatic ring
▪ The ring possesses more electrons
▪ The carbocation intermediate is stabilized
▪ Activation energy is lowered
▪ Deactivating groups withdraw electrons from the
aromatic ring
▪ The ring possesses lesser electrons
▪ The carbocation intermediate is destabilized
▪ Activation energy is increased
© 2016 Cengage Learning. All Rights Reserved.
Origins of Substituent Effects
▪ Inductive effect: Withdrawal or donation of
electrons by a sigma bond due to
electronegativity
▪ Prevalent in halobenzenes and phenols
© 2016 Cengage Learning. All Rights Reserved.
Resonance Effects - Electron
Withdrawal
▪ Resonance effect: Withdrawal or donation of
electrons through a bond due to the overlap of
a p orbital on the substituent with a p orbital on
the aromatic ring
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Explain why Freidel-Crafts alkylations often give
polymer substitution but Freidel-Crafts
acylations do not
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution
▪ An acyl substituent if deactivating
▪ Once an aromatic ring has been acylated, it is less
reactive to further substitution
▪ An alkyl substituent is activating, however, an alkyl-
substituted ring is more reactive than an
unsubstituted ring
▪ Polysubstitution occurs readily
© 2016 Cengage Learning. All Rights Reserved.
Ortho- and Para-Directing
Activators: Alkyl Groups
▪ Alkyl groups possess an electron-dating
inductive effect
© 2016 Cengage Learning. All Rights Reserved.
Ortho- and Para-Directing
Activators: OH and NH2
▪ Hydroxyl, alkoxyl, and amino groups possess a
strong, electron-donating resonance effect
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Explain why acetanilide is less reactive than
aniline toward electrophilic substitution
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ For acetanilide, resonance delocalization of the
nitrogen lone pair electrons to the aromatic ring is
less favoured
▪ Positive charge on nitrogen is next to the positively
polarized carbonyl group
▪ The electronegativity of oxygen favors resonance
delocalization to the carbonyl oxygen
▪ In aniline, the decreased availability of nitrogen
lone pair electrons results in decreased reactivity
of the ring toward electrophilic substitution
© 2016 Cengage Learning. All Rights Reserved.
Ortho- and Para-Directing
Deactivators: Halogens
▪ Caused by the dominance of the stronger
electron-withdrawing inductive effect over their
weaker electron-donating resonance effect
▪ Electron donating resonance effect is present
only at the ortho and para positions
▪ Ortho and para reactions can cause stabilization
of the positive charge in the carbocation
intermediates
▪ Meta intermediates take more time to form
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.15 - Carbocation Intermediates in the
Nitration of Chlorobenzene
© 2016 Cengage Learning. All Rights Reserved.
Meta-Directing Deactivators
▪ The meta intermediate possesses three
favourable resonance forms
▪ Ortho and para intermediates possess only two
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Draw resonance structures for the intermediates
from the reaction of an electrophile at the ortho,
meta, and para positions of nitrobenzene
▪ Determine which intermediates are most stable
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Table 16.2 - Substituent Effects in
Electrophilic Aromatic Substitution
© 2016 Cengage Learning. All Rights Reserved.
Trisubstituted Benzenes:
Additivity of Effects
▪ Additivity effects are based on three rules:
▪ The situation is straightforward if the directing
effects of the groups reinforce each other
© 2016 Cengage Learning. All Rights Reserved.
Trisubstituted Benzenes:
Additivity of Effects
▪ If the directing effects of two groups oppose each
other, the more powerful activating group decides
the principal outcome
▪ Usually gives mixtures of products
© 2016 Cengage Learning. All Rights Reserved.
Trisubstituted Benzenes:
Additivity of Effects
▪ Further substitution is rare when two groups are
in a meta-disubstituted compound as the site is
too hindered
▪ An alternate route must be taken in the preparation
of aromatic rings with three adjacent substituents
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Determine the position at which electrophilic
substitution occurs in the following substance
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
▪ Both groups are ortho-para directors and direct
substitution to the same positions
▪ Attack does not occur between the two groups for
steric reasons
© 2016 Cengage Learning. All Rights Reserved.
