Professional Documents
Culture Documents
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Uploaded by
eastareaCopyright:
Available Formats
You might also like
- Hypnotherapy ScriptsDocument109 pagesHypnotherapy ScriptsSabina Lupasco97% (31)
- Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma BiomarkersDocument10 pagesPrediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma BiomarkersAndrade GuiNo ratings yet
- Jamaneurology Ashton 2024 Oi 230097 1703782257.54322Document10 pagesJamaneurology Ashton 2024 Oi 230097 1703782257.54322HermesNo ratings yet
- Blood biomarkers for Alzheimer's disease in clinical practice and trials (科研通-ablesci.com)Document14 pagesBlood biomarkers for Alzheimer's disease in clinical practice and trials (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- 10.1515 - Almed 2020 0090Document11 pages10.1515 - Almed 2020 0090reggioamelia adaptacioncurricularNo ratings yet
- NFL Strongly Correlates With TNF R1 in The Plasma of AD Patients, But Not With Cognitive DeclineDocument7 pagesNFL Strongly Correlates With TNF R1 in The Plasma of AD Patients, But Not With Cognitive Declinemuralim_phy1986No ratings yet
- Differential Levels of Plasma Biomarkers of Neurodegeneration in Lewy Body Dementia, Alzheimer's Disease, Frontotemporal Dementia and Progressive Supranuclear PalsyDocument8 pagesDifferential Levels of Plasma Biomarkers of Neurodegeneration in Lewy Body Dementia, Alzheimer's Disease, Frontotemporal Dementia and Progressive Supranuclear PalsyAlexandra CastellanosNo ratings yet
- Ijms 22 02761Document12 pagesIjms 22 02761Juberiya AjazNo ratings yet
- Evaluation of CSF Biomarkers As Predictors of Alzheimer's Disease - A Clinical Follow-Up Study of 4.7 YearsDocument10 pagesEvaluation of CSF Biomarkers As Predictors of Alzheimer's Disease - A Clinical Follow-Up Study of 4.7 YearsJuliano Flávio Rubatino RodriguesNo ratings yet
- Tau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageDocument13 pagesTau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageBruno MañonNo ratings yet
- Ossenkoppele 2019 PET Tau Pet Amyloid Cortical ThicknessDocument13 pagesOssenkoppele 2019 PET Tau Pet Amyloid Cortical ThicknessAngela SilvaNo ratings yet
- Biomakers For The Early Detection.1Document19 pagesBiomakers For The Early Detection.1MARIA DE FATIMA TEIXEIRA GORDILLONo ratings yet
- Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's diseaseDocument16 pagesPlasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's diseaseSteven Condor CruzNo ratings yet
- NEUROLOGY2018894063Document12 pagesNEUROLOGY2018894063leilaNo ratings yet
- MOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseDocument9 pagesMOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseeastareaNo ratings yet
- 10 1016@j Neuroscience 2017 07 017Document26 pages10 1016@j Neuroscience 2017 07 017Anggraeni Arum SNo ratings yet
- Changes in Plasma Amyloid and Tau in ADocument16 pagesChanges in Plasma Amyloid and Tau in AeastareaNo ratings yet
- 2017 - Differential Diagnosis of AD Using Spectrochemical Analysis of BloodDocument10 pages2017 - Differential Diagnosis of AD Using Spectrochemical Analysis of BloodM JNo ratings yet
- Schweitzer 2006Document5 pagesSchweitzer 2006emilNo ratings yet
- Blood Biomarkers in Neurodegenerative Diseases (科研通-ablesci.com)Document9 pagesBlood Biomarkers in Neurodegenerative Diseases (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- Spal LazziDocument13 pagesSpal LazziPriscila SelingardiNo ratings yet
- PE Como Predictores de Discapacidad en EM - 2012Document5 pagesPE Como Predictores de Discapacidad en EM - 20122j5f2y76g2No ratings yet
- Advantage of Modified MRI Protocol For HDocument148 pagesAdvantage of Modified MRI Protocol For HasasakopNo ratings yet
- 1 s2.0 S0300289622006640 MainDocument7 pages1 s2.0 S0300289622006640 MainInda MegaNo ratings yet
- Nejme 2400102Document3 pagesNejme 2400102linjingwang912No ratings yet
- AlzaimerDocument13 pagesAlzaimerAfni Panggar BesiNo ratings yet
- Counts ADbiomarkerreview2016Document20 pagesCounts ADbiomarkerreview2016MARIA DE FATIMA TEIXEIRA GORDILLONo ratings yet
- Tomasi Et Al 2017Document5 pagesTomasi Et Al 2017Cristiane TomasiNo ratings yet
- Tunel Del CarpoDocument6 pagesTunel Del CarpojoaquinNo ratings yet
- 576 Friday, 15 June 2018 Scientific AbstractsDocument2 pages576 Friday, 15 June 2018 Scientific AbstractsDanna Carvajal GaitanNo ratings yet
- Falgàs 2019 - Comparison Between Visual and Quantitative Hippocampal Atrophy AssessmentDocument7 pagesFalgàs 2019 - Comparison Between Visual and Quantitative Hippocampal Atrophy AssessmentljdNo ratings yet
- Increased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological DiseasesDocument9 pagesIncreased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological Diseasesnreena aslamNo ratings yet
- Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKDDocument15 pagesRandomized Placebo-Controlled EPPIC Trials of AST-120 in CKDPROF. ERWIN M. GLOBIO, MSITNo ratings yet
- Early Hippocampal Atrophy Is An Important Signal For Clinicians But Not Necessarily A Harbinger of Alzheimer DiseaseDocument2 pagesEarly Hippocampal Atrophy Is An Important Signal For Clinicians But Not Necessarily A Harbinger of Alzheimer DiseaseCarlos Hernan Castañeda RuizNo ratings yet
- Association Neurofilament and Alzheimer Progression Mattsson2019Document9 pagesAssociation Neurofilament and Alzheimer Progression Mattsson2019eastareaNo ratings yet
- Autonomic Neuroscience: Basic and Clinical: Jose-Alberto Palma, Lucy Norcli Ffe-Kaufmann, Horacio KaufmannDocument11 pagesAutonomic Neuroscience: Basic and Clinical: Jose-Alberto Palma, Lucy Norcli Ffe-Kaufmann, Horacio KaufmannCalvin Tanuwijaya Stick BolaNo ratings yet
- Newly Diagnosed Anemia Increases Risk of Parkinson's Disease: A Population-Based Cohort StudyDocument7 pagesNewly Diagnosed Anemia Increases Risk of Parkinson's Disease: A Population-Based Cohort Studydiah_budiarti_1No ratings yet
- 1 s2.0 S1053811921005103 MainDocument11 pages1 s2.0 S1053811921005103 Maincryxynhe18No ratings yet
- Ferritin 7Document2 pagesFerritin 7SuryaNo ratings yet
- 2018 - Circulating microRNAs As Novel Biomarkers of Alzheimer's DiseaseDocument6 pages2018 - Circulating microRNAs As Novel Biomarkers of Alzheimer's DiseaseM JNo ratings yet
- Jad - 2018 - 61 4 - Jad 61 4 Jad170553 - Jad 61 Jad170553Document13 pagesJad - 2018 - 61 4 - Jad 61 4 Jad170553 - Jad 61 Jad170553Quang NguyễnNo ratings yet
- Clinical Predictors and Differential Diagnosis of Posterior Reversible Encephalopathy SyndromeDocument7 pagesClinical Predictors and Differential Diagnosis of Posterior Reversible Encephalopathy SyndromekidNo ratings yet
- 1516 4446 RBP 1516444620200013Document3 pages1516 4446 RBP 1516444620200013Luisa RamirezNo ratings yet
- Konduksi DSP NcsDocument7 pagesKonduksi DSP NcsMutiara Kristiani PutriNo ratings yet
- Pietro Bon IDocument5 pagesPietro Bon Ibeealoner07No ratings yet
- May 2021 1624011543 9200117Document4 pagesMay 2021 1624011543 9200117Manoj KumarNo ratings yet
- Calmodulin Levels in Blood Cells As A Potential Biomarker of Alzheimer 'S DiseaseDocument10 pagesCalmodulin Levels in Blood Cells As A Potential Biomarker of Alzheimer 'S DiseaseFanel PutraNo ratings yet
- Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingDocument8 pagesAssociation of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingeastareaNo ratings yet
- Clinica Encefalitis Nmda InglesDocument7 pagesClinica Encefalitis Nmda InglesOskr MezaNo ratings yet
- Moll Cellproteomic PDFDocument49 pagesMoll Cellproteomic PDFGLORIA ESTEFANI YANTAS ALCANTARANo ratings yet
- Blood-based biomarkers in Alzheimer's disease - a mini-review (科研通-ablesci.com)Document9 pagesBlood-based biomarkers in Alzheimer's disease - a mini-review (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- 2022 Clinical Features and Risk Factors of Mortality in Patients With Posterior Reversible Encephalopathy SyndromeDocument7 pages2022 Clinical Features and Risk Factors of Mortality in Patients With Posterior Reversible Encephalopathy SyndromeSafitri MuhlisaNo ratings yet
- Jpad 2022 101Document9 pagesJpad 2022 101leilaNo ratings yet
- White Matter Hyperintensities A Marker For Apathy in Parkinson Without DementiaDocument10 pagesWhite Matter Hyperintensities A Marker For Apathy in Parkinson Without DementiamutiahmuftihNo ratings yet
- 3 - Evolution of Genetic Testing Supports Precision Medicine For Caring Alzheimer's Disease PatientsDocument6 pages3 - Evolution of Genetic Testing Supports Precision Medicine For Caring Alzheimer's Disease Patientsveidamororo17No ratings yet
- A/T/N: An Unbiased Descriptive Classification Scheme For Alzheimer Disease BiomarkersDocument11 pagesA/T/N: An Unbiased Descriptive Classification Scheme For Alzheimer Disease BiomarkersSatya YudhayanaNo ratings yet
- Laske2015Document18 pagesLaske2015Paco SaavedraNo ratings yet
- Multifactorial Diseases.: Chair of Medical Genetics Department of Clinical Genetics Karol P. Ruszel, PHDDocument51 pagesMultifactorial Diseases.: Chair of Medical Genetics Department of Clinical Genetics Karol P. Ruszel, PHDTimyNo ratings yet
- Reciprocal Predictive Relationships Between AmyloidDocument10 pagesReciprocal Predictive Relationships Between AmyloidCristhian RodriguezNo ratings yet
- A Multicenter Cohort StudyDocument9 pagesA Multicenter Cohort Studyfatchurhamzah62No ratings yet
- 69th AACC Annual Scientific Meeting Abstract eBookFrom Everand69th AACC Annual Scientific Meeting Abstract eBookNo ratings yet
- A Power-Law Model of Psychological Memory Strength in Short - and Long-Term RecognitionDocument11 pagesA Power-Law Model of Psychological Memory Strength in Short - and Long-Term RecognitioneastareaNo ratings yet
- A Critical Review LTF en EpilepsiaDocument26 pagesA Critical Review LTF en EpilepsiaeastareaNo ratings yet
- Audrain - Samantha - 201511 - MA - Thesis Nvestigating Accelerated Long-Term ForgettingDocument73 pagesAudrain - Samantha - 201511 - MA - Thesis Nvestigating Accelerated Long-Term ForgettingeastareaNo ratings yet
- Alexander2016.pdf A Psychometrical Model For Psychodiagnostic Assessment of Memory DeficitsDocument15 pagesAlexander2016.pdf A Psychometrical Model For Psychodiagnostic Assessment of Memory DeficitseastareaNo ratings yet
- Putcha Fractionating The Rey Auditory Verbal Learning Test Distinct Roles of Largescale Cortical Networks in Prodromal Alzheimer's DiseaseDocument27 pagesPutcha Fractionating The Rey Auditory Verbal Learning Test Distinct Roles of Largescale Cortical Networks in Prodromal Alzheimer's DiseaseeastareaNo ratings yet
- Atherton2018 Encoding-Related Brain Activity AFL Epilepsy PDFDocument45 pagesAtherton2018 Encoding-Related Brain Activity AFL Epilepsy PDFeastareaNo ratings yet
- Papp 2014Document17 pagesPapp 2014eastareaNo ratings yet
- Accelerated Long-Term Forgetting After TIA or MinorDocument7 pagesAccelerated Long-Term Forgetting After TIA or MinoreastareaNo ratings yet
- Sperling 2009Document22 pagesSperling 2009eastareaNo ratings yet
- Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer DiseaseDocument11 pagesLongitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer DiseaseeastareaNo ratings yet
- Neurofilament Light Chain Gaetani2019Document12 pagesNeurofilament Light Chain Gaetani2019eastareaNo ratings yet
- Rentz2011 FNAMEDocument8 pagesRentz2011 FNAMEeastareaNo ratings yet
- Correlates of Recognition Memory Performance in Amnestic Mild Cognitive ImpairmentDocument8 pagesCorrelates of Recognition Memory Performance in Amnestic Mild Cognitive ImpairmenteastareaNo ratings yet
- Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingDocument8 pagesAssociation of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingeastareaNo ratings yet
- Comparing Progression Biomarkers in Clinical Trials of Early Alzheimer’ S DiseaseDocument13 pagesComparing Progression Biomarkers in Clinical Trials of Early Alzheimer’ S DiseaseeastareaNo ratings yet
- Age and Sex Impact Plasma NFL and T-Tau Trajectories in Individuals With Subjective Memory Complaints - A 3-Year Follow-Up StudyDocument12 pagesAge and Sex Impact Plasma NFL and T-Tau Trajectories in Individuals With Subjective Memory Complaints - A 3-Year Follow-Up StudyeastareaNo ratings yet
- Changes in Plasma Amyloid and Tau in ADocument16 pagesChanges in Plasma Amyloid and Tau in AeastareaNo ratings yet
- Association Neurofilament and Alzheimer Progression Mattsson2019Document9 pagesAssociation Neurofilament and Alzheimer Progression Mattsson2019eastareaNo ratings yet
- MOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseDocument9 pagesMOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseeastareaNo ratings yet
- Accelerated Long-Term Forgetting in Transient Epileptic Amnesia Acquision or Consolidation DeficitDocument7 pagesAccelerated Long-Term Forgetting in Transient Epileptic Amnesia Acquision or Consolidation DeficiteastareaNo ratings yet
- Relaccem - Msard152 2014Document7 pagesRelaccem - Msard152 2014eastareaNo ratings yet
- Moreno-Manso2020 Child AbuseDocument17 pagesMoreno-Manso2020 Child AbuseeastareaNo ratings yet
- Nihms 1045384Document20 pagesNihms 1045384eastareaNo ratings yet
- The Dutch Review Process For Evaluating The QualitDocument25 pagesThe Dutch Review Process For Evaluating The QualiteastareaNo ratings yet
- No Association Between Loneliness Episodic MemoryDocument13 pagesNo Association Between Loneliness Episodic MemoryeastareaNo ratings yet
- The Paradoxical Effect of COVID-19 Outbreak On LonDocument4 pagesThe Paradoxical Effect of COVID-19 Outbreak On LoneastareaNo ratings yet
- Jamaneurology Liu 2022 Oi 220011 1651870402.27767Document9 pagesJamaneurology Liu 2022 Oi 220011 1651870402.27767eastareaNo ratings yet
- Jama Caricchio 2021 Oi 210064 1626283669.23567Document10 pagesJama Caricchio 2021 Oi 210064 1626283669.23567eastareaNo ratings yet
- Jamapsychiatry Frontera 2022 SC 220001 1659381701.66875Document7 pagesJamapsychiatry Frontera 2022 SC 220001 1659381701.66875eastareaNo ratings yet
- Oosterhuis 2015 Sample Size Requirements For Traditional and Regression-Based NormDocument12 pagesOosterhuis 2015 Sample Size Requirements For Traditional and Regression-Based NormeastareaNo ratings yet
- 12th PNHRS Program Book As of AugustDocument47 pages12th PNHRS Program Book As of AugustChristine Rodriguez-GuerreroNo ratings yet
- Ebook Braddoms Physical Medicine and Rehabilitation Sixth Edition PDF Full Chapter PDFDocument67 pagesEbook Braddoms Physical Medicine and Rehabilitation Sixth Edition PDF Full Chapter PDFmelinda.warren862100% (34)
- Chronic MeningitisDocument7 pagesChronic Meningitisneurojuancarfab100% (1)
- SDS PAC LiquidDocument4 pagesSDS PAC LiquidQuality AssuranceNo ratings yet
- NSTP Group 3 First Aid ReportingDocument44 pagesNSTP Group 3 First Aid ReportingCindy GeverolaNo ratings yet
- Ethics or Necessity Continuing Animal Testing For Scientific BreakthroughsDocument4 pagesEthics or Necessity Continuing Animal Testing For Scientific Breakthroughsperezcarlj1010No ratings yet
- Domestic ViolenceDocument13 pagesDomestic ViolenceJENNIFER YBAÑEZNo ratings yet
- English TM 8Document11 pagesEnglish TM 8Budi AtmikaNo ratings yet
- Crisis and Nursing InterventionDocument44 pagesCrisis and Nursing InterventionArchana100% (4)
- Emergency ActionDocument14 pagesEmergency ActionferozNo ratings yet
- 2021 DPharm - Clinical InkDocument8 pages2021 DPharm - Clinical InkEd SeguineNo ratings yet
- Delrosario 2017Document33 pagesDelrosario 2017Pande Agung MahariskiNo ratings yet
- COPD Case Pres2Document29 pagesCOPD Case Pres2Kelly Queenie Andres100% (2)
- Eng1503 Mock Exam Sample Answers: THANK YOU For Answering The Mock Examination Question BelowDocument14 pagesEng1503 Mock Exam Sample Answers: THANK YOU For Answering The Mock Examination Question BelowOlwethu PhikeNo ratings yet
- What Philippine Law Was Created To Include in It The Code of Ethics of Filipino Nurses?Document3 pagesWhat Philippine Law Was Created To Include in It The Code of Ethics of Filipino Nurses?Shirlene Benigra GorospeNo ratings yet
- Gensler Design Forecast 2013Document43 pagesGensler Design Forecast 2013Pete PetrášNo ratings yet
- The Who Five Keys To Safer Food A Tool For Food Safety Health PromotionDocument15 pagesThe Who Five Keys To Safer Food A Tool For Food Safety Health PromotionCarlos RuizNo ratings yet
- OsteoarthritisDocument37 pagesOsteoarthritisChikezie Onwukwe100% (1)
- Nursing Care Plan - Fatigue (Antepartum)Document3 pagesNursing Care Plan - Fatigue (Antepartum)kaimimiyaNo ratings yet
- Communiqué Émis Par La PSCDocument4 pagesCommuniqué Émis Par La PSCL'express MauriceNo ratings yet
- Garlic Benefits - Can Garlic Lower Your Cholesterol?Document4 pagesGarlic Benefits - Can Garlic Lower Your Cholesterol?Jipson VargheseNo ratings yet
- A Management Algorithm For Esophageal PerforationDocument4 pagesA Management Algorithm For Esophageal Perforationoscar chirinosNo ratings yet
- Birth StoryDocument7 pagesBirth StoryKaty Whipple100% (1)
- Ramadan and DiabetesDocument14 pagesRamadan and DiabetesDitoLinggoNo ratings yet
- 19 Disaster SurgeryDocument45 pages19 Disaster SurgeryAhmed noor Ahmed noorNo ratings yet
- WritingDocument8 pagesWritingRukshan GunasenaNo ratings yet
- Home EconomicsDocument5 pagesHome EconomicsNicole Kathleen Caranto100% (1)
- Green Environment: Love Our EarthDocument3 pagesGreen Environment: Love Our EarthSoon JimmyNo ratings yet
- Y12 - Prophet's Methadology of Health Care (Solved)Document5 pagesY12 - Prophet's Methadology of Health Care (Solved)Umair HibatullahNo ratings yet
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Uploaded by
eastareaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Discriminative Accuracy of Plasma Phospho Tau217 For Alzheimer Disease Vs Other Neurodegenerative Disorders
Uploaded by
eastareaCopyright:
Available Formats
Research
JAMA | Original Investigation
Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease
vs Other Neurodegenerative Disorders
Sebastian Palmqvist, MD, PhD; Shorena Janelidze, PhD; Yakeel T. Quiroz, PhD; Henrik Zetterberg, MD, PhD; Francisco Lopera, MD;
Erik Stomrud, MD, PhD; Yi Su, PhD; Yinghua Chen, MSc; Geidy E. Serrano, PhD; Antoine Leuzy, PhD; Niklas Mattsson-Carlgren, MD, PhD;
Olof Strandberg, PhD; Ruben Smith, MD, PhD; Andres Villegas, MD; Diego Sepulveda-Falla, MD; Xiyun Chai, MD; Nicholas K. Proctor, BS;
Thomas G. Beach, MD, PhD; Kaj Blennow, MD, PhD; Jeffrey L. Dage, PhD; Eric M. Reiman, MD; Oskar Hansson, MD, PhD
Supplemental content
IMPORTANCE There are limitations in current diagnostic testing approaches for Alzheimer
disease (AD).
OBJECTIVE To examine plasma tau phosphorylated at threonine 217 (P-tau217) as a diagnostic
biomarker for AD.
DESIGN, SETTING, AND PARTICIPANTS Three cross-sectional cohorts: an Arizona-based
neuropathology cohort (cohort 1), including 34 participants with AD and 47 without AD
(dates of enrollment, May 2007-January 2019); the Swedish BioFINDER-2 cohort (cohort 2),
including cognitively unimpaired participants (n = 301) and clinically diagnosed patients with
mild cognitive impairment (MCI) (n = 178), AD dementia (n = 121), and other
neurodegenerative diseases (n = 99) (April 2017-September 2019); and a Colombian
autosomal-dominant AD kindred (cohort 3), including 365 PSEN1 E280A mutation carriers
and 257 mutation noncarriers (December 2013-February 2017).
EXPOSURES Plasma P-tau217.
MAIN OUTCOMES AND MEASURES Primary outcome was the discriminative accuracy of plasma
P-tau217 for AD (clinical or neuropathological diagnosis). Secondary outcome was the
association with tau pathology (determined using neuropathology or positron emission
tomography [PET]).
RESULTS Mean age was 83.5 (SD, 8.5) years in cohort 1, 69.1 (SD, 10.3) years in cohort 2, and
35.8 (SD, 10.7) years in cohort 3; 38% were women in cohort 1, 51% in cohort 2, and 57% in
cohort 3. In cohort 1, antemortem plasma P-tau217 differentiated neuropathologically defined
AD from non-AD (area under the curve [AUC], 0.89 [95% CI, 0.81-0.97]) with significantly
higher accuracy than plasma P-tau181 and neurofilament light chain (NfL) (AUC range,
0.50-0.72; P < .05). The discriminative accuracy of plasma P-tau217 in cohort 2 for clinical AD
dementia vs other neurodegenerative diseases (AUC, 0.96 [95% CI, 0.93-0.98]) was
significantly higher than plasma P-tau181, plasma NfL, and MRI measures (AUC range,
0.50-0.81; P < .001) but not significantly different compared with cerebrospinal fluid (CSF)
P-tau217, CSF P-tau181, and tau-PET (AUC range, 0.90-0.99; P > .15). In cohort 3, plasma
P-tau217 levels were significantly greater among PSEN1 mutation carriers, compared with
noncarriers, from approximately 25 years and older, which is 20 years prior to estimated
onset of MCI among mutation carriers. Plasma P-tau217 levels correlated with tau tangles in
participants with (Spearman ρ = 0.64; P < .001), but not without (Spearman ρ = 0.15;
P = .33), β-amyloid plaques in cohort 1. In cohort 2, plasma P-tau217 discriminated abnormal
vs normal tau-PET scans (AUC, 0.93 [95% CI, 0.91-0.96]) with significantly higher accuracy
than plasma P-tau181, plasma NfL, CSF P-tau181, CSF Aβ42:Aβ40 ratio, and MRI measures
(AUC range, 0.67-0.90; P < .05), but its performance was not significantly different
compared with CSF P-tau217 (AUC, 0.96; P = .22).
CONCLUSIONS AND RELEVANCE Among 1402 participants from 3 selected cohorts, plasma
P-tau217 discriminated AD from other neurodegenerative diseases, with significantly higher
Author Affiliations: Author
accuracy than established plasma- and MRI-based biomarkers, and its performance was not affiliations are listed at the end of this
significantly different from key CSF- or PET-based measures. Further research is needed to article.
optimize the assay, validate the findings in unselected and diverse populations, and Corresponding Author: Oskar
determine its potential role in clinical care. Hansson, MD, PhD, Memory Clinic,
Skåne University Hospital, SE-20502
JAMA. doi:10.1001/jama.2020.12134 Malmö, Sweden (Oskar.Hansson@
Published online July 28, 2020. med.lu.se).
