Professional Documents
Culture Documents
10.1055@s 0039 1693992
10.1055@s 0039 1693992
Uploaded by
Claudia Sughey Herrera PalominoCopyright:
Available Formats
You might also like
- Gerd, Peptic Ulcer, Ulcerative Colitis, Crohn's DiseaseDocument11 pagesGerd, Peptic Ulcer, Ulcerative Colitis, Crohn's DiseasekamipimaNo ratings yet
- Surgery OsceDocument69 pagesSurgery OsceRebecca BrandonNo ratings yet
- Jung 2014Document8 pagesJung 2014Fabrizzio BardalesNo ratings yet
- Annrcse01521 0053Document1 pageAnnrcse01521 0053Muhammad Fiqhi HardiansyahNo ratings yet
- Alargamiento Del Complejo Gastronemio SoleoDocument8 pagesAlargamiento Del Complejo Gastronemio SoleoC Martin TraumatoNo ratings yet
- Usg 17050Document7 pagesUsg 17050Amit Kumar RanoNo ratings yet
- JTD 06 S3Document8 pagesJTD 06 S3DH SiriruiNo ratings yet
- A Rare Variant Case of Pure Esophageal Atresia With An Atretic SegmentDocument3 pagesA Rare Variant Case of Pure Esophageal Atresia With An Atretic SegmentDetry Kala'lembangNo ratings yet
- Kunio Et Al. - 2015 - Short EsophagusDocument12 pagesKunio Et Al. - 2015 - Short EsophagusDaniel PredaNo ratings yet
- Imaging LGEADocument10 pagesImaging LGEANuanSyafrinaNo ratings yet
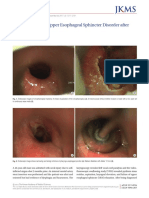
- Dysphagia Due To Upper Esophageal Sphincter DisordDocument3 pagesDysphagia Due To Upper Esophageal Sphincter DisordEustakia Rini Kartika DewiNo ratings yet
- 1 s2.0 S0016510711024308 MainDocument6 pages1 s2.0 S0016510711024308 Mainpainkiller78No ratings yet
- Cutaneous Needle Aspirations in Liver DiseaseDocument6 pagesCutaneous Needle Aspirations in Liver DiseaseRhian BrimbleNo ratings yet
- Clinical Anatomy - 2020 - Le Saint Grant - Arterial Anatomy of The Anterior Abdominal Wall Ultrasound Evaluation As ADocument6 pagesClinical Anatomy - 2020 - Le Saint Grant - Arterial Anatomy of The Anterior Abdominal Wall Ultrasound Evaluation As AarifNo ratings yet
- Role of Open Surgical Drainage or Aspiration in Management of Amoebic Liver AbscessDocument9 pagesRole of Open Surgical Drainage or Aspiration in Management of Amoebic Liver AbscessIJAR JOURNALNo ratings yet
- Update On Foregut Molecular Embryology and Role of Regenerative Medicine TherapiesDocument9 pagesUpdate On Foregut Molecular Embryology and Role of Regenerative Medicine TherapiesmikeNo ratings yet
- Poh 2011Document8 pagesPoh 2011DH SiriruiNo ratings yet
- What Proportion of Children With Complex Oesophageal Atresia Require Oesophageal Lengthening ProceduresDocument5 pagesWhat Proportion of Children With Complex Oesophageal Atresia Require Oesophageal Lengthening ProceduresGunduz AgaNo ratings yet
- PuIpal and Periapical Tissue Reactions AfterDocument4 pagesPuIpal and Periapical Tissue Reactions AfterANGEL ADALBERTO GALVAN GARCIANo ratings yet
- Ultrasonographic Atlas of Splenic LesionsDocument14 pagesUltrasonographic Atlas of Splenic LesionsFlavio RojasNo ratings yet
- 1 s2.0 S0039610903001191 MainDocument15 pages1 s2.0 S0039610903001191 MainJuan Jos� Beltr�n Hern�ndezNo ratings yet
- Proposal New - 060952Document33 pagesProposal New - 060952sommylydiiNo ratings yet
- Synthetic Versus Biological Mesh in Laparoscopic and Open Ventral Hérnia RepairDocument9 pagesSynthetic Versus Biological Mesh in Laparoscopic and Open Ventral Hérnia RepairRhuan AntonioNo ratings yet
- Ureteral ReconstructionDocument6 pagesUreteral ReconstructiongumNo ratings yet