Nucleophilic Aromatic
Substitution
▪ Aryl halides with electron-withdrawing
substituents can also undergo a nucleophilic
substitution reaction
© 2016 Cengage Learning. All Rights Reserved.
Nucleophilic Aromatic
Substitution
▪ Not very common
▪ Uses
▪ Reaction of proteins with Sanger’s reagent
results in a label being attached to one end of the
protein chain
▪ Reaction is superficially similar to the SN1 and
SN2 nucleophilic substitutions
▪ Aryl halides are inert to both SN1 and SN2 conditions
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.17 - Mechanism of
Nucleophilic Aromatic Substitution
© 2016 Cengage Learning. All Rights Reserved.
Figure 16.18 - Nucleophilic Aromatic
Substitution of Nitrochlorobenzenes
© 2016 Cengage Learning. All Rights Reserved.
Differences Between Electrophilic and
Nucleophilic Aromatic Substitutions
Electrophilic substitutions Nucleophilic substitutions
▪ Favored by electron- ▪ Favored by electron-
donating substituents withdrawing substituents
▪ Electron-withdrawing ▪ Electron-withdrawing
groups cause ring groups cause ring
deactivation activation
▪ Electron-withdrawing ▪ Electron withdrawing
groups are meta directors groups are ortho-para
▪ Replace hydrogen on the directors
ring ▪ Replace a leaving group
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Propose a mechanism for the preparation of
oxyfluorfen, a herbicide, through the reaction
between phenol and an aryl fluoride
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
▪ Step 1: Addition of the nucleophile
▪ Step 2: Elimination of the fluoride ion
© 2016 Cengage Learning. All Rights Reserved.
Benzyne
▪ On a general basis, there are no reactions
between nucleophiles and halobenzenes that do
not have electron withdrawing substituents
▪ High temperatures can be used to make
chlorobenzene react
© 2016 Cengage Learning. All Rights Reserved.
Benzyne
▪ A Diels-Adler reaction occurs when
bromobenzene reacts with KNH2 in the
presence of a conjugated diene, such as furan
▪ Elimination of HBr from bromobenzene forms a
benzyne as the chemical intermediate
© 2016 Cengage Learning. All Rights Reserved.
Benzyne
▪ Benzyne has the electronic structure of a highly
distorted alkyne
▪ The benzyne triple bond uses sp2-hybridized
carbon atoms
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Explain why the treatment of p-toluene with
NaOH at 300°C yields a mixture of two
products, but treatment of m-bromotoluene with
NaOH yields a mixture of two or three products
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Oxidation of Aromatic
Compounds
▪ In the presence of an aromatic ring, alkyl side
chains are converted to carboxyl groups through
oxidation
▪ Alkylbenzene is converted to benzoic acid
© 2016 Cengage Learning. All Rights Reserved.
Oxidation of Aromatic
Compounds
▪ Side-chain oxidation involves a complex
mechanism wherein C–H bonds next to the
aromatic ring react to form intermediate benzylic
radicals
▪ Analogous side-chain reactions are a part of
many biosynthetic pathways
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Mention the aromatic substance that is obtained
if KMnO4 undergoes oxidation with the following
substance
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
▪ Oxidation takes place at the benzylic position
© 2016 Cengage Learning. All Rights Reserved.
Bromination of Alkylbenzene
Side Chains
▪ Occurs when an alkylbenzene is treated with N-
bromosuccinimide (NBS)
© 2016 Cengage Learning. All Rights Reserved.
Mechanism of NBS (Radical)
Reaction
▪ Abstraction of a benzylic hydrogen atom
generates an intermediate benzylic radical
▪ Benzylic radical reacts with Br2 to yield product
and a Br- radical
▪ Br- radical cycles back into reaction to carry on
the chain
▪ Br2 is produced when HBr reacts with NBS
© 2016 Cengage Learning. All Rights Reserved.
Mechanism of NBS (Radical)
Reaction
··
© 2016 Cengage Learning. All Rights Reserved.
