Professional Documents
Culture Documents
First.: (2, 4, Is Be Is Is Be Is
First.: (2, 4, Is Be Is Is Be Is
Uploaded by
Aitor PastorOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
First.: (2, 4, Is Be Is Is Be Is
First.: (2, 4, Is Be Is Is Be Is
Uploaded by
Aitor PastorCopyright:
Available Formats
scientist first.
She taught that “good stuff5 about molal 25 °C (2, 4, 5, 3); this is almost 10 times smaller than the
freezing-point depression and allotropic forms of sulfur and square of the solubility. Meites et al. (2) make the pertinent
the combined gas law. I loved it and many of the kids I teach points that (1) the solubility of CaS04 is analogous to that of
today love it, too. a sparingly soluble weak acid such as 2,4,6-triehlorophenol,
My students do complain, but complaints are not rare in (2) it would be better to confine the solubility-product prin-
their other classes, either, the teachers tell me. Driver Ed ciple to univalent salts such as silver bromide or thallous
and Health are “too hard” or too “dull”. History is “not iodide where there is negligible ion-pairing, and (3) teachers
useful”, and foreign language requires “too much memori- should seriously consider whether the solubility-product
zing”, etc. principle is in fact closely enough related to solubility to
Make no mistake about it, the argument in Krajcik and justify its presentation in that context.
Yager’s article is anti-intellectualism. Its roots go back a long The above comments may seem to be rather pedantic, but
way in professional education. It seeks to draw strength from their neglect is illustrated in an illuminating way by the
popular appeal. Its adherents are kids who want an easier value of the “Solubility Product” for CaS04 quoted in the
program, parents who want the appearance of success on CRC Handbook of Chemistry and Physics (7) (hereafter
report cards without hassle, a few teachers whose back- called the CRC Handbook). I noticed that the value of K&p =
ground is weak or who prefer the authority of newspapers to 1.9 X 10-4 quoted from it by Masterman (1) was not the same
textbooks and research articles. It is “democratic” in the as that in my own copy. On examining the various editions of
negative, divisive sense of the word. the CRC Handbook available in our laboratories, I obtained
Teaching objective material does not make a teacher un- the results shown in the table. Over a period of 40 years the
popular or turn off the students. When the graduated high value given in the CRC Handbook has changed at least four
school students come back from college to see you, they will times (possibly more, I haven’t looked at all editions) with a
thank you for giving them a solid base of understanding and range of almost an order of magnitude.
skills for that tough first year of college chemistry. We can now speculate on the reasons for these changes.
Please, give us the “lumber” to build our courses, but let There is no information in the CRC Handbook about
the “sawdust” filter down into education journals. sources, except that the three latest editions have used AGf°
values to calculate Ksp (K&p exp (—ArG°/RT) where ArG0/
=
S. T. Bond RT AGf°(Ca2+,aq) + AGf°(Sq42-,aq)
=
AGf°(CaS04, c)).
-
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
Bridgeport High School The previous value of 2,45 X 10“5 probably comes from ref 5
Bridgeport, WV 26330 which was published in 1970; the CRC Handbook value
Downloaded via UNIV DE ALICANTE on October 16, 2022 at 11:59:25 (UTC).
changed somewhere between the 1969-70 and the 1972-73
editions. The earlier value of 1.95 X 10-4 was almost certain-
ly the square of the solubility, whose value quoted in the
“Physical Constants of Inorganic Compounds” table in the
CRC Handbook has remained constant over the whole peri-
od.
There are several morals to this tale. Always treat tabulat-
Solubility Products and Solubility: Plus 9a Change? ed data in textbooks and compilations with skepticism, espe-
To the Editor:
cially if the original source is not given; there is no guarantee
that the compilers have always appreciated the approxima-
tions they have made. Quote the edition as well as the book
Although Masterman (1) in his article “Ksp Determination from which you obtain data. And do not assume that the
of Calcium Sulfate” has measured the solubility of CaSC>4 in data quoted in the latest edition are necessarily more reli-
water, he has not determined the solubility product Ksp. The able than those from previous editions; in this case the value
relationship (or lack of it) between solubility and Ksp per- of 2.45 X 105 is closest to the “true” thermodynamic value of
haps needs to be emphasized once again, because it appears ref 5, which was obtained by careful experiments on CaS04
to be something that is discovered and subsequently forgot-
and is, I suspect, more reliable than the values in the most
ten with monotonous regularity. In this Journal alone, the recent editions that were calculated from general thermody-
point was fully discussed by Meites et al (2), overlooked by namic data obtained from a variety of sources.