(Reprinted) E1
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Research Original Investigation Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders
A
major global challenge is the increasing prevalence of
dementia, especially dementia caused by Alzheimer Key Points
disease (AD). It is estimated that approximately 100 mil-
Question What is the discriminative accuracy of plasma
lion people worldwide will have AD dementia in 2050.1 In the phospho-tau217 (P-tau217) for differentiating Alzheimer disease
global action plan against dementia, the World Health Orga- from other neurodegenerative disorders?
nization has specified improved diagnostics as a key area,2 since
Findings In this cross-sectional study that included 1402
it is important for optimal disease management and treat-
participants from 3 selected cohorts, plasma P-tau217
ment. Early, accurate, and biomarker-based diagnosis of AD discriminated Alzheimer disease from other neurodegenerative
will likely become even more important when disease- diseases (area under the receiver operating characteristic curve of
modifying treatments become available.3,4 0.89 in a neuropathologically defined cohort and 0.96 in a
Recent improvements in AD diagnostics include biomark- clinically defined cohort), with performance that was significantly
ers that can identify its underlying disease pathologies better than established Alzheimer disease plasma- and MRI-based
biomarkers but not significantly different from key CSF- or
(ie, β-amyloid [Aβ] and tau), using positron emission tomog-
PET-based biomarkers.
raphy (PET) or cerebrospinal fluid (CSF) analysis. Specifically
Aβ-PET,5 tau-PET,6 the CSF Aβ42:Aβ40 ratio, and CSF tau phos- Meaning Although plasma P-tau217 was able to discriminate
phorylated at threonine 181 (P-tau181)7 have shown high di- Alzheimer disease from other neurodegenerative diseases, further
research is needed to validate the findings in unselected and
agnostic accuracy and been incorporated in the diagnostic
diverse populations, optimize the assay, and determine its
framework for AD.8 Recently, CSF P-tau217 (phosphorylated potential role in clinical care.
at threonine 217) was found more accurate than CSF P-tau181.9
The global use of these biomarkers, however, is still limited be-
cause of high costs, insufficient availability, and invasive
nature.10 There is therefore a great interest in blood-based bio- (CERAD) Aβ-plaque scores17 and Braak (neurofibrillary tau-
markers, and research on plasma Aβ42:Aβ40 ratio11,12 and tangle) stage.18 Participants with NIA-RI intermediate likeli-
P-tau18113,14 suggests potential value. hood of AD (tangles in limbic regions [Braak III-IV] and
The main objective of this study was to determine the di- moderate-to-frequent Aβ plaques) or high likelihood (tangles
agnostic accuracy of plasma P-tau217 for AD; both for discrimi- in neocortex [Braak V-VI] and moderate-to-frequent Aβ
nating clinically diagnosed AD dementia from other neurode- plaques)16 were categorized as having AD. “Non-AD” was used
generative diseases and neuropathologically defined AD from to describe participants with none-to-sparse Aβ plaques.17
non-AD individuals. The accuracy of plasma P-tau217 was com-
pared with other key plasma, CSF, PET, and magnetic reso- Cohort 2 (Swedish BioFINDER-2 Study)
nance imaging (MRI) biomarkers for AD. Secondary objec- The participants from the prospective Swedish BioFINDER-2
tives were to investigate the age at which plasma P-tau217 levels study (NCT03174938) were recruited at Skåne University
increase in autosomal-dominant AD and if plasma P-tau217 lev- Hospital and the Hospital of Ängelholm in Sweden (dates of
els were associated with AD-like tau pathology, determined enrollment, April 2017-September 2019) and included
using neuropathology or tau-PET. cognitively unimpaired controls and patients with mild
cognitive impairment (MCI), AD with dementia (fulfilling the
Diagnostic and Statistical Manual of Mental Disorders [Fifth
Edition] AD criteria19 and Aβ-positive), and various other
Methods neurodegenerative diseases. Cognitively unimpaired
Participants and Clinical Assessments participants and participants with MCI were subdivided into
Additional cohort descriptions can be found in the eMethods Aβ-positive/negative participants as well as preclinical AD
in the Supplement. All participants or their legal representa- (Aβ-positive and tau-positive participants without cognitive
tives provided written informed consent. Ethical approval was impairment)8 and AD with MCI (Aβ-positive and tau-positive
given by the Western Institutional Review Board of Puyallup, participants with MCI).8 See the eMethods in the Supplement
Washington (cohort 1), the Regional Ethical Committee in Lund, for details on diagnostic criteria and eFigure 1 in the
Sweden (cohort 2) or the institutional review board at the Uni- Supplement for the enrollment flowchart.
versity of Antioquia, Colombia (cohort 3).
Cohort 3 (Colombian Autosomal-Dominant AD Registry)
Cohort 1 (Arizona-Based Neuropathology Cohort) Cohort 3 included cognitively unimpaired and impaired
Cohort 1 consisted of neuropathologically classified partici- PSEN1 E280A mutation carriers and age- and sex-matched
pants from an antemortem-postmortem donor cohort cognitively unimpaired noncarriers from the same kindred.
(the Arizona Study of Aging and Neurodegenerative Disorders/ They were enrolled and assessed in the Alzheimer Prevention
Brain and Body Donation Program) with dates of enrollment Initiative Colombia Registry, with dates of enrollment from
from May 2007 to January 2019.15 Plasma samples were col- December 2013 to February 2017.20 Memory function was
lected 0.02 to 2.9 years prior to death. Neuropathological di- assessed using the CERAD 10-word delayed recall test scored
agnosis of AD was based on National Institute on Aging– from 0 (worst) to 10 (best), 21 and global cognition was
Reagan Institute (NIA-RI) criteria,16 which are dependent on assessed with the Mini-Mental State Examination scored
the Consortium to Establish a Registry for Alzheimer Disease from 0 (worst) to 30 (best).
E2 JAMA Published online July 28, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders Original Investigation Research
Plasma and CSF Sampling and Analysis of included participants, except for Aβ-PET, which, per study de-
Blood samples were collected and handled as described in the sign, was not performed on all participants. When using Aβ-PET
eMethods in the Supplement. Concentrations of plasma as outcome, only those with Aβ-PET data were included. In co-
P-tau217 (all 3 cohorts) were measured using immunoassays hort 2, the cases with missing biomarker data were not included
at Lilly Research Laboratories.13,22,23 Details of the plasma in the study (eFigure 1 in the Supplement). In cohort 2, sensitiv-
P-tau217 analysis and analysis of the other plasma and CSF bio- ity, specificity, correctly classified participants (“accuracy”), and
markers are described in the eMethods in the Supplement. All likelihood ratios were reported using biomarker cutoffs defined
biomarker assays are summarized in eTable 1 in the Supple- as the mean value +2 standard deviations in Aβ-negative controls.
ment. The plasma and CSF analyses were performed by tech- Cohort 1 had no control sample and therefore the cutoffs were
nicians blinded to the clinical and imaging data. instead established at the highest Youden Index (sensitivity +
specificity – 1) when comparing AD with non-AD cases.
Imaging Procedures in Cohort 2 (BioFINDER-2) P<.05 (corrected, 2-sided) was considered statistically sig-
The procedures of MRI, tau-PET (using RO948 labeled with ra- nificant. SPM12 (Wellcome Department of Imaging Neurosci-
dioactive fluorine [18F])24 and Aβ-PET (using flutemetamol la- ence, Institute of Neurology) was used for voxel-based analy-
beled with 18F) are described in the eMethods in the Supple- ses. SPSS version 26 (IBM) and R version 3.6.1 (R Foundation
ment. All assessments of imaging and clinical data were for Statistical Computing) were used for all other statistical
performed blinded to plasma P-tau217 data. analyses. Additional statistics are described in the eMethods
in the Supplement.
Outcomes
Detailed definitions of all outcomes are described in the
eMethods in the Supplement. The primary outcomes/
reference standards were intermediate-to-high likelihood of AD
Results
vs non-AD according to neuropathology (cohort 1), or clinical Participants
AD dementia vs other neurodegenerative diseases (cohort 2). The neuropathology cohort (cohort 1) with antemortem plasma
Neuropathological AD criteria were used, because these still rep- samples included 81 participants. Eighteen (22%) had inter-
resent the gold standard of AD diagnosis. However, a clinical, mediate likelihood and 16 (20%) had high likelihood of AD.
Aβ biomarker–supported AD dementia diagnosis better repre- These 34 (42%) comprised the AD group; of these, 27 had de-
sents the tests’ accuracy in clinical practice and was therefore mentia. Forty-seven (58%) comprised the non-AD group. The
chosen as an additional primary outcome. A neuropathologi- BioFINDER-2 study (cohort 2) included 699 clinically diag-
cally high likelihood of AD vs non-AD was used as secondary nosed participants, of whom 301 (43%) were controls (ie, cog-
outcome to examine the discriminative accuracy of plasma nitively unimpaired), 178 (25%) had MCI, 121 (17%) had AD with
P-tau217 for the most definite AD diagnosis. Tau tangle den- dementia, and 99 (14%) had various other neurodegenerative
sity at autopsy (cohort 1) and tau-PET or Aβ-PET status (cohort 2) diseases. The Colombian autosomal-dominant AD registry
were also used as secondary outcomes to examine the associa- (cohort 3) included 622 participants, of whom 365 (59%) were
tion of plasma P-tau217 with both neuropathological and in vivo PSEN1 mutation carriers and 257 (41%) age- and sex-matched
state-of-the-art biomarkers for tau or Aβ pathology. Further sec- noncarriers (controls). Among the mutation carriers, 259 (71%)
ondary outcome included age at onset of increased plasma were cognitively unimpaired and 106 (29%) cognitively im-
P-tau217 levels in autosomal-dominant AD (cohort 3) to exam- paired. Participant characteristics across all 3 cohorts are sum-
ine how early the plasma P-tau217 levels change in familial AD. marized in eTable 2 in the Supplement and cohort-wise char-
Exploratory outcomes included preclinical AD8 and AD with MCI acteristics in eTables 3-7 in the Supplement. The mean age was
(ie, prodromal AD)8 as reference standards (cohort 2). 83.5 (SD, 8.5) years in cohort 1, 69.1 (SD, 10.3) years in cohort
2, and 35.8 (SD, 10.7) years in cohort 3; 38% were women in
Statistical Analysis cohort 1, 51% in cohort 2, and 57% in cohort 3. The diagnoses
Participants with plasma P-tau217 values below the lower detec- among the non-AD groups in cohort 1 and cohort 2 are de-
tion limit of the assay (0.48 pg/mL) were included in the main scribed in eTables 8 and 9 in the Supplement.
analysis (Supplement). A sensitivity analysis excluding these
participants was also performed (Supplement). Correlation co- Association Between Plasma P-tau217 and AD
efficients were calculated using Spearman rank tests. All group in the Neuropathology Cohort (Cohort 1)
comparisons were adjusted for age and sex (and time between For the primary outcome intermediate-to-high likelihood of
collection of plasma sample and death in cohort 1) in linear re- AD vs non-AD, the AUC was 0.89 (95% CI, 0.81-0.97; 85% cor-
gression models using plasma P-tau217 levels as outcome. The rectly classified) using plasma P-tau217 levels, which was sig-
primary method for examining the discriminative performance nificantly higher than the AUCs for plasma levels of P-tau181
of the biomarkers was the area under the receiver operating char- (0.72 [95% CI, 0.60 to 0.84]; ΔAUC, 0.17 [95% CI, 0.04 to 0.30];
acteristic curve (AUC). Significant differences between the AUCs P = .04) and neurofilament light chain (NfL) (0.50 [95% CI, 0.37
were tested using DeLong statistics,25 and Bonferroni correction to 0.63]; ΔAUC, 0.39 [95% CI, 0.26 to 0.52]; P < .001) (Figure 1;
was applied to account for multiple comparisons. The correction eFigure 2A and eTable 10 in the Supplement).
was applied per research question/analysis (specified in each In secondary analyses that compared participants with high
eTable in the Supplement). There were no missing biomarker data likelihood of AD vs non-AD, the AUC for plasma P-tau217 levels
jama.com (Reprinted) JAMA Published online July 28, 2020 E3
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Research Original Investigation Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders
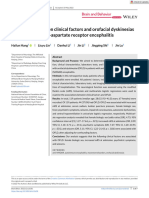
Figure 1. Plasma P-tau217 Concentrations in the Neuropathology Cohort (Cohort 1)
A Correlations between plasma P-tau217 concentration and B Antemortem plasma P-tau217
total tangle density score concentration
25 25
AD
20 20
Plasma P-tau217, pg/mL Non-AD
Plasma P-tau217, pg/mL
15 15
10 10
5 5
0 0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Non-AD AD
Tangle density score (n = 47) (n = 34)
A, Correlations between plasma tau phosphorylated at threonine 217 (P-tau217) indicate 95% confidence intervals around the regression lines. B, Antemortem
concentration and total tangle density score in the Alzheimer disease (AD; National plasma P-tau217 concentrations in the AD (NIA-RI intermediate or high likelihood
Institute on Aging–Reagan Institute [NIA-RI] intermediate or high likelihood of AD) of AD) (n = 34) and non-AD (no or sparse β-amyloid plaques) (n = 47) groups
and non-AD (no or sparse β-amyloid plaques) groups. For participants in the AD according to neuropathology.16 Box ends denote the 25th and 75th percentiles,
group, Spearman ρ was 0.64 (P < .001); for those in the non-AD group there was and the horizontal line within each box represents the median. Whiskers extend to
no significant correlation (Spearman ρ = 0.15, P = .33). The tangle density score the upper and lower adjacent values or the most extreme points within
(x-axis; 0-15) is the sum of neurofibrillary tau-tangle density score (0-3) in standard 1.5 × interquartile range of the 25th and 75th percentiles. P < .001 for comparison
regions of the frontal, temporal, and parietal lobes; hippocampal CA1; and of non-AD and AD groups. Corresponding receiver operating characteristic curve
entorhinal/transentorhinal regions (eMethods in the Supplement). Shaded areas analyses are shown in eFigure 1A in the Supplement.
was 0.98 (95% CI, 0.94 to 1.00; 94% correctly classified), which of CSF P-tau217 (0.99 [95% CI, 0.98 to 1.00]; ΔAUC, –0.03 [95%
was significantly higher than for plasma levels of P-tau181 (0.85 CI, –0.06 to –0.01]; P = .15), CSF P-tau181 (0.90 [95% CI, 0.86 to
[95% CI, 0.76 to 0.95]; ΔAUC, 0.12 [95% CI, 0.04 to 0.20]; 0.95]; ΔAUC, 0.05 [95% CI, 0.01 to 0.10]; P = .21) or tau-PET (0.98
P = .003) and NfL (0.51 [95% CI, 0.35 to 0.67]; ΔAUC, 0.46 [95% [95% CI, 0.97 to 0.99]; ΔAUC, –0.02 [95% CI, –0.05 to 0.00];
CI, 0.31 to 0.61]; P < .001) (eFigure 2B and 2C and eTable 10 in P = .72) (Figure 2C; eTable 12 in the Supplement). Separate AUCs
the Supplement). Furthermore, antemortem plasma P-tau217 of plasma P-tau217 for discriminating AD dementia vs other spe-
levels correlated significantly with the density of cortical tau- cific neurodegenerative diseases, vs Aβ-negative controls and vs
containing neurofibrillary tangles postmortem in AD (Spear- Aβ-negative MCI, are reported in the Table and in eFigure 3 in
man ρ = 0.64, P < .001), while there was no significant corre- the Supplement. Overall, plasma P-tau217 had AUCs of 0.92 to
lation in non-AD (Spearman ρ = 0.15, P = .33) (Figure 1). 0.98 in these analyses.