Bromination of Alkylbenzene
Side Chains
▪ The reaction of HBr with NBS occurs only at the
benzylic position
▪ The benzylic radical intermediate is stabilized by
resonance
▪ The p orbital of the benzyl radical overlaps with the
ringed electron system
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Styrene, the simplest alkenylbenzene, is
prepared for commercial use in plastics
manufacture by catalytic dehydrogenation of
ethylbenzene
▪ Prepare styrene from benzene
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Reduction of Aromatic
Compounds
▪ Aromatic rings are inert to catalytic
hydrogenation under conditions that reduce
alkene double bonds
▪ They can selectively reduce an alkene double
bond in the presence of an aromatic ring
© 2016 Cengage Learning. All Rights Reserved.
Reduction of Aromatic
Compounds
▪ Reduction of an aromatic ring requires either:
▪ A platinum catalyst and a pressure of several
hundred atmospheres
▪ A catalyst such as rhodium or carbon
© 2016 Cengage Learning. All Rights Reserved.
Reduction of Aryl Alkyl Ketones
▪ An aromatic ring can activate neighboring
carbonyl group toward reduction
▪ An aryl alkyl ketone can be converted into an
alkylbenzene by catalytic hydrogenation over a
palladium catalyst
© 2016 Cengage Learning. All Rights Reserved.
Reduction of Aryl Alkyl Ketones
▪ Only aryl alkyl ketones can be converted into a
methylene group by catalytic hydrogenation
▪ Nitro substituents hinder the catalytic reduction
of aryl alkyl ketones
▪ Nitro group undergoes reduction to form an
amino group
© 2016 Cengage Learning. All Rights Reserved.
Worked Example
▪ Prepare diphenylmethane, (Ph)2CH2, from
benzene and an acid chloride
▪ Solution:
© 2016 Cengage Learning. All Rights Reserved.
Synthesis of Polysubstituted
Benzenes
▪ Working synthesis reactions is one of the best
ways to learn organic chemistry
▪ Knowledge on using the right reactions at the
right time is vital to a successful scheme
▪ Ability to plan a sequence of reactions in right
order is valuable to synthesis of substituted
aromatic rings
© 2016 Cengage Learning. All Rights Reserved.
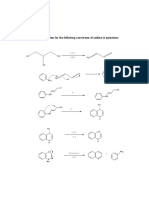
Worked Example
▪ Synthesize m-Chloronitrobenzene from benzene
▪ Solution:
▪ In order to synthesize the product with the correct
orientation of substituents, benzene must be
nitrated before it is chlorinated
© 2016 Cengage Learning. All Rights Reserved.
Summary
▪ There are two phases in an electrophilic
aromatic substitution reaction:
▪ Initial reaction of an electrophile E+
▪ Loss of H+ from the resonance-stabilized
carbocation intermediate
▪ The Friedel-Crafts alkylation and acylation
reactions are important electrophilic aromatic
substitution reactions that involve the reaction of
an aromatic ring with carbocation electrophiles
© 2016 Cengage Learning. All Rights Reserved.
Summary
▪ Resonance and inductive effects are the means
by which substituents influence aromatic rings
▪ Nucleophilic aromatic substitution is a reaction
that halobenzenes go through and involve an
addition of a nucleophile to the ring
▪ In halobenzenes that are not activated by
electron-withdrawing substituents, nucleophilic
aromatic substitutions occur by elimination of
HX which yields a benzene
© 2016 Cengage Learning. All Rights Reserved.