Sawyer (3), discussed afresh by Martin (4) and neglected by
Masterman (I). Briefly, to equate Ksp to the square of the
solubility of CaS04 is grossly in error, because it ignores (1) Literature Cited
the appreciable ionic strength of the solution (because both 1. Masterman, D. J. Chem. Educ, 1987,64,408-409.
ions are divalent and the salt is not highly insoluble), which 2. Meites, L.; Pode, J. S. F.; Thomas, H. C. J. Chem. Educ. 1966, 43, 667-672.
3. Sawyer, A. K. J. Chem. Educ. 1983,60, 416.
leads to activity coefficients very different from unity, and 4. Martin, R. B. J. Chem. Educ. 1986,63, 471-472.
(2) the fact that an appreciable proportion of the compound 5. Gardner, A. W.; Glueckauf, E. Trans. Faraday Soc. 1970, 66,1081-1087.
6. Guenther, W. B. Chemical Equilibrium; Plenum: New York, 1975; pp 172-176.
is present in solution as the undissociated molecule or ion- 7. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL.
pair CaS04(aq), i.e., it is not a strong electrolyte. If these
factors are taken into account then the true thermodynamic John H. Carpenter
solubility product K$p° aca2+aso42- about 2.5 X 10-5 at
=
Newcastle University
Newcastle upon Tyne, NE1 7RU, UK
Value of Solubility Product of CaS04 in the CRC Handbook.
Edition Ksp Temp.
Editor's Note: A review article "Ion Association, Solubilities, and
30th (1947) 6.1 X 10-5 10 °c Reduction Potentials in Aqueous Solution” by Russo and Hanania,
39th, 41st, 44th, 49th, 50tha 1.95 X 10"4 10 °C
which begins on page 148 of this issue, also discusses the confusion
53rd, 55-60th, 62nd 2.45 X 10“5 25 °C
about CaS04 and places it in the larger context of how aqueous
65th, 66th 3.73 X 10"5 25 °C
solution concepts should be taught in general chemistry.
67th (1986-1987) 7.1 X 10"5 25 °C
• No table of solubility products in 46th and 47th editions.
184 Journal of Chemical Education
You might also like
- University Grammar of English Workbook R A CloseDocument92 pagesUniversity Grammar of English Workbook R A CloseAndrea Mijatovic93% (15)
- Letters: Mystery Molecules or What's in A Name?Document2 pagesLetters: Mystery Molecules or What's in A Name?10CH1-25- Võ Lê Hoàn VũNo ratings yet
- What Is Special About Academic English?: A Everyday Words and Academic UsesDocument18 pagesWhat Is Special About Academic English?: A Everyday Words and Academic UsesDrago's channelNo ratings yet
- unit-1-2-vocabDocument4 pagesunit-1-2-vocabAn Nguyen Thai HongNo ratings yet
- University Chemistry Mahan Bruce H Journal of AcsDocument2 pagesUniversity Chemistry Mahan Bruce H Journal of Acshabib ahmed0% (3)
- Lesson Plan Format CouldDocument4 pagesLesson Plan Format CouldJUAN DAVID ABRIL PATARROYONo ratings yet
- Chemistry 1 Sylabus MGNDocument10 pagesChemistry 1 Sylabus MGNBarbie GuerreroNo ratings yet
- Module 4. Normality, PPM, And, PPBDocument3 pagesModule 4. Normality, PPM, And, PPBKenneth Roy MatuguinaNo ratings yet
- S S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateDocument7 pagesS S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateNic CarlsonNo ratings yet
- Grade 11 Daily Lesson LOGDocument11 pagesGrade 11 Daily Lesson LOGFudge FajardoNo ratings yet
- Video Explana On: Explana On:: Next Exit Review PreviousDocument50 pagesVideo Explana On: Explana On:: Next Exit Review PreviousCharlie GoyalNo ratings yet
- Kerr 1997 Using ConcordancesDocument3 pagesKerr 1997 Using ConcordancesClaudiaNo ratings yet