- Leiomyoma of The Esophagus: Open Versus Thoracoscopic EnucleationDocument5 pagesLeiomyoma of The Esophagus: Open Versus Thoracoscopic EnucleationYacine Tarik AizelNo ratings yet
- Clinical Study of Bowel ObstructionDocument6 pagesClinical Study of Bowel ObstructionhorzhanNo ratings yet
- Liver Abscess: Catheter Drainage V/s Needle AspirationDocument6 pagesLiver Abscess: Catheter Drainage V/s Needle AspirationMishel Rodriguez GuzmanNo ratings yet
- KumarasingheDocument4 pagesKumarasingheVitta Kusma WijayaNo ratings yet
- Artigo 5Document8 pagesArtigo 5doctorbanNo ratings yet
- Scholars Journal of Medical Case Reports: ISSN 2347-6559 (Online) ISSN 2347-9507 (Print)Document2 pagesScholars Journal of Medical Case Reports: ISSN 2347-6559 (Online) ISSN 2347-9507 (Print)galihmuchlishermawanNo ratings yet
- Ureteric-Urethral Engraftment As A New Surgical Technique For Management of Incontinence in Bladder Exstrophy Complex A Retrospective CohortDocument6 pagesUreteric-Urethral Engraftment As A New Surgical Technique For Management of Incontinence in Bladder Exstrophy Complex A Retrospective CohortJad DegheiliNo ratings yet
- Abdalla 2017Document3 pagesAbdalla 2017dewaprasatyaNo ratings yet
- Abdominal Wall Hernia ThesisDocument7 pagesAbdominal Wall Hernia Thesisaflpaftaofqtoa100% (2)
- Testicular BloodDocument6 pagesTesticular Bloodsalcor00No ratings yet
- (2020) Colonography Evaluation of The Relationship Between Colon Anatomy and DiverticulaDocument7 pages(2020) Colonography Evaluation of The Relationship Between Colon Anatomy and DiverticulaChristian ToalongoNo ratings yet
- A New Method For The Removal of Safety Pins Ingested by ChildrenDocument2 pagesA New Method For The Removal of Safety Pins Ingested by ChildrenAnusha SainiNo ratings yet
- Elipse Balloon The Pitfalls of Excessive SimplicityDocument3 pagesElipse Balloon The Pitfalls of Excessive SimplicityTony Miguel Saba SabaNo ratings yet
- Manavella 2018Document10 pagesManavella 2018dimiz77No ratings yet
- Comparison of Flank and Midline Approaches To OvarDocument6 pagesComparison of Flank and Midline Approaches To Ovartrisnaput3No ratings yet
- Bove-Chapelle ViscMobilizAdhesionRatDocument7 pagesBove-Chapelle ViscMobilizAdhesionRatQuiroprácticaParaTodosNo ratings yet
- Anatomy. Eight Edition. Saint Louis: Mosby.: Ped PDFDocument10 pagesAnatomy. Eight Edition. Saint Louis: Mosby.: Ped PDFannisa ussalihahNo ratings yet
- A Dental Nightmare, Resolved - What A Radiologist Needs To Know When Consulted About Ingestion of Dental Foreign Body Material - PMCDocument17 pagesA Dental Nightmare, Resolved - What A Radiologist Needs To Know When Consulted About Ingestion of Dental Foreign Body Material - PMCsj5h4vwxxfNo ratings yet
- Treatment For Failed Epispadias Repair Presenting in AdultsDocument6 pagesTreatment For Failed Epispadias Repair Presenting in AdultsDhonz R AdiwaramanNo ratings yet
- Figures 1Document8 pagesFigures 1Arman KozhakhmetNo ratings yet
- Gastrointestinal Emergency in NeonatesDocument15 pagesGastrointestinal Emergency in NeonatesGuillermo Andrés AriasNo ratings yet
- Effects of Tracheostomy Tube On SwallowingDocument21 pagesEffects of Tracheostomy Tube On SwallowingPaola A. MartinezNo ratings yet
- Role of Endoscopic Stent Insertion On Managmeent of Gastric Twist After Sleeve GastrectomyDocument6 pagesRole of Endoscopic Stent Insertion On Managmeent of Gastric Twist After Sleeve GastrectomyJorge SalazarNo ratings yet