You might also like
- Seeley's Chapter 8 Nervous SystemDocument100 pagesSeeley's Chapter 8 Nervous SystemChristine Tapawan89% (9)
- LAB QO 4 - Nitration of ChlorobenzeneDocument9 pagesLAB QO 4 - Nitration of Chlorobenzenemario100% (1)
- Chapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurryDocument20 pagesChapter 16: Benzene - Electrophilic Aromatic Substitution: Chem231 Study Notes On McmurrykjjkimkmkNo ratings yet
- Reactions of Alkenes and Alkynes Study GuideDocument17 pagesReactions of Alkenes and Alkynes Study GuideMelissa GarciaNo ratings yet
- CHM 2210 Practice Exam 1Document12 pagesCHM 2210 Practice Exam 1Shaima MossamatNo ratings yet
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- McMurry Chapter 8Document72 pagesMcMurry Chapter 8Christine TapawanNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Carboxylic Acids and Acid Derivatives Carbonyl PDFDocument124 pagesCarboxylic Acids and Acid Derivatives Carbonyl PDFrvignesh2809No ratings yet
- Unit 5 Organic Chemistry ReactionsDocument9 pagesUnit 5 Organic Chemistry ReactionsRobbing_Hood100% (1)
- McMurry Chapter 7Document70 pagesMcMurry Chapter 7Christine TapawanNo ratings yet
- Organic Chemistry Midterm 1 Dir+eff++keyDocument1 pageOrganic Chemistry Midterm 1 Dir+eff++keyNorma Leticia RamosNo ratings yet
- Carboxylic AcidDocument21 pagesCarboxylic AcidMuhammad AjmalNo ratings yet
- Alkyl Halides SN1,2 and E1,2,1cbDocument45 pagesAlkyl Halides SN1,2 and E1,2,1cblorrainebarandonNo ratings yet
- Introduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)Document24 pagesIntroduction of Organic Chemistry by Eyes of Ajnish Kumar Gupta (AKG)ajju_208180% (5)
- Reactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionDocument51 pagesReactions of Aldehydes and Ketones: Oxidation Reduction Nucleophilic AdditionmacybnzNo ratings yet
- Mesomeric EffectDocument21 pagesMesomeric EffectAwais Arshad100% (2)
- Organic Chemistry IIDocument7 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- Organic Chemistry Reaction Mechanism Group AssignmentDocument3 pagesOrganic Chemistry Reaction Mechanism Group AssignmentSourabh Dhavala0% (1)
- Foundations of Organic ChemistryDocument4 pagesFoundations of Organic Chemistryeager18No ratings yet
- FullDocument1,175 pagesFull43 Trần Công VinhNo ratings yet
- Revision of IsomerismDocument20 pagesRevision of IsomerismAjnish GuptaNo ratings yet
- Resonance and Mesomeric EffectDocument13 pagesResonance and Mesomeric Effectapoorva singhNo ratings yet
- MCAT Organic Chemistry ReviewDocument43 pagesMCAT Organic Chemistry ReviewVetina LirioNo ratings yet
- Intro To Organic ChemDocument91 pagesIntro To Organic ChemMiguel Marquez GelacioNo ratings yet
- Organometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiDocument23 pagesOrganometallic Compunds: D. JIM LIVINGSTON, Asst - Prof in Chemistry, ST - John's College, PalaiJim LivingstonNo ratings yet
- McMurry Chapter 15Document59 pagesMcMurry Chapter 15Christine TapawanNo ratings yet
- Alkyl Halides PDFDocument66 pagesAlkyl Halides PDFAhmed Sideeg83% (6)
- Hetero-Cyclic CompoundsDocument69 pagesHetero-Cyclic CompoundsNaveed SajidNo ratings yet
- Grignard ReagntDocument18 pagesGrignard ReagntSiddarth Singh100% (1)
- Structure and Synthesis of Alcohols: Organic Chemistry, 7Document52 pagesStructure and Synthesis of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Welcome To Chem 206: Fall Term, 2005, David A. EvansDocument22 pagesWelcome To Chem 206: Fall Term, 2005, David A. EvanseraborNo ratings yet