- Science 3 Module Q1Document41 pagesScience 3 Module Q1AKo Si NikKoNo ratings yet
- Dup Lesson Plan 2 1Document8 pagesDup Lesson Plan 2 1api-417232384No ratings yet
- Science Education Lesson Plan Format: NGSS Performance ExpectationDocument5 pagesScience Education Lesson Plan Format: NGSS Performance Expectationapi-532228090No ratings yet
- Rizal SyllabiDocument8 pagesRizal SyllabiLerramie Dela PeñaNo ratings yet
- 766-Beveridge - Alignment TemplateDocument10 pages766-Beveridge - Alignment Templateapi-379231471No ratings yet
- Q3 - Week 6 - March 4 - 8Document10 pagesQ3 - Week 6 - March 4 - 8Lucky RemosNo ratings yet
- Lab Manual Gen ChemDocument2 pagesLab Manual Gen Chemjpda2004No ratings yet
- Cuadernillo Senderos 6 U1 Leccion4Document25 pagesCuadernillo Senderos 6 U1 Leccion4Natalia Romero QNo ratings yet
- The Wonders of The World: ObjectivesDocument8 pagesThe Wonders of The World: ObjectivesJavier OrellanaNo ratings yet
- Comparison of WP 2 Portfolio Draft Copy of Writing Project 2 Submission DraftDocument4 pagesComparison of WP 2 Portfolio Draft Copy of Writing Project 2 Submission Draftapi-515447837No ratings yet
- Illuminating A Path For Organic Synthesis Towards SustainabilityDocument18 pagesIlluminating A Path For Organic Synthesis Towards Sustainabilitydanielsad100No ratings yet
- Eportfolio Case Standard 5Document6 pagesEportfolio Case Standard 5api-384664466No ratings yet
- Test Tube GeologyDocument2 pagesTest Tube GeologyJavier Andres Esteban MuñozNo ratings yet
- Colloid 4395323 PDFDocument8 pagesColloid 4395323 PDFDianaRoseAcupeadoNo ratings yet
- OPT B1 CLIL Units9-10 TNDocument1 pageOPT B1 CLIL Units9-10 TNAna Gabriela Cortès BarreraNo ratings yet
- Everyday or Academic Use Meaning Academic Use Meaning: Lesson 1. Academic Word 1.1. Everyday Words and Academic UsesDocument3 pagesEveryday or Academic Use Meaning Academic Use Meaning: Lesson 1. Academic Word 1.1. Everyday Words and Academic UsesThành HàNo ratings yet
- The Kite Runner Theme Analysis Lesson: PreparationDocument8 pagesThe Kite Runner Theme Analysis Lesson: PreparationHoàng Tâm LêNo ratings yet
- Vocab 10-15Document8 pagesVocab 10-15binbin19012008No ratings yet
- 2015 Using IL Threshold Concepts For Biology - Bees, Butterflies, and BeetlesDocument5 pages2015 Using IL Threshold Concepts For Biology - Bees, Butterflies, and BeetlesJavier PNo ratings yet
- Department of Education: Carpenito Integrated School Science 8-Second Quarter Performance TaskDocument5 pagesDepartment of Education: Carpenito Integrated School Science 8-Second Quarter Performance TaskGERRY CHEL LAURENTENo ratings yet
- Properties of Water Lesson Plan TemplateDocument5 pagesProperties of Water Lesson Plan Templateapi-529884372No ratings yet
- Science10 Q3 W6 D5Document18 pagesScience10 Q3 W6 D5Marl Rina EsperanzaNo ratings yet
- GE 109 Lesson 1 (Studying Rizal in The Classroom)Document6 pagesGE 109 Lesson 1 (Studying Rizal in The Classroom)Angel Cuacko GacmatanNo ratings yet
- The Concept of Sublimation - Iodine As An Example: Marina Stojanovska, Vladimir M. Petruševski, Bojan ŠoptrajanovDocument5 pagesThe Concept of Sublimation - Iodine As An Example: Marina Stojanovska, Vladimir M. Petruševski, Bojan ŠoptrajanovRezita RamadhaniNo ratings yet
- Second Languages in The Primary School TDocument14 pagesSecond Languages in The Primary School Tmonday bluesNo ratings yet
- Ecological Society of AmericaDocument6 pagesEcological Society of AmericalucasNo ratings yet