According to the updated diagnostic framework for AD
Discriminative Accuracy of Plasma P-tau217 for AD diagnosis, AD can be diagnosed before the dementia stage if
vs Other Neurodegenerative Diseases in the BioFINDER-2 biomarkers provide evidence for both Aβ and tau pathologies.8
Study (Cohort 2) When using these criteria as exploratory outcomes, the pre-
The levels of plasma P-tau217 by diagnostic groups are shown clinical AD and AD with MCI groups had significantly higher
in Figure 2A (P values for group comparisons are reported in plasma P-tau217 levels than Aβ-negative controls and
eTable 11 in the Supplement). The AUCs for the primary clinical Aβ-negative MCI (eFigure 4A, eTable 13 in the Supplement).
outcome of AD dementia vs other neurodegenerative diseases Plasma P-tau217 differentiated preclinical AD from Aβ-
are shown in Figure 2, panels B and C; corresponding sensitivi- negative controls (AUC, 0.90 [95% CI, 0.85 to 0.94]) and AD
ties, specificities, likelihood ratios, and accuracies are reported with MCI (prodromal AD) from Aβ-negative MCI (AUC, 0.91
in the Table. For plasma P-tau217, 89% of the participants were [95% CI, 0.86 to 0.95]). Comparisons of plasma P-tau217 with
correctly classified and the AUC was 0.96 (95% CI, 0.93 to 0.98), other biomarkers are shown in eFigure 4, panels B-E and in
which was significantly higher than for plasma levels of P-tau181 eTables 14 and 15 in the Supplement.
(0.81 [95% CI, 0.74 to 0.87]; ΔAUC, 0.15 [95% CI, 0.10 to 0.21];
P < .001) and NfL (0.50 [95% CI, 0.42 to 0.58]; ΔAUC, 0.46 [95% Relationship With Tau-PET, Aβ-PET, and CSF P-tau217
CI, 0.38 to 0.50]; P < .001) and the MRI measures of cortical thick- in the BioFINDER-2 Study (Cohort 2)
ness of AD signature regions (0.78 [95% CI, 0.72 to 0.85]; ΔAUC, When examining tau-PET as a function of increasing plasma
0.17 [95% CI, 0.10 to 0.25]; P < .001) and hippocampal volume P-tau217 levels (eFigure 5A in the Supplement), a significant
(0.74 [95% CI, 0.67 to 0.81]; ΔAUC, 0.22 [95% CI, 0.14 to 0.30]; association was seen in Aβ-positive (r2 = 0.56, P < .001), but not
P < .001) (Figure 2B; eTable 12 in the Supplement). However, the Aβ-negative (r2 = 0.00, P = .53), participants. Voxel-wise asso-
AUC of plasma P-tau217 was not significantly different from that ciations with tau-PET and Aβ-PET are shown in eFigure 6,
E4 JAMA Published online July 28, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders Original Investigation Research
Figure 2. Plasma P-tau217 in the BioFINDER-2 Study (Cohort 2)
A Levels of P-tau217 in plasma across diagnostic groups B AD dementia vs other neurodegenerative diseases: comparison of
plasma P-tau217 vs other plasma and MRI biomarkers
Aβ– Cognitively
unimpaired 100
(N = 244)
80
Aβ– Mild cognitive
impairment
(N = 86)
60
Sensitivity, %
Aβ+ Cognitively
unimpaired
(N = 77) 40
Plasma P-tau217 (AUC = 0.96)
Plasma P-tau181 (AUC = 0.81)
Aβ+ Mild cognitive AD signature cortical thickness
impairment 20 (AUC = 0.78)
(N = 92)
Hippocampal volumes
(AUC = 0.74)
Plasma NfL (AUC = 0.50)
Aβ+ Alzheimer 0
disease dementia 100 80 60 40 20 0
(N = 121) Specificity, %
C AD dementia vs other neurodegenerative diseases: comparison of
Parkinson disease/
plasma P-tau217 vs CSF and tau-PET biomarkers
Parkinson disease dementia/
multiple system atrophy 100
(N = 45)
Progressive supranuclear 80
palsy/corticobasal
syndrome
(N = 21)
60
Sensitivity, %
Behavioral variant
frontotemporal dementia/
40
primary progressive aphasia
(N = 21)
20 CSF P-tau217 (AUC = 0.99)
Vascular dementia
Tau-PET (AUC = 0.98)
(N = 12)
Plasma P-tau217 (AUC = 0.96)
CSF P-tau181 (AUC = 0.90)
0
0 10 20 30 40 100 80 60 40 20 0
Plasma P-tau217, pg/mL Specificity, %
A, Plasma tau phosphorylated at threonine 217 (P-tau217) concentrations across plasma P-tau217 with other plasma biomarkers and magnetic resonance
the different diagnostic groups. P values from group comparisons are shown in imaging (MRI) (B); and comparing plasma P-tau217 with cerebrospinal fluid
eTable 11 in the Supplement. Box ends denote the 25th and 75th percentiles, biomarkers and tau-PET (C). Statistical comparisons between areas under the
the vertical lines are medians, and the whiskers extend to the upper and lower ROC curve (AUCs) are reported in eTable 12 in the Supplement. Separate ROC
adjacent values or the most extreme points within 1.5 × interquartile range of curve analysis for AD dementia vs other specific diagnostic groups are shown in
the 25th and 75th percentiles. B and C show receiver operating characteristic eFigure 3 in the Supplement. Aβ+ indicates β-amyloid positive; Aβ–, β-amyloid
(ROC) curve analyses with clinical Alzheimer disease (AD) dementia (n = 121) vs negative; CSF, cerebrospinal fluid; NfL, neurofilament light chain; PET, positron
all other neurodegenerative diseases (n = 99) as reference standard comparing emission tomography.
panels A-D in the Supplement, revealing that plasma P-tau217 the AUCs for plasma levels of P-tau181 (0.83 [95% CI, 0.80 to
levels were most strongly associated with the tau-PET signal 0.87]; ΔAUC, 0.10 [95% CI, 0.06 to 0.13]; P < .001) and NfL (0.67
in temporoparietal regions and with Aβ-PET in medial fronto- [95% CI, 0.63 to 0.72]; ΔAUC, 0.26 [95% CI, 0.21 to 0.30];
parietal regions. Correlations with different tau-PET regions of P < .001); for CSF levels of P-tau181 (0.85 [95% CI, 0.81 to 0.88];
interest and other biomarkers are reported in eTable 16 in the ΔAUC, 0.09 [95% CI, 0.05 to 0.12]; P < .001) and Aβ42:Aβ40
Supplement and the association with CSF P-tau217 in eFig- ratio (0.90 [95% CI, 0.87 to 0.92]; ΔAUC, 0.04 [95% CI, –0.01
ure 5B and eTable 16 in the Supplement. to 0.08]; P = .04); and for MRI measures of cortical thickness
Plasma P-tau217 levels discriminated abnormal vs nor- of AD signature regions (0.84 [95% CI, 0.81 to 0.88]; ΔAUC, 0.09
mal tau-PET status with an AUC of 0.93 (95% CI, 0.91 to 0.96; [95% CI, 0.04 to 0.13]; P < .001) and hippocampal volume (0.80
86% correctly classified), which was significantly higher than [95% CI, 0.77 to 0.84]; ΔAUC, 0.13 [95% CI, 0.08 to 0.17];
jama.com (Reprinted) JAMA Published online July 28, 2020 E5
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Research Original Investigation Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders
Table. Diagnostic Performance of Plasma P-tau217 for Differentiating Alzheimer Disease From Other Neurodegenerative Diseases
in the BioFINDER-2 Cohort (Cohort 2)a
% (95% CI) Likelihood ratio (95% CI)
Correctly classified Specificity
AD dementia (n = 121) vs No. AUC (95% CI) participants (at 93% sensitivity)b Positive Negative
All other neurodegenerative diseases 99 0.96 (0.94-0.98)c 89 (86-92) 83 (77-89) 5.4 (4.1-8.1) 0.08 (0.03-0.12)
All Aβ-negative other neurodegenerative diseases 84 0.96 (0.94-0.99) 92 (89-95) 89 (84-95) 8.7 (5.9-16.8) 0.06 (0.03-0.11)
All Aβ-positive other neurodegenerative diseases 15 0.93 (0.89-0.96) 88 (84-92) 47 (25-67) 1.8 (1.2-2.8) 0.14 (0.06-0.28)
Behavioral variant of frontotemporal dementia 21 0.92 (0.87-0.99) 92 (88-95) 81 (67-93) 4.9 (2.9-12.7) 0.09 (0.03-0.13)
or primary progressive aphasia
Vascular dementia 12 0.97 (0.94-0.99) 92 (89-96) 83 (67-100) 5.6 (2.8-9.3) 0.08 (0.03-0.13)
Parkinson disease or multiple system atrophy 45 0.97 (0.95-0.99) 90 (87-94) 82 (74-92) 5.3 (3.5-11.4) 0.08 (0.03-0.12)
Progressive supranuclear palsy 21 0.96 (0.93-0.99) 92 (89-96) 86 (75-100) 6.5 (3.6-14.9) 0.08 (0.03-0.12)
or corticobasal syndrome
Aβ-negative mild cognitive impairment 86 0.97 (0.96-0.99) 93 (91-96) 93 (89-98) 13.4 (8.4-44.0) 0.07 (0.03-0.11)
Aβ-negative controls 224 0.98 (0.97-0.99) 95 (93-97) 96 (94-98) 20.9 (14.4-43.1) 0.07 (0.03-0.10)
Abbreviations: Aβ, β-amyloid; AD, Alzheimer disease; AUC, area under the receiver identifying AD and the sensitivity was consequently 93% in all analyses with
operating characteristic curve; P-tau217, plasma tau phosphorylated at threonine 217. AD vs other groups. The 95% CI for the sensitivity was 90%-97%.
a c
The reference standard was clinical Alzheimer disease diagnosis vs other The 95% CIs in the table were calculated using the percentile method (due to
diseases/conditions (specified in the first column). A cutoff of greater than small sample sizes in some of the other neurodegenerative groups), which
2.5 pg/mL was used for plasma P-tau217, which was established using the explains the small discrepancy in 95% CIs; 0.94-0.98 in this table and
mean +2 standard deviations in Aβ-negative control participants. 0.93-0.98 in the text (from eTable 12 in the Supplement) when comparing
b
The predefined cutoff (2.5 pg/mL) produced a sensitivity of 93% for with other biomarkers.