- General Chemistry ReviewDocument15 pagesGeneral Chemistry ReviewPhirun ChengNo ratings yet
- PDFDocument155 pagesPDFHifza shairwani100% (1)
- Reactions of Alcohols: Organic Chemistry, 7Document53 pagesReactions of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Organic Chemistry II Chapter22Document8 pagesOrganic Chemistry II Chapter22RangikaNo ratings yet
- C F C CL C - BR: HalogenoalkanesDocument11 pagesC F C CL C - BR: HalogenoalkanesMufaro MutotiNo ratings yet
- Chapter 9 - HybridizationDocument15 pagesChapter 9 - HybridizationJing Yi PangNo ratings yet
- Reaction SummaryDocument5 pagesReaction SummaryShafaqatRahmanNo ratings yet
- Synthesis Review - Undergraduate Organic Synthesis GuideDocument19 pagesSynthesis Review - Undergraduate Organic Synthesis GuidePhạm Thị Thùy NhiênNo ratings yet
- Organic ChemistryDocument24 pagesOrganic ChemistryNivas KaruppananNo ratings yet
- Organic Chemistry 2021Document76 pagesOrganic Chemistry 2021Arah Mae BonillaNo ratings yet
- Protecting Group HandoutDocument5 pagesProtecting Group HandoutRafaelle Sanvictores SilongNo ratings yet
- Carbon 13 SpectrosDocument53 pagesCarbon 13 SpectrossaheedvkNo ratings yet
- Organic Chemistry Practice ProblemsDocument1 pageOrganic Chemistry Practice ProblemsSushant KumarNo ratings yet
- Reduction Agents Organic ChemistryDocument55 pagesReduction Agents Organic ChemistryvgvijuNo ratings yet
- Organic Chemistry Wade 8th Edition Chapter 18Document30 pagesOrganic Chemistry Wade 8th Edition Chapter 18이서영No ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 11Document58 pagesVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmNo ratings yet
- CH2.2 - AlkeneDocument48 pagesCH2.2 - AlkeneNur Ain SyuhadaNo ratings yet
- Inductive EffectDocument38 pagesInductive EffectJoe JNo ratings yet
- 19 Enolates EnaminesDocument59 pages19 Enolates EnaminesTwas Anassin100% (2)
- 1 BenzeneDocument41 pages1 Benzeneraj royelNo ratings yet
- Aromatic CompoundsDocument53 pagesAromatic CompoundsAshutoshNo ratings yet
- Huckel Rule of Aromaticity 2 PDFDocument25 pagesHuckel Rule of Aromaticity 2 PDFUmar Farooq100% (1)
- Lecture 1Document11 pagesLecture 1Fang GaoNo ratings yet
- Organometallic ChemistryDocument31 pagesOrganometallic ChemistrySadiaKhan100% (1)
- Electron Delocalization and ResonanceDocument9 pagesElectron Delocalization and ResonanceMariana LizethNo ratings yet
- Organometallic CompoundsDocument40 pagesOrganometallic CompoundsHalida SophiaNo ratings yet
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersFrom EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- 2 - Chemistry of BenzeneDocument22 pages2 - Chemistry of BenzeneGrgtNo ratings yet
- 18 Aromatic SubstitutionsDocument67 pages18 Aromatic SubstitutionsJun LyNo ratings yet
- McMurry Chapter 15Document59 pagesMcMurry Chapter 15Christine TapawanNo ratings yet
- McMurry Chapter 7Document70 pagesMcMurry Chapter 7Christine TapawanNo ratings yet
- Org Chem Lab Experiment 2: ExtractionDocument14 pagesOrg Chem Lab Experiment 2: ExtractionChristine TapawanNo ratings yet
- Heterocyclic ChemistryDocument47 pagesHeterocyclic ChemistrySalik KhanNo ratings yet
- Alcohol Phenol Ether Concise - OptDocument18 pagesAlcohol Phenol Ether Concise - Optaleena'No ratings yet
- Jurna Brominasi Eugenol 458-2493-1-PBDocument7 pagesJurna Brominasi Eugenol 458-2493-1-PBAnonymous g7yHQeB36No ratings yet