- TEACHING SCIENCE IN ELEMENTARY GRADES (Biology&chemistry)Document14 pagesTEACHING SCIENCE IN ELEMENTARY GRADES (Biology&chemistry)Jobelle NovesterasNo ratings yet
- Surviving Your Stupid Stupid Decision To Go To Grad School by Adam Ruben - ExcerptDocument31 pagesSurviving Your Stupid Stupid Decision To Go To Grad School by Adam Ruben - ExcerptCrown Publishing Group80% (10)
- Chemistry Lesson Plan 2Document6 pagesChemistry Lesson Plan 2api-550644778No ratings yet
- Org ChemDocument6 pagesOrg ChemBABYLEN BAHALANo ratings yet
- Lesson 2Document1 pageLesson 2api-450908456No ratings yet
- Lesson 3Document7 pagesLesson 3api-525586323No ratings yet
- Cambridge English Empower Empower C1 Reading Plus Teacher U09 WorksheetDocument2 pagesCambridge English Empower Empower C1 Reading Plus Teacher U09 WorksheetGERESNo ratings yet
- The Signature Pedagogy in Chemistry Education: September 2013Document6 pagesThe Signature Pedagogy in Chemistry Education: September 2013Nsikan SaturdayNo ratings yet
- Class Ix - Science PDFDocument15 pagesClass Ix - Science PDFDeborah YNo ratings yet
- Lyceum of Iligan FoundationDocument12 pagesLyceum of Iligan Foundation줄리엔ien7goNo ratings yet
- Ed073pa313 1Document3 pagesEd073pa313 1Noor FatimaNo ratings yet
- S S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateDocument7 pagesS S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateNic CarlsonNo ratings yet
- Lessonplan2 ChemicalreactionsteamobeygibbsDocument7 pagesLessonplan2 Chemicalreactionsteamobeygibbsapi-352579919No ratings yet
- Ed061pa208 1Document1 pageEd061pa208 1MisaelNo ratings yet
- S S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateDocument7 pagesS S C P Science L P T: Ingle Ubject Redential Rogram Esson LAN EmplateNic CarlsonNo ratings yet
- Personal Development 1 July 8 and 10 WEEK 3Document4 pagesPersonal Development 1 July 8 and 10 WEEK 3Jessa De JesusNo ratings yet
- Academic Essay WritingDocument26 pagesAcademic Essay WritingFahad Areeb100% (1)
- Building Comprehension - Grade 5: High-Interest ReadingFrom EverandBuilding Comprehension - Grade 5: High-Interest ReadingRating: 5 out of 5 stars5/5 (1)
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- Ed066p853 2Document1 pageEd066p853 2Aitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- For Conceptualization The Franck-Condon PrincipleDocument1 pageFor Conceptualization The Franck-Condon PrincipleAitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Appendix A - Conversion From Molar To Molal: PL PLDocument57 pagesAppendix A - Conversion From Molar To Molal: PL PLAitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
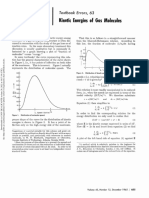
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Acs Joc 8b00707Document14 pagesAcs Joc 8b00707Aitor PastorNo ratings yet
- Microscale Preparation of Alcl3 Journal of ChemicaDocument2 pagesMicroscale Preparation of Alcl3 Journal of ChemicaAitor PastorNo ratings yet
- Kinetics: Electrode ProcessesDocument8 pagesKinetics: Electrode ProcessesAitor PastorNo ratings yet
- The Multistep: Is Rate-Limiting ofDocument5 pagesThe Multistep: Is Rate-Limiting ofAitor PastorNo ratings yet
- Textbook: ForumDocument5 pagesTextbook: ForumAitor PastorNo ratings yet
- Lecture Notes On Thermodynamics. Joseph M. PowersDocument383 pagesLecture Notes On Thermodynamics. Joseph M. PowersGustavo CoronelNo ratings yet