P < .001). However, the AUC of plasma P-tau217 was not sig- Nonetheless, the main analysis was repeated in all 3 cohorts
nificantly different from the AUC for CSF P-tau217 (0.96 [95% excluding participants with plasma P-tau217 levels below de-
CI, 0.94 to 0.97]; ΔAUC, –0.02 [95% CI, –0.05 to 0.00]; P = .22) tection (eMethods in the Supplement), which resulted in simi-
(Figure 3, panels A and C; eTable 17 in the Supplement). lar findings (eFigures 8-11 and eResults in the Supplement). We
Using Aβ-PET status as outcome (n = 488 [70%]), plasma also investigated the potential added value of combining
P-tau217 discriminated abnormal vs normal scans (AUC, 0.87 plasma P-tau217 with other plasma biomarkers (eTables 19 and
[95% CI, 0.83 to 0.90]) significantly better than all other bio- 20 in the Supplement) or when using plasma ratios of P-tau217
markers (AUC range, 0.66-0.80) except CSF P-tau217 (0.93 [95% to total tau (T-tau) or Aβ42 (eTables 10 and 12 in the Supple-
CI, 0.91 to 0.96]; ΔAUC, –0.06 [95% CI, –0.10 to –0.03]; P = .003) ment) for discriminating AD vs non-AD in cohort 1 and cohort
and CSF Aβ42:Aβ40 ratio (0.97 [95% CI, 0.95 to 0.98]; ΔAUC, 2. No biomarker ratio or combination of biomarkers were sig-
–0.10 [95% CI, –0.05 to –0.14]; P < .001), which performed sig- nificantly better than plasma P-tau217 alone.
nificantly better than plasma P-tau217 (Figure 3, panels B and
D; eTable 18 in the Supplement).
Discussion
Findings From the Autosomal-Dominant AD Cohort (Cohort 3)
Mean plasma P-tau217 concentrations were 1.9 pg/mL (95% CI, The main findings of this study were that plasma P-tau217 dif-
1.4 to 1.8) in noncarriers, 4.5 pg/mL (95% CI, 4.1 to 5.0) in cog- ferentiated clinically diagnosed AD dementia from other neu-
nitively unimpaired mutation carriers, and 16.8 pg/mL (95% CI, rodegenerative disorders (Figure 2, panels A-C; Table), and dis-
15.8 to 17.8) in cognitively impaired mutation carriers. Plasma tinguished participants with neuropathologically defined AD
P-tau217 levels increased by age in PSEN1 E280A mutation car- from participants without diagnostic levels of AD histopathol-
riers, and a significant difference from noncarriers was seen at ogy (Figure 1; eFigure 2, panels A-C, and eTable 10 in the
age 24.9 years (Figure 4; eFigure 7 in the Supplement), about 20 Supplement). Further, plasma P-tau217 had significantly higher
years before the mutation carriers’ median age of MCI onset.20 diagnostic accuracy for clinical AD compared with plasma
Plasma P-tau217 levels correlated significantly with lower P-tau181, plasma NfL, and MRI measures and did not perform
Mini-Mental State Examination scores in cognitively impaired significantly differently compared with CSF P-tau181, CSF
(Spearman ρ = –0.28, P = .02), but not unimpaired (Spearman P-tau217, and tau-PET (eTable 12 in the Supplement). Addi-
ρ = –0.11; P = .16), carriers. Further, plasma P-tau217 levels cor- tionally, plasma P-tau217 levels correlated with cerebral tau
related significantly with memory performance both in cogni- tangles (Figure 1) and discriminated abnormal vs normal tau-
tively impaired (Spearman ρ = –0.34; P = .003) and unim- PET scans with significantly higher accuracy than plasma P-
paired (Spearman ρ = –0.31; P < .001) mutation carriers. tau181, plasma NfL, CSF P-tau181, CSF Aβ42:Aβ40 ratio, and
MRI measures (Figure 3, panels A and C). Plasma P-tau217 lev-
Sensitivity Analyses els were significantly greater among PSEN1 mutation carri-
P-tau217 values below the lower detection limit of the assay ers, compared with noncarriers, from approximately age 25
are not thought to be attributable to measurement error or years and older, which is 20 years prior to the estimated on-
sample interference but are believed to be true low values. set of MCI among mutation carriers.
E6 JAMA Published online July 28, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders Original Investigation Research
Figure 3. Discriminative Accuracy of Plasma P-tau217 for Tau-PET and Aβ-PET in the BioFINDER-2 Study (Cohort 2)
Plasma P-tau217 vs other plasma and MRI biomarkers
A Tau-PET (temporal meta-ROI) B Aβ-PET (neocortical meta-ROI)
100 100
80 80
60 60
Sensitivity, %
Sensitivity, %
40 40
Plasma P-tau217 (AUC = 0.93) Plasma P-tau217 (AUC = 0.87)
20 AD signature cortical thickness (AUC = 0.84) 20 Plasma P-tau181 (AUC = 0.76)
Plasma P-tau181 (AUC = 0.83) AD signature cortical thickness (AUC = 0.71)
Hippocampal volumes (AUC = 0.80) Plasma NfL (AUC = 0.69)
Plasma NfL (AUC = 0.67) Hippocampal volumes (AUC = 0.66)
0 0
100 80 60 40 20 0 100 80 60 40 20 0
Specificity, % Specificity, %
Plasma P-tau217 vs CSF biomarkers
C Tau-PET (temporal meta-ROI) D Aβ-PET (neocortical meta-ROI)
100 100
80 80
60 60
Sensitivity, %
Sensitivity, %
40 40
20 CSF P-tau217 (AUC = 0.96) 20 CSF Aβ42:Aβ40 ratio (AUC = 0.97)
Plasma P-tau217 (AUC = 0.93) CSF P-tau217 (AUC = 0.93)
CSF Aβ42:Aβ40 ratio (AUC = 0.90) Plasma P-tau217 (AUC = 0.87)
CSF P-tau181 (AUC = 0.85) CSF P-tau181 (AUC = 0.80)
0 0
100 80 60 40 20 0 100 80 60 40 20 0
Specificity, % Specificity, %
Receiver operating characteristic (ROC) curve analyses of plasma tau values ratio 1.36 and 0.53, respectively (eMethods in the Supplement). Plasma
phosphorylated at threonine 217 (P-tau217) and other biomarkers using P-tau217 was compared with other plasma biomarkers and magnetic resonance
tau–positron emission tomography (PET) positivity in the temporal meta–region imaging (A and B); and with cerebrospinal fluid (CSF) biomarkers (C and D).
of interest (ROI) as reference standard (A and C; n = 699 [tau-PET–, n = 532; Comparisons between areas under the ROC curve (AUCs) with sensitivities and
tau-PET+, n = 167]) and Aβ-PET positivity in the neocortical meta-ROI as specificities are shown in eTables 17 and 18 in the Supplement. Aβ indicates
reference standard (B and D; n = 488 [Aβ-PET–, n = 326; Aβ-PET+, n = 162]). β-amyloid; CSF, cerebrospinal fluid; NfL, neurofilament light chain.
The tau-PET and Aβ-PET cutoffs for abnormality were standardized uptake
The suggested advantage of using plasma P-tau217 over in previous studies. Further, the discriminative accuracy of
plasma P-tau181 is in agreement with findings comparing CSF plasma P-tau217 was not significantly different compared with
levels of P-tau217 and P-tau181, which also show a higher dis- using ratios to either plasma T-tau or Aβ42 (eTables 10 and 12
criminative accuracy of P-tau217 for AD vs other neurodegen- in the Supplement), which is probably explained by the much
erative diseases, tau-PET status, and Aβ-PET status.9,27 Re- more altered levels of plasma P-tau217 in AD dementia vs
cent studies have also suggested that CSF P-tau217 levels may non-AD conditions compared with the other 2 plasma bio-
change earlier than CSF P-tau181 levels in AD.28,29 Both plasma markers. Taken together, these results indicate that plasma
P-tau assays, however, performed well for detecting AD and P-tau217 might be useful in the differential diagnosis of pa-
significantly better than plasma T-tau and NfL as expected, tients with cognitive impairment, and future studies need to
given the performance of plasma T-tau30-32 and plasma NfL33-35 examine how this might improve case management and
jama.com (Reprinted) JAMA Published online July 28, 2020 E7
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Research Original Investigation Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders
Figure 4. Plasma P-tau217 and NfL Levels as a Function of Age in the Autosomal-Dominant Alzheimer Disease Kindred (Cohort 3)
A Plasma P-tau217 levels by age B Plasma neurofilament light chain levels by age
200 200
100 100
Plasma P-tau217, pg/mL
10 10
Plasma NfL, pg/mL
1 1
0.1 0.1 Dementia carriers
MCI carriers
Unimpaired carriers
Noncarriers
0.01 0.01
10 20 30 40 50 60 10 20 30 40 50 60
Age, y Age, y
A and B, Plasma tau phosphorylated at threonine 217 (P-tau217) and lines indicate the median onset of mild cognitive impairment in mutation
neurofilament light chain (NfL) levels in PSEN1 E280A mutation carriers carriers (at age 44 years).20 The plasma NfL results are included for comparison
(orange) and noncarriers (blue) and as a function of age. Shaded areas indicate with plasma P-tau217 and have partly been included in another analysis.26
the 99% credible intervals around the spline model estimates. Vertical dotted
treatment of patients with symptomatic AD. Plasma P-tau217 observed in the neuropathology cohort. Here, 91% of the par-
might be especially useful at facilities with limited access to ticipants without AD exhibited Braak stage III or greater tau pa-
CSF or PET testing, such as in primary care and most memory thology (eTables 3 and 8 in the Supplement), but the mean lev-
clinics globally, including those in low- and middle-income els of plasma P-tau217 were still higher in the AD group vs this
countries, but assay development and validation in such set- non-AD group and there was no correlation between plasma
tings are needed first. P-tau217 and tau pathology in the non-AD group (Figure 1).
Plasma P-tau217 levels were elevated already early in the Recently, tau-PET has been shown to perform very well in
disease process (during the presymptomatic stages) in both distinguishing AD dementia from other neurodegenerative
sporadic AD (Figure 2A; eFigure 4A in the Supplement) and in diseases.6 In the present study, plasma P-tau217 and tau-PET
autosomal-dominant AD (Figure 4A; eFigure 7 in the Supple- did not perform significantly differently in distinguishing these
ment). The results also supported that plasma P-tau217 might groups (Figure 2C; eTable 12 in the Supplement). Further, the
identify preclinical AD (eFigure 4, panels B and D, and eTable 14 accuracy of plasma P-tau217 for discriminating between abnor-
in the Supplement). These findings suggest that plasma mal vs normal tau-PET scan findings was significantly higher
P-tau217 is an early marker of AD pathophysiology, which is than that of the most commonly used CSF P-tau biomarker, CSF
similar to what has previously been shown for CSF P-tau, but P-tau181 (Figure 3C; eTable 17 in the Supplement). This high-
in contrast to tau-PET, which changes mainly during the later lights the potential use of P-tau217 as a substitute marker for tau-
stages of the disease.6,29,36,37 In the present study, plasma PET when regional cerebral tau analysis is not required.
P-tau217 levels increased further during the symptomatic
stages both in sporadic AD (Figure 2A; eFigure 4A and eTable 13 Limitations
in the Supplement) and autosomal-dominant AD (Figure 4A; This study has several limitations. First, the study involved 3
eFigure 7 in the Supplement). selected cohorts, and the results should be validated in unse-
Plasma P-tau217 correlated with cerebral tau pathology mea- lected primary care populations and ethnically more diverse
sured postmortem using neuropathology (Figure 1) or in life populations. Second, the main analysis included participants
using tau-PET or CSF P-tau217 (eFigure 5 and eTable 16 in the with plasma P-tau217 concentrations too low to be accurately
Supplement). However, these correlations were only present in determined (ie, below the detection limit). However, the re-
cases with evident Aβ pathology, indicating that the increase sults were similar in a sensitivity analysis excluding these par-
in plasma P-tau217 levels appears to be associated with Aβ pa- ticipants (eFigures 8-11 in the Supplement). Optimization of
thology. The findings are in line with those from CSF studies sug- the sensitivity of the assay is currently ongoing. Third, the cur-
gesting that neurons exposed to Aβ pathology exhibit in- rent assay is a research-grade assay. The next step is to de-
creased production and secretion of soluble tau, which occurs velop a fully validated clinical-grade assay together with a cer-
before increased tau-PET signal.29,38 Similarly, plasma P-tau217 tified reference material39 and establish universal cutoffs,
levels were increased in AD but not in neurodegenerative dis- before the assay can be used in clinical practice. Further, trans-
eases characterized by other types of cerebral tau pathology, in- fer of the assay to fully automated platforms may facilitate po-
cluding progressive supranuclear palsy and corticobasal syn- tential implementation in clinical practice worldwide. Fourth,
drome (Figure 2A; eFigure 3, panels D and J in the Supplement). the present design was cross-sectional, and to confirm the value
The specificity of P-tau217 to AD-related tau-pathology was also of plasma P-tau217 levels as a marker of disease progression
E8 JAMA Published online July 28, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders Original Investigation Research
and to determine how early in the disease trajectory it starts
to increase in sporadic AD, future studies should include lon- Conclusions
gitudinal analyses of P-tau217. Fifth, although the group with
other neurodegenerative diseases in cohort 2 was of ad- Among 1402 participants from 3 selected cohorts, plasma
equate size (n = 99), the separate diagnostic groups were rela- P-tau217 discriminated AD from other neurodegenerative dis-
tively small (n = 12-45) and the discriminative accuracy against eases, with significantly higher accuracy than established
a specific other neurodegenerative disease should be inter- plasma- and MRI-based biomarkers, and its performance was
preted with caution. Sixth, established plasma and CSF as- not significantly different from key CSF- or PET-based mea-
says were used, but discriminative differences between plasma sures. Further research is needed to optimize the assay, vali-
P-tau217 and the other fluid biomarkers may to some degree date the findings in unselected and diverse populations, and
depend on the assay design and platform used. determine its potential role in clinical care.