- B. Pharm 3rd Semester Previous Year Question PaperDocument20 pagesB. Pharm 3rd Semester Previous Year Question PaperAkanksha MadhaleNo ratings yet
- Aromatic Cmpds AnskeyDocument6 pagesAromatic Cmpds AnskeyAaron LeeNo ratings yet
- Sulfonation Mechanism of Benzene With SO3 in Sulfuric Acid or Oleum PDFDocument37 pagesSulfonation Mechanism of Benzene With SO3 in Sulfuric Acid or Oleum PDFLaely Dian MarlindawatiNo ratings yet
- Marking Scheme: Single Correct (+3,-1) M M: 138 Time: 1 HR 30 MinDocument7 pagesMarking Scheme: Single Correct (+3,-1) M M: 138 Time: 1 HR 30 MinPRITHVIRAJ GHOSHNo ratings yet
- Hydrocarbons PDFDocument19 pagesHydrocarbons PDFNeha ChaudharyNo ratings yet
- Alcohol Phenols and EthersDocument13 pagesAlcohol Phenols and EthersShivaanee SK100% (1)
- UNIT 6 HALO ALKANES & Halo Arenes LatestDocument50 pagesUNIT 6 HALO ALKANES & Halo Arenes Latestsukaina fatimaNo ratings yet
- Bischler Napieralski ReactionDocument3 pagesBischler Napieralski ReactionUmesh RangaNo ratings yet
- Haloalkanes and HaloarenesDocument3 pagesHaloalkanes and HaloarenesRAUNAK DEYNo ratings yet
- Chapter 12: Reactions of Arenes - Electrophilic Aromatic SubstitutionDocument29 pagesChapter 12: Reactions of Arenes - Electrophilic Aromatic SubstitutionRahma AshrafNo ratings yet
- (Download PDF) Organic Chemistry 11Th Edition Francis A Carey Full Chapter PDFDocument60 pages(Download PDF) Organic Chemistry 11Th Edition Francis A Carey Full Chapter PDFghelaiacmad7100% (11)
- Organic Chemistry 11Th Edition Francis A Carey Full ChapterDocument51 pagesOrganic Chemistry 11Th Edition Francis A Carey Full Chapterthomas.robinson634100% (8)
- Essay On StalinDocument8 pagesEssay On Stalinfz6zke9m100% (2)
- Electrophilic Aromatic Substitution: Ortho/para Directors Meta DirectorsDocument8 pagesElectrophilic Aromatic Substitution: Ortho/para Directors Meta DirectorsLuis Felipe Mera GrandasNo ratings yet
- Pyridine Reactions: University College of Pharmaceutialsciences K.U. CampusDocument16 pagesPyridine Reactions: University College of Pharmaceutialsciences K.U. CampusVã RãNo ratings yet
- Topic:-Nomenclature: 1. Give IUPAC Name ofDocument16 pagesTopic:-Nomenclature: 1. Give IUPAC Name ofkannan2030No ratings yet
- POC II Unit 1Document29 pagesPOC II Unit 1adapasivagangaNo ratings yet
- CHEM 238 Experiment #4: Formal Lab Report: Electrophilic Aromatic Substitution-The Nitration of Methyl BenzoateDocument18 pagesCHEM 238 Experiment #4: Formal Lab Report: Electrophilic Aromatic Substitution-The Nitration of Methyl Benzoateapi-592105594No ratings yet
- Pharmaceutical Organic Chemistry Lab 1 PHC464Document6 pagesPharmaceutical Organic Chemistry Lab 1 PHC464beyonduckNo ratings yet
- Allylic and Benzylic Reactivity: Instructor Supplemental Solutions To ProblemsDocument11 pagesAllylic and Benzylic Reactivity: Instructor Supplemental Solutions To ProblemsSandipan SahaNo ratings yet
- Substituent Effects - Organic ChemistryDocument8 pagesSubstituent Effects - Organic ChemistrytracyymendozaNo ratings yet
- Organic Chemistry 3 Assignment 1, B1748443Document7 pagesOrganic Chemistry 3 Assignment 1, B1748443zacksNo ratings yet
- Aromatic CompoundsDocument30 pagesAromatic CompoundsMA Masum HossainNo ratings yet
- 09 Stereo NotesDocument48 pages09 Stereo NotesZoran PavlovicNo ratings yet
- CBSE NCERT Solutions For Class 12 Chemistry Chapter 11: Back of Chapter QuestionsDocument51 pagesCBSE NCERT Solutions For Class 12 Chemistry Chapter 11: Back of Chapter QuestionsVivin Sansuri YNo ratings yet