- Peter Atkins Julio de Paula Physical Chemistry 1 - 180-205-1-11Document11 pagesPeter Atkins Julio de Paula Physical Chemistry 1 - 180-205-1-11Akbar Hidayatullah ZainiNo ratings yet
- Pulse Tube Cryocooler-SeminarDocument26 pagesPulse Tube Cryocooler-SeminarUtsav RaoNo ratings yet
- Study On ePTFE With Poros and MorphologicalDocument8 pagesStudy On ePTFE With Poros and MorphologicalJay KumarNo ratings yet
- MCQ - Class 9 - Atoms and MoleculesDocument28 pagesMCQ - Class 9 - Atoms and Moleculesget2maniNo ratings yet
- Exergy Analysis of Energy Systems PDFDocument15 pagesExergy Analysis of Energy Systems PDFGrecia SuffoNo ratings yet
- 8 and 18 Revision Test MsDocument7 pages8 and 18 Revision Test MsTrần Thị Diễm HươngNo ratings yet
- C3abmb000027 (26P)Document1 pageC3abmb000027 (26P)Smith VelásquezNo ratings yet
- 14.3 ClassworkDocument3 pages14.3 Classworkisabe;llaNo ratings yet
- Shape Control of Ag Shell Growth On Au NanodisksDocument4 pagesShape Control of Ag Shell Growth On Au NanodisksAdrianoDSNo ratings yet
- J Jpowsour 2006 02 003Document4 pagesJ Jpowsour 2006 02 003Customer Tech Support / Product Development Sales/CCT Dept. AECPLNo ratings yet
- June 2018 MS - Paper 1 OCR (A) Chemistry AS-LevelDocument18 pagesJune 2018 MS - Paper 1 OCR (A) Chemistry AS-LevelRunNo ratings yet
- Reheat Cycle: Schematic DiagramDocument2 pagesReheat Cycle: Schematic DiagramDave BalladoNo ratings yet
- Ap Chemistry Midterm: Section 1 Multiple Choice Questions 75 Questions 50% of Total GradeDocument15 pagesAp Chemistry Midterm: Section 1 Multiple Choice Questions 75 Questions 50% of Total Grade소피아No ratings yet
- Diffusion Rate of Dye Using Different Types of LiquidDocument14 pagesDiffusion Rate of Dye Using Different Types of LiquidTroy SparksNo ratings yet
- Ferroelectricity: Spontaneous Dipole MomentDocument24 pagesFerroelectricity: Spontaneous Dipole Momentaliazab100% (2)
- Class 11 Chemistry Revision Notes Some Basic Concepts of ChemistryDocument15 pagesClass 11 Chemistry Revision Notes Some Basic Concepts of ChemistryPriyanshuNo ratings yet
- 2011-04-26 - 05-10-27-PM - Iit Jee ChemistryDocument21 pages2011-04-26 - 05-10-27-PM - Iit Jee ChemistryemmaNo ratings yet
- Metal SolubilityDocument30 pagesMetal Solubilityarvin4dNo ratings yet
- Manfacture OF: Cyclo HexaneDocument91 pagesManfacture OF: Cyclo HexaneNikhil Kumar Chennuri100% (4)
- Acid Base BalanceDocument21 pagesAcid Base BalancevampirekawaiiNo ratings yet
- Analysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationDocument15 pagesAnalysis of Aspirin - Infrared (Ir) Spectroscopy and Melting Point DeterminationMahmoud ElshahawyNo ratings yet
- Material Balance Applied To Oil ReservoirsDocument25 pagesMaterial Balance Applied To Oil ReservoirsHamaamNo ratings yet
- SS2 Chemistry (2nd Term)Document7 pagesSS2 Chemistry (2nd Term)kazosky4topNo ratings yet
- Total Internal Reflection and LensesDocument69 pagesTotal Internal Reflection and LensesRodel VerzosaNo ratings yet
- Rate Law Worksheet PDFDocument3 pagesRate Law Worksheet PDFJunghoon Lee100% (1)
- Quantum Mechanics II - Homework 2Document6 pagesQuantum Mechanics II - Homework 2Ale GomezNo ratings yet
- NEET 11 PT 2 SolutionDocument12 pagesNEET 11 PT 2 SolutionPriya SuriyakumarNo ratings yet
- Student-Energy Balance PDFDocument15 pagesStudent-Energy Balance PDFAdeniran JoshuaNo ratings yet
- Pharmaceutical Applications of Hot Melt Extrusion Part IDocument19 pagesPharmaceutical Applications of Hot Melt Extrusion Part ITabare CostaNo ratings yet