ARTICLE INFORMATION Obtained funding: Palmqvist, Zetterberg, Foundation-supported research studies.
Accepted for Publication: June 22, 2020. Mattsson-Carlgren, Beach, Blennow, Dage, Reiman, Dr Hansson reported receiving grants from Roche,
Hansson. Biogen, and Pfizer and receiving nonfinancial
Published Online: July 28, 2020. Administrative, technical, or material support: support from GE Healthcare, AVID
doi:10.1001/jama.2020.12134 Palmqvist, Lopera, Strandberg, Smith, Villegas, Radiopharmaceuticals, and Euroimmun. No other
Author Affiliations: Clinical Memory Research Sepulveda-Falla, Chai, Beach, Dage, Reiman, disclosures were reported.
Unit, Department of Clinical Sciences, Malmö, Lund Hansson. Funding/Support: Work at the authors’ research
University, Lund, Sweden (Palmqvist, Janelidze, Supervision: Dage, Reiman, Hansson. centers was supported by the Swedish Research
Stomrud, Leuzy, Mattsson-Carlgren, Strandberg, Conflict of Interest Disclosures: Dr Quiroz Council, the Knut and Alice Wallenberg Foundation,
Smith, Hansson); Memory Clinic, Skåne University reported receiving grants from the National Region Skåne, the Marianne and Marcus Wallenberg
Hospital, Malmö, Sweden (Palmqvist, Stomrud, Institutes of Health (NIH) and Massachusetts Foundation, the Strategic Research Area MultiPark
Hansson); Massachusetts General Hospital, Harvard General Hospital. Dr Zetterberg reported receiving (Multidisciplinary Research in Parkinson’s disease)
Medical School, Boston (Quiroz); Grupo de grants from The Knut and Alice Wallenberg at Lund University, the Swedish Alzheimer
Neurociencias de Antioquia of Universidad de Foundation, European Research Council, and Foundation, the Swedish Brain Foundation, The
Antioquia, Medellin, Colombia (Quiroz, Lopera, Swedish Research Council; receiving personal fees Parkinson Foundation of Sweden, The Parkinson
Villegas, Sepulveda-Falla); Department of from Samumed, Roche Diagnostics, CogRx, Wave, Research Foundation, the Skåne University Hospital
Psychiatry and Neurochemistry, the Sahlgrenska Alzecure, and Biogen; and that he is cofounder of Foundation, the Greta and Johan Kock Foundation,
Academy at the University of Gothenburg, Mölndal, Brain Biomarker Solutions in Gothenburg AB. the Swedish federal government under the ALF
Sweden (Zetterberg, Blennow); Clinical Dr Lopera reported receiving grants from the NIH agreement, the NIH Office of the Director, the
Neurochemistry Laboratory, Sahlgrenska University and Genentech/Roche/Banner and receiving Alzheimer’s Association, the Massachusetts General
Hospital, Mölndal, Sweden (Zetterberg, Blennow); personal fees from the NIH. Dr Su reported Hospital Executive Committee on Research, the
Department of Neurodegenerative Disease, UCL receiving grants from the NIH, the State of Arizona, National Institute of Neurological Disorders and
Institute of Neurology, Queen Square, London, BrightFocus Foundation, and Alzheimer's Stroke (U24 NS072026), National Institute on
United Kingdom (Zetterberg); UK Dementia Association and receiving personal fees from Green Aging (P30 AG19610), the Arizona Department of
Research Institute at UCL, London, United Kingdom Valley Pharmaceutical LLC. Dr Chai reported a Health Services, the Michael J. Fox Foundation for
(Zetterberg); Banner Alzheimer’s Institute, Phoenix, patent to pTau217 assay and its use, antibodies Parkinson’s Research, Banner Alzheimer’s
Arizona (Su, Chen, Reiman); Banner Sun Health pending. Dr Beach reported receiving grants from Foundation, Sun Health Foundation, the State of
Research Institute, Sun City, Arizona (Serrano, the State of Arizona; receiving personal fees from Arizona, and Eli Lilly and Company. Eli Lilly and
Beach); Wallenberg Center for Molecular Medicine, Prothena Biosciences, Vivid Genomics, and Avid Company provided material support for P-tau217
Lund University, Lund, Sweden (Mattsson-Carlgren); Radiopharmaceuticals; and holding stock options sample analysis and salary for Dr Chai, Mr Proctor,
Department of Neurology, Skåne University with Vivid Genomics. Dr Blennow reported and Dr Dage. The precursor of 18F-flutemetamol
Hospital, Lund, Sweden (Mattsson-Carlgren, receiving personal fees from Abcam, Axon, Biogen, was provided by GE Healthcare and the precursor
Smith); Molecular Neuropathology of Alzheimer’s Lilly, MagQu, Novartis, and Roche Diagnostics and of 18F-RO948 was provided by Roche.
Disease (MoNeA), Institute of Neuropathology, that he is cofounder of Brain Biomarker Solutions in
University Medical Center Hamburg-Eppendorf, Role of the Funder/Sponsor: The funders had no
Gothenburg AB, a GU Ventures-based platform role in the design and conduct of the study;
Hamburg, Germany (Sepulveda-Falla); Eli Lilly and company at the University of Gothenburg. Dr Dage
Company, Indianapolis, Indiana (Chai, Proctor, collection, management, analysis, and
reported a patent pending for compounds and interpretation of the data; preparation, review, or
Dage); University of Arizona, Phoenix (Reiman); methods targeting human tau. Dr Reiman reported
Arizona State University, Phoenix (Reiman); approval of the manuscript; and decision to submit
receiving grants from National Institute on Aging the manuscript for publication. In addition, Eli Lilly
Translational Genomics Research Institute, Phoenix, and the State of Arizona; receiving philanthropic
Arizona (Reiman). and company had the opportunity to review the
funding from the Banner Alzheimer’s Foundation, manuscript before submission but had no veto
Author Contributions: Drs Hansson and Palmqvist Sun Health Foundation, and Roche/Roche power. Dr Hansson made the final decision to
had full access to all of the data in the study and Diagnostics; receiving personal fees from Alkahest, submit the manuscript to JAMA for publication.
take responsibility for the integrity of the data and Alzheon, Aural Analytics, Denali, Green Valley,
the accuracy of the data analysis. Drs Palmqvist and MagQ, Takeda/Zinfandel, United Neuroscience; Meeting Presentation: This study was presented
Janelidze contributed equally as first authors. that he has since submission of manuscript become online at the Alzheimer's Association International
Drs Hansson and Reiman (nonequally) contributed a cofounder of AlzPath, which aims to further Conference; July 28, 2020.
as senior authors. develop P-tau217 and fluid biomarkers and advance
Concept and design: Hansson. their use in research, drug development, and REFERENCES
Acquisition, analysis, or interpretation of data: All clinical settings; holding a patent owned by Banner 1. Brookmeyer R, Johnson E, Ziegler-Graham K,
authors. Health for a strategy to use biomarkers to Arrighi HM. Forecasting the global burden of
Drafting of the manuscript: Palmqvist, Janelidze, accelerate evaluation of Alzheimer prevention Alzheimer’s disease. Alzheimers Dement. 2007;3
Hansson. therapies; and that he is a principal investigator of (3):186-191. doi:10.1016/j.jalz.2007.04.381
Critical revision of the manuscript for important prevention trials that include research agreements 2. Global Action Plan on the Public Health Response
intellectual content: All authors. with Genentech/Roche and Novartis/Amgen, PET to Dementia 2017-2025. World Health Organization;
Statistical analysis: Palmqvist, Janelidze, Su, Chen, studies that include research agreements with 2017.
Strandberg. Avid/Lilly, and several NIH and
jama.com (Reprinted) JAMA Published online July 28, 2020 E9
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
Research Original Investigation Accuracy of Plasma P-tau217 for Distinguishing Alzheimer Disease From Other Neurodegenerative Disorders
3. Abbasi J. Promising results in 18-month analysis 16. National Institute on Aging and Reagan 28. Barthélemy NR, Li Y, Joseph-Mathurin N, et al;
of Alzheimer drug candidate. JAMA. 2018;320 Institute Working Group on Diagnostic Criteria for Dominantly Inherited Alzheimer Network. A soluble
(10):965. doi:10.1001/jama.2018.13027 the Neuropathological Assessment of Alzheimer’s phosphorylated tau signature links tau, amyloid and
4. Sevigny J, Chiao P, Bussière T, et al. The antibody Disease. Consensus recommendations for the the evolution of stages of dominantly inherited
aducanumab reduces Aβ plaques in Alzheimer’s postmortem diagnosis of Alzheimer’s disease. Alzheimer’s disease. Nat Med. 2020;26(3):398-407.
disease. Nature. 2016;537(7618):50-56. doi:10. Neurobiol Aging. 1997;18(4)(suppl):S1-S2. doi:10.1038/s41591-020-0781-z
1038/nature19323 17. Mirra SS, Heyman A, McKeel D, et al. The 29. Mattsson-Carlgren N, Andersson E, Janelidze S,
5. Rabinovici GD, Gatsonis C, Apgar C, et al. Consortium to Establish a Registry for Alzheimer’s et al. Aβ deposition is associated with increases in
Association of amyloid positron emission Disease (CERAD), II: standardization of the soluble and phosphorylated tau that precede a
tomography with subsequent change in clinical neuropathologic assessment of Alzheimer’s positive tau PET in Alzheimer’s disease. Sci Adv.
management among Medicare beneficiaries with disease. Neurology. 1991;41(4):479-486. doi:10. 2020;6(16):eaaz2387. doi:10.1126/sciadv.aaz2387
mild cognitive impairment or dementia. JAMA. 1212/WNL.41.4.479 30. Mattsson N, Zetterberg H, Janelidze S, et al;
2019;321(13):1286-1294. doi:10.1001/jama.2019. 18. Braak H, Braak E. Neuropathological stageing of Alzheimer’s Disease Neuroimaging Initiative
2000 Alzheimer-related changes. Acta Neuropathol. Investigators. Plasma tau in Alzheimer disease.
6. Ossenkoppele R, Rabinovici GD, Smith R, et al. 1991;82(4):239-259. doi:10.1007/BF00308809 Neurology. 2016;87(17):1827-1835. doi:10.1212/WNL.
Discriminative accuracy of [18F]flortaucipir 19. Diagnostic and Statistical Manual of Mental 0000000000003246
positron emission tomography for Alzheimer Disorders (Fifth Edition). American Psychiatric 31. Palmqvist S, Insel PS, Zetterberg H, et al;
disease vs other neurodegenerative disorders. JAMA. Association; 2013. Alzheimer’s Disease Neuroimaging Initiative;
2018;320(11):1151-1162. doi:10.1001/jama.2018.12917 20. Tariot PN, Lopera F, Langbaum JB, et al; Swedish BioFINDER study. Accurate risk estimation
7. Mattsson N, Zetterberg H, Hansson O, et al. CSF Alzheimer’s Prevention Initiative. The Alzheimer’s of β-amyloid positivity to identify prodromal
biomarkers and incipient Alzheimer disease in Prevention Initiative Autosomal-Dominant Alzheimer’s disease: cross-validation study of
patients with mild cognitive impairment. JAMA. Alzheimer’s Disease Trial: a study of crenezumab practical algorithms. Alzheimers Dement. 2019;15
2009;302(4):385-393. doi:10.1001/jama.2009.1064 versus placebo in preclinical PSEN1 E280A mutation (2):194-204. doi:10.1016/j.jalz.2018.08.014
8. Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA carriers to evaluate efficacy and safety in the 32. Zetterberg H, Wilson D, Andreasson U, et al.
research framework: toward a biological definition treatment of autosomal-dominant Alzheimer’s Plasma tau levels in Alzheimer’s disease. Alzheimers
of Alzheimer’s disease. Alzheimers Dement. 2018;14 disease, including a placebo-treated noncarrier Res Ther. 2013;5(2):9. doi:10.1186/alzrt163
(4):535-562. doi:10.1016/j.jalz.2018.02.018 cohort. Alzheimers Dement (N Y). 2018;4:150-160. 33. Chatterjee P, Goozee K, Sohrabi HR, et al.
9. Janelidze S, Stomrud E, Smith R, et al. 21. Morris JC, Heyman A, Mohs RC, et al. The Association of plasma neurofilament light chain
Cerebrospinal fluid p-tau217 performs better than Consortium to Establish a Registry for Alzheimer’s with neocortical amyloid-β load and cognitive
p-tau181 as a biomarker of Alzheimer’s disease. Nat Disease (CERAD), I: clinical and neuropsychological performance in cognitively normal elderly
Commun. 2020;11(1):1683. doi:10.1038/s41467- assessment of Alzheimer’s disease. Neurology. participants. J Alzheimers Dis. 2018;63(2):479-487.
020-15436-0 1989;39(9):1159-1165. doi:10.1212/WNL.39.9.1159 doi:10.3233/JAD-180025
10. Duits FH, Martinez-Lage P, Paquet C, et al. 22. Palmqvist S, Insel PS, Stomrud E, et al. 34. Mattsson N, Andreasson U, Zetterberg H,
Performance and complications of lumbar puncture Cerebrospinal fluid and plasma biomarker Blennow K; Alzheimer’s Disease Neuroimaging
in memory clinics: results of the multicenter lumbar trajectories with increasing amyloid deposition in Intiative. Association of plasma neurofilament light
puncture feasibility study. Alzheimers Dement. Alzheimer’s disease. EMBO Mol Med. 2019;11(12): with neurodegeneration in patients with Alzheimer
2016;12(2):154-163. doi:10.1016/j.jalz.2015.08.003 e11170. doi:10.15252/emmm.201911170 disease. JAMA Neurol. 2017;74(5):557-566. doi:10.
23. Mielke MM, Hagen CE, Xu J, et al. Plasma 1001/jamaneurol.2016.6117
11. Ovod V, Ramsey KN, Mawuenyega KG, et al.
Amyloid β concentrations and stable isotope phospho-tau181 increases with Alzheimer’s disease 35. Zhou W, Zhang J, Ye F, et al; Alzheimer’s
labeling kinetics of human plasma specific to central clinical severity and is associated with tau- and Disease Neuroimaging Initiative. Plasma
nervous system amyloidosis. Alzheimers Dement. amyloid-positron emission tomography. Alzheimers neurofilament light chain levels in Alzheimer’s
2017;13(8):841-849. doi:10.1016/j.jalz.2017.06.2266 Dement. 2018;14(8):989-997. doi:10.1016/j.jalz. disease. Neurosci Lett. 2017;650:60-64. doi:10.
2018.02.013 1016/j.neulet.2017.04.027
12. Palmqvist S, Janelidze S, Stomrud E, et al.
Performance of fully automated plasma assays as 24. Leuzy A, Smith R, Ossenkoppele R, et al. 36. Gordon BA, Blazey TM, Christensen J, et al.
screening tests for Alzheimer disease–related Diagnostic performance of RO948 F 18 tau positron Tau PET in autosomal dominant Alzheimer’s
β-amyloid status. JAMA Neurol. 2019;76(9):1060- emission tomography in the differentiation of disease: relationship with cognition, dementia and
1069. doi:10.1001/jamaneurol.2019.1632 Alzheimer disease from other neurodegenerative other biomarkers. Brain. 2019;142(4):1063-1076.
disorders. JAMA Neurol. Published online May 11, doi:10.1093/brain/awz019
13. Janelidze S, Mattsson N, Palmqvist S, et al. 2020. doi:10.1001/jamaneurol.2020.0989
Plasma P-tau181 in Alzheimer’s disease: relationship 37. Quiroz YT, Sperling RA, Norton DJ, et al.
to other biomarkers, differential diagnosis, 25. DeLong ER, DeLong DM, Clarke-Pearson DL. Association between amyloid and tau accumulation
neuropathology and longitudinal progression to Comparing the areas under two or more correlated in young adults with autosomal dominant
Alzheimer’s dementia. Nat Med. 2020;26(3):379- receiver operating characteristic curves: Alzheimer disease. JAMA Neurol. 2018;75(5):548-
386. doi:10.1038/s41591-020-0755-1 a nonparametric approach. Biometrics. 1988;44(3): 556. doi:10.1001/jamaneurol.2017.4907
837-845. doi:10.2307/2531595 38. Sato C, Barthelemy NR, Mawuenyega KG, et al
14. Thijssen EH, La Joie R, Wolf A, et al; Advancing
Research and Treatment for Frontotemporal Lobar 26. Quiroz YT, Zetterberg H, Reiman EM, et al. Tau kinetics in neurons and the human central
Degeneration (ARTFL) Investigators. Diagnostic Plasma neurofilament light chain in the presenilin 1 nervous system. Neuron. 2018;97(6):1284-1298.
value of plasma phosphorylated tau181 in E280A autosomal dominant Alzheimer’s disease 39. Kuhlmann J, Andreasson U, Pannee J, et al;
Alzheimer’s disease and frontotemporal lobar kindred: a cross-sectional and longitudinal cohort IFCC Working Group on Standardization of CSF
degeneration. Nat Med. 2020;26(3):387-397. doi: study. Lancet Neurol. 2020;19(6):513-521. doi:10. proteins (WG-CSF). CSF Aβ1-42—an excellent but
10.1038/s41591-020-0762-2 1016/S1474-4422(20)30137-X complicated Alzheimer’s biomarker—a route to
15. Beach TG, Adler CH, Sue LI, et al. Arizona study 27. Barthélemy NR, Bateman RJ, Hirtz C, et al. standardisation. Clin Chim Acta. 2017;467:27-33.
of aging and neurodegenerative disorders and brain Cerebrospinal fluid phospho-tau T217 outperforms doi:10.1016/j.cca.2016.05.014
and body donation program. Neuropathology. T181 as a biomarker for the differential diagnosis of
2015;35(4):354-389. doi:10.1111/neup.12189 Alzheimer’s disease and PET amyloid-positive
patient identification. Alzheimers Res Ther. 2020;12
(1):26. doi:10.1186/s13195-020-00596-4
E10 JAMA Published online July 28, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ on 07/29/2020
You might also like
- Hypnotherapy ScriptsDocument109 pagesHypnotherapy ScriptsSabina Lupasco97% (31)
- Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma BiomarkersDocument10 pagesPrediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma BiomarkersAndrade GuiNo ratings yet
- Jamaneurology Ashton 2024 Oi 230097 1703782257.54322Document10 pagesJamaneurology Ashton 2024 Oi 230097 1703782257.54322HermesNo ratings yet
- Blood biomarkers for Alzheimer's disease in clinical practice and trials (科研通-ablesci.com)Document14 pagesBlood biomarkers for Alzheimer's disease in clinical practice and trials (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- 10.1515 - Almed 2020 0090Document11 pages10.1515 - Almed 2020 0090reggioamelia adaptacioncurricularNo ratings yet
- NFL Strongly Correlates With TNF R1 in The Plasma of AD Patients, But Not With Cognitive DeclineDocument7 pagesNFL Strongly Correlates With TNF R1 in The Plasma of AD Patients, But Not With Cognitive Declinemuralim_phy1986No ratings yet
- Differential Levels of Plasma Biomarkers of Neurodegeneration in Lewy Body Dementia, Alzheimer's Disease, Frontotemporal Dementia and Progressive Supranuclear PalsyDocument8 pagesDifferential Levels of Plasma Biomarkers of Neurodegeneration in Lewy Body Dementia, Alzheimer's Disease, Frontotemporal Dementia and Progressive Supranuclear PalsyAlexandra CastellanosNo ratings yet
- Ijms 22 02761Document12 pagesIjms 22 02761Juberiya AjazNo ratings yet
- Evaluation of CSF Biomarkers As Predictors of Alzheimer's Disease - A Clinical Follow-Up Study of 4.7 YearsDocument10 pagesEvaluation of CSF Biomarkers As Predictors of Alzheimer's Disease - A Clinical Follow-Up Study of 4.7 YearsJuliano Flávio Rubatino RodriguesNo ratings yet
- Tau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageDocument13 pagesTau PET and Multimodal Brain Imaging in Patients at Risk F - 2019 - NeuroImageBruno MañonNo ratings yet
- Ossenkoppele 2019 PET Tau Pet Amyloid Cortical ThicknessDocument13 pagesOssenkoppele 2019 PET Tau Pet Amyloid Cortical ThicknessAngela SilvaNo ratings yet
- Biomakers For The Early Detection.1Document19 pagesBiomakers For The Early Detection.1MARIA DE FATIMA TEIXEIRA GORDILLONo ratings yet
- Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's diseaseDocument16 pagesPlasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's diseaseSteven Condor CruzNo ratings yet
- NEUROLOGY2018894063Document12 pagesNEUROLOGY2018894063leilaNo ratings yet
- MOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseDocument9 pagesMOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseeastareaNo ratings yet
- 10 1016@j Neuroscience 2017 07 017Document26 pages10 1016@j Neuroscience 2017 07 017Anggraeni Arum SNo ratings yet
- Changes in Plasma Amyloid and Tau in ADocument16 pagesChanges in Plasma Amyloid and Tau in AeastareaNo ratings yet
- 2017 - Differential Diagnosis of AD Using Spectrochemical Analysis of BloodDocument10 pages2017 - Differential Diagnosis of AD Using Spectrochemical Analysis of BloodM JNo ratings yet
- Schweitzer 2006Document5 pagesSchweitzer 2006emilNo ratings yet
- Blood Biomarkers in Neurodegenerative Diseases (科研通-ablesci.com)Document9 pagesBlood Biomarkers in Neurodegenerative Diseases (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- Spal LazziDocument13 pagesSpal LazziPriscila SelingardiNo ratings yet
- PE Como Predictores de Discapacidad en EM - 2012Document5 pagesPE Como Predictores de Discapacidad en EM - 20122j5f2y76g2No ratings yet
- Advantage of Modified MRI Protocol For HDocument148 pagesAdvantage of Modified MRI Protocol For HasasakopNo ratings yet
- 1 s2.0 S0300289622006640 MainDocument7 pages1 s2.0 S0300289622006640 MainInda MegaNo ratings yet
- Nejme 2400102Document3 pagesNejme 2400102linjingwang912No ratings yet
- AlzaimerDocument13 pagesAlzaimerAfni Panggar BesiNo ratings yet
- Counts ADbiomarkerreview2016Document20 pagesCounts ADbiomarkerreview2016MARIA DE FATIMA TEIXEIRA GORDILLONo ratings yet
- Tomasi Et Al 2017Document5 pagesTomasi Et Al 2017Cristiane TomasiNo ratings yet
- Tunel Del CarpoDocument6 pagesTunel Del CarpojoaquinNo ratings yet
- 576 Friday, 15 June 2018 Scientific AbstractsDocument2 pages576 Friday, 15 June 2018 Scientific AbstractsDanna Carvajal GaitanNo ratings yet
- Falgàs 2019 - Comparison Between Visual and Quantitative Hippocampal Atrophy AssessmentDocument7 pagesFalgàs 2019 - Comparison Between Visual and Quantitative Hippocampal Atrophy AssessmentljdNo ratings yet
- Increased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological DiseasesDocument9 pagesIncreased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological Diseasesnreena aslamNo ratings yet
- Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKDDocument15 pagesRandomized Placebo-Controlled EPPIC Trials of AST-120 in CKDPROF. ERWIN M. GLOBIO, MSITNo ratings yet
- Early Hippocampal Atrophy Is An Important Signal For Clinicians But Not Necessarily A Harbinger of Alzheimer DiseaseDocument2 pagesEarly Hippocampal Atrophy Is An Important Signal For Clinicians But Not Necessarily A Harbinger of Alzheimer DiseaseCarlos Hernan Castañeda RuizNo ratings yet
- Association Neurofilament and Alzheimer Progression Mattsson2019Document9 pagesAssociation Neurofilament and Alzheimer Progression Mattsson2019eastareaNo ratings yet
- Autonomic Neuroscience: Basic and Clinical: Jose-Alberto Palma, Lucy Norcli Ffe-Kaufmann, Horacio KaufmannDocument11 pagesAutonomic Neuroscience: Basic and Clinical: Jose-Alberto Palma, Lucy Norcli Ffe-Kaufmann, Horacio KaufmannCalvin Tanuwijaya Stick BolaNo ratings yet
- Newly Diagnosed Anemia Increases Risk of Parkinson's Disease: A Population-Based Cohort StudyDocument7 pagesNewly Diagnosed Anemia Increases Risk of Parkinson's Disease: A Population-Based Cohort Studydiah_budiarti_1No ratings yet
- 1 s2.0 S1053811921005103 MainDocument11 pages1 s2.0 S1053811921005103 Maincryxynhe18No ratings yet
- Ferritin 7Document2 pagesFerritin 7SuryaNo ratings yet
- 2018 - Circulating microRNAs As Novel Biomarkers of Alzheimer's DiseaseDocument6 pages2018 - Circulating microRNAs As Novel Biomarkers of Alzheimer's DiseaseM JNo ratings yet
- Jad - 2018 - 61 4 - Jad 61 4 Jad170553 - Jad 61 Jad170553Document13 pagesJad - 2018 - 61 4 - Jad 61 4 Jad170553 - Jad 61 Jad170553Quang NguyễnNo ratings yet
- Clinical Predictors and Differential Diagnosis of Posterior Reversible Encephalopathy SyndromeDocument7 pagesClinical Predictors and Differential Diagnosis of Posterior Reversible Encephalopathy SyndromekidNo ratings yet
- 1516 4446 RBP 1516444620200013Document3 pages1516 4446 RBP 1516444620200013Luisa RamirezNo ratings yet
- Konduksi DSP NcsDocument7 pagesKonduksi DSP NcsMutiara Kristiani PutriNo ratings yet
- Pietro Bon IDocument5 pagesPietro Bon Ibeealoner07No ratings yet
- May 2021 1624011543 9200117Document4 pagesMay 2021 1624011543 9200117Manoj KumarNo ratings yet
- Calmodulin Levels in Blood Cells As A Potential Biomarker of Alzheimer 'S DiseaseDocument10 pagesCalmodulin Levels in Blood Cells As A Potential Biomarker of Alzheimer 'S DiseaseFanel PutraNo ratings yet
- Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingDocument8 pagesAssociation of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingeastareaNo ratings yet
- Clinica Encefalitis Nmda InglesDocument7 pagesClinica Encefalitis Nmda InglesOskr MezaNo ratings yet
- Moll Cellproteomic PDFDocument49 pagesMoll Cellproteomic PDFGLORIA ESTEFANI YANTAS ALCANTARANo ratings yet
- Blood-based biomarkers in Alzheimer's disease - a mini-review (科研通-ablesci.com)Document9 pagesBlood-based biomarkers in Alzheimer's disease - a mini-review (科研通-ablesci.com)Pei-Hao ChenNo ratings yet
- 2022 Clinical Features and Risk Factors of Mortality in Patients With Posterior Reversible Encephalopathy SyndromeDocument7 pages2022 Clinical Features and Risk Factors of Mortality in Patients With Posterior Reversible Encephalopathy SyndromeSafitri MuhlisaNo ratings yet
- Jpad 2022 101Document9 pagesJpad 2022 101leilaNo ratings yet
- White Matter Hyperintensities A Marker For Apathy in Parkinson Without DementiaDocument10 pagesWhite Matter Hyperintensities A Marker For Apathy in Parkinson Without DementiamutiahmuftihNo ratings yet
- 3 - Evolution of Genetic Testing Supports Precision Medicine For Caring Alzheimer's Disease PatientsDocument6 pages3 - Evolution of Genetic Testing Supports Precision Medicine For Caring Alzheimer's Disease Patientsveidamororo17No ratings yet
- A/T/N: An Unbiased Descriptive Classification Scheme For Alzheimer Disease BiomarkersDocument11 pagesA/T/N: An Unbiased Descriptive Classification Scheme For Alzheimer Disease BiomarkersSatya YudhayanaNo ratings yet
- Laske2015Document18 pagesLaske2015Paco SaavedraNo ratings yet
- Multifactorial Diseases.: Chair of Medical Genetics Department of Clinical Genetics Karol P. Ruszel, PHDDocument51 pagesMultifactorial Diseases.: Chair of Medical Genetics Department of Clinical Genetics Karol P. Ruszel, PHDTimyNo ratings yet
- Reciprocal Predictive Relationships Between AmyloidDocument10 pagesReciprocal Predictive Relationships Between AmyloidCristhian RodriguezNo ratings yet
- A Multicenter Cohort StudyDocument9 pagesA Multicenter Cohort Studyfatchurhamzah62No ratings yet
- 69th AACC Annual Scientific Meeting Abstract eBookFrom Everand69th AACC Annual Scientific Meeting Abstract eBookNo ratings yet
- A Power-Law Model of Psychological Memory Strength in Short - and Long-Term RecognitionDocument11 pagesA Power-Law Model of Psychological Memory Strength in Short - and Long-Term RecognitioneastareaNo ratings yet
- A Critical Review LTF en EpilepsiaDocument26 pagesA Critical Review LTF en EpilepsiaeastareaNo ratings yet
- Audrain - Samantha - 201511 - MA - Thesis Nvestigating Accelerated Long-Term ForgettingDocument73 pagesAudrain - Samantha - 201511 - MA - Thesis Nvestigating Accelerated Long-Term ForgettingeastareaNo ratings yet
- Alexander2016.pdf A Psychometrical Model For Psychodiagnostic Assessment of Memory DeficitsDocument15 pagesAlexander2016.pdf A Psychometrical Model For Psychodiagnostic Assessment of Memory DeficitseastareaNo ratings yet
- Putcha Fractionating The Rey Auditory Verbal Learning Test Distinct Roles of Largescale Cortical Networks in Prodromal Alzheimer's DiseaseDocument27 pagesPutcha Fractionating The Rey Auditory Verbal Learning Test Distinct Roles of Largescale Cortical Networks in Prodromal Alzheimer's DiseaseeastareaNo ratings yet
- Atherton2018 Encoding-Related Brain Activity AFL Epilepsy PDFDocument45 pagesAtherton2018 Encoding-Related Brain Activity AFL Epilepsy PDFeastareaNo ratings yet
- Papp 2014Document17 pagesPapp 2014eastareaNo ratings yet
- Accelerated Long-Term Forgetting After TIA or MinorDocument7 pagesAccelerated Long-Term Forgetting After TIA or MinoreastareaNo ratings yet
- Sperling 2009Document22 pagesSperling 2009eastareaNo ratings yet
- Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer DiseaseDocument11 pagesLongitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer DiseaseeastareaNo ratings yet
- Neurofilament Light Chain Gaetani2019Document12 pagesNeurofilament Light Chain Gaetani2019eastareaNo ratings yet
- Rentz2011 FNAMEDocument8 pagesRentz2011 FNAMEeastareaNo ratings yet
- Correlates of Recognition Memory Performance in Amnestic Mild Cognitive ImpairmentDocument8 pagesCorrelates of Recognition Memory Performance in Amnestic Mild Cognitive ImpairmenteastareaNo ratings yet
- Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingDocument8 pagesAssociation of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in The Mayo Clinic Study On AgingeastareaNo ratings yet
- Comparing Progression Biomarkers in Clinical Trials of Early Alzheimer’ S DiseaseDocument13 pagesComparing Progression Biomarkers in Clinical Trials of Early Alzheimer’ S DiseaseeastareaNo ratings yet
- Age and Sex Impact Plasma NFL and T-Tau Trajectories in Individuals With Subjective Memory Complaints - A 3-Year Follow-Up StudyDocument12 pagesAge and Sex Impact Plasma NFL and T-Tau Trajectories in Individuals With Subjective Memory Complaints - A 3-Year Follow-Up StudyeastareaNo ratings yet
- Changes in Plasma Amyloid and Tau in ADocument16 pagesChanges in Plasma Amyloid and Tau in AeastareaNo ratings yet
- Association Neurofilament and Alzheimer Progression Mattsson2019Document9 pagesAssociation Neurofilament and Alzheimer Progression Mattsson2019eastareaNo ratings yet
- MOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseDocument9 pagesMOCA The Validation of Multifactor Model of Plasma Aβ 42 and Total-Tau in Combination With MoCA for Diagnosing Probable Alzheimer DiseaseeastareaNo ratings yet
- Accelerated Long-Term Forgetting in Transient Epileptic Amnesia Acquision or Consolidation DeficitDocument7 pagesAccelerated Long-Term Forgetting in Transient Epileptic Amnesia Acquision or Consolidation DeficiteastareaNo ratings yet
- Relaccem - Msard152 2014Document7 pagesRelaccem - Msard152 2014eastareaNo ratings yet
- Moreno-Manso2020 Child AbuseDocument17 pagesMoreno-Manso2020 Child AbuseeastareaNo ratings yet
- Nihms 1045384Document20 pagesNihms 1045384eastareaNo ratings yet
- The Dutch Review Process For Evaluating The QualitDocument25 pagesThe Dutch Review Process For Evaluating The QualiteastareaNo ratings yet
- No Association Between Loneliness Episodic MemoryDocument13 pagesNo Association Between Loneliness Episodic MemoryeastareaNo ratings yet
- The Paradoxical Effect of COVID-19 Outbreak On LonDocument4 pagesThe Paradoxical Effect of COVID-19 Outbreak On LoneastareaNo ratings yet
- Jamaneurology Liu 2022 Oi 220011 1651870402.27767Document9 pagesJamaneurology Liu 2022 Oi 220011 1651870402.27767eastareaNo ratings yet
- Jama Caricchio 2021 Oi 210064 1626283669.23567Document10 pagesJama Caricchio 2021 Oi 210064 1626283669.23567eastareaNo ratings yet
- Jamapsychiatry Frontera 2022 SC 220001 1659381701.66875Document7 pagesJamapsychiatry Frontera 2022 SC 220001 1659381701.66875eastareaNo ratings yet
- Oosterhuis 2015 Sample Size Requirements For Traditional and Regression-Based NormDocument12 pagesOosterhuis 2015 Sample Size Requirements For Traditional and Regression-Based NormeastareaNo ratings yet
- 12th PNHRS Program Book As of AugustDocument47 pages12th PNHRS Program Book As of AugustChristine Rodriguez-GuerreroNo ratings yet
- Ebook Braddoms Physical Medicine and Rehabilitation Sixth Edition PDF Full Chapter PDFDocument67 pagesEbook Braddoms Physical Medicine and Rehabilitation Sixth Edition PDF Full Chapter PDFmelinda.warren862100% (34)
- Chronic MeningitisDocument7 pagesChronic Meningitisneurojuancarfab100% (1)
- SDS PAC LiquidDocument4 pagesSDS PAC LiquidQuality AssuranceNo ratings yet
- NSTP Group 3 First Aid ReportingDocument44 pagesNSTP Group 3 First Aid ReportingCindy GeverolaNo ratings yet
- Ethics or Necessity Continuing Animal Testing For Scientific BreakthroughsDocument4 pagesEthics or Necessity Continuing Animal Testing For Scientific Breakthroughsperezcarlj1010No ratings yet
- Domestic ViolenceDocument13 pagesDomestic ViolenceJENNIFER YBAÑEZNo ratings yet
- English TM 8Document11 pagesEnglish TM 8Budi AtmikaNo ratings yet
- Crisis and Nursing InterventionDocument44 pagesCrisis and Nursing InterventionArchana100% (4)
- Emergency ActionDocument14 pagesEmergency ActionferozNo ratings yet
- 2021 DPharm - Clinical InkDocument8 pages2021 DPharm - Clinical InkEd SeguineNo ratings yet
- Delrosario 2017Document33 pagesDelrosario 2017Pande Agung MahariskiNo ratings yet
- COPD Case Pres2Document29 pagesCOPD Case Pres2Kelly Queenie Andres100% (2)
- Eng1503 Mock Exam Sample Answers: THANK YOU For Answering The Mock Examination Question BelowDocument14 pagesEng1503 Mock Exam Sample Answers: THANK YOU For Answering The Mock Examination Question BelowOlwethu PhikeNo ratings yet
- What Philippine Law Was Created To Include in It The Code of Ethics of Filipino Nurses?Document3 pagesWhat Philippine Law Was Created To Include in It The Code of Ethics of Filipino Nurses?Shirlene Benigra GorospeNo ratings yet
- Gensler Design Forecast 2013Document43 pagesGensler Design Forecast 2013Pete PetrášNo ratings yet
- The Who Five Keys To Safer Food A Tool For Food Safety Health PromotionDocument15 pagesThe Who Five Keys To Safer Food A Tool For Food Safety Health PromotionCarlos RuizNo ratings yet
- OsteoarthritisDocument37 pagesOsteoarthritisChikezie Onwukwe100% (1)
- Nursing Care Plan - Fatigue (Antepartum)Document3 pagesNursing Care Plan - Fatigue (Antepartum)kaimimiyaNo ratings yet
- Communiqué Émis Par La PSCDocument4 pagesCommuniqué Émis Par La PSCL'express MauriceNo ratings yet
- Garlic Benefits - Can Garlic Lower Your Cholesterol?Document4 pagesGarlic Benefits - Can Garlic Lower Your Cholesterol?Jipson VargheseNo ratings yet
- A Management Algorithm For Esophageal PerforationDocument4 pagesA Management Algorithm For Esophageal Perforationoscar chirinosNo ratings yet
- Birth StoryDocument7 pagesBirth StoryKaty Whipple100% (1)
- Ramadan and DiabetesDocument14 pagesRamadan and DiabetesDitoLinggoNo ratings yet
- 19 Disaster SurgeryDocument45 pages19 Disaster SurgeryAhmed noor Ahmed noorNo ratings yet
- WritingDocument8 pagesWritingRukshan GunasenaNo ratings yet
- Home EconomicsDocument5 pagesHome EconomicsNicole Kathleen Caranto100% (1)
- Green Environment: Love Our EarthDocument3 pagesGreen Environment: Love Our EarthSoon JimmyNo ratings yet
- Y12 - Prophet's Methadology of Health Care (Solved)Document5 pagesY12 - Prophet's Methadology of Health Care (Solved)Umair HibatullahNo ratings yet