- Undescended TestisDocument3 pagesUndescended TestisIoannis ValioulisNo ratings yet
- Managemen Esofagitis Corosive 1Document6 pagesManagemen Esofagitis Corosive 1yunia chairunnisaNo ratings yet
- Journal of Pediatric Surgery: Shabnam Sabetkish, Nastaran Sabetkish, Abdol-Mohammad KajbafzadehDocument7 pagesJournal of Pediatric Surgery: Shabnam Sabetkish, Nastaran Sabetkish, Abdol-Mohammad KajbafzadehAmatystNo ratings yet
- Hum. Reprod.-2005-Remorgida-2317-20Document4 pagesHum. Reprod.-2005-Remorgida-2317-20lediayukimeNo ratings yet
- Massive Congenital Lymphatic Malformation of The SM - 2013 - Journal of PediatriDocument3 pagesMassive Congenital Lymphatic Malformation of The SM - 2013 - Journal of PediatriGunduz AgaNo ratings yet
- Pi Is 0022534714035575Document2 pagesPi Is 0022534714035575Andri Feisal NasutionNo ratings yet
- Complete Penile DisassemblyDocument2 pagesComplete Penile DisassemblyGunduz AgaNo ratings yet
- ABRAVAS 5 de 9 - Hernandez Divers 2007 Liver BXDocument5 pagesABRAVAS 5 de 9 - Hernandez Divers 2007 Liver BXCamilo SantanderNo ratings yet
- OHT Flank ApproachDocument6 pagesOHT Flank ApproachMarinaJiménezNo ratings yet
- Cannulation Attempts and The Development of Post EDocument2 pagesCannulation Attempts and The Development of Post EMarcio MullerNo ratings yet
- Ostomia Intestinal PDFDocument7 pagesOstomia Intestinal PDFDaniela Pérez LópezNo ratings yet
- Prosthetic Repair of Acutely Incarcerated Groin Hernias: A Prospective Clinical Observational Cohort StudyDocument6 pagesProsthetic Repair of Acutely Incarcerated Groin Hernias: A Prospective Clinical Observational Cohort Studynh2411No ratings yet
- Laparoscopic Extraperitoneal Resection of Urachal Cyst: Sun-Il Lee, M.D., Sung-Soo Kim, M.DDocument3 pagesLaparoscopic Extraperitoneal Resection of Urachal Cyst: Sun-Il Lee, M.D., Sung-Soo Kim, M.DNurul Rezki Fitriani AzisNo ratings yet
- Punção No Hipocôndrio Esquerdo Com Agulha de Veress para A Criação Do PneumoperitônioDocument8 pagesPunção No Hipocôndrio Esquerdo Com Agulha de Veress para A Criação Do PneumoperitônioSINAN SHAWKATNo ratings yet
- Esophageal Preservation and Replacement in ChildrenFrom EverandEsophageal Preservation and Replacement in ChildrenAshwin PimpalwarNo ratings yet
- Chapter 1Document20 pagesChapter 1luiperdvrouNo ratings yet
- 2015 Management of Ingested Foreign Bodies in Children - A Clinical Report of The NASPGHAN Endoscopy CommitteeDocument13 pages2015 Management of Ingested Foreign Bodies in Children - A Clinical Report of The NASPGHAN Endoscopy CommitteeCarlos CuadrosNo ratings yet
- PREP ICU 2013 Answers and Critiques - 1 - Jan & FebDocument47 pagesPREP ICU 2013 Answers and Critiques - 1 - Jan & FebNicholasHuffNo ratings yet
- Turtle Manual English PDFDocument25 pagesTurtle Manual English PDFDulce AmorNo ratings yet
- 00 GIT NotesDocument81 pages00 GIT NotesHythem Hashim100% (1)
- Molluscan Studies: Journal ofDocument19 pagesMolluscan Studies: Journal ofWidi SetyogatiNo ratings yet
- Vascular ImpressionsDocument3 pagesVascular ImpressionsfiameliaaNo ratings yet
- Lecture7 V SemesterDocument28 pagesLecture7 V SemestersreelekshmiNo ratings yet
- Diagnosis and Management of Functional HeartburnDocument9 pagesDiagnosis and Management of Functional Heartburnal ghiffari muhammad rayhanNo ratings yet
- Fisiologi Menelan Dan Keluarnya Suara1Document27 pagesFisiologi Menelan Dan Keluarnya Suara1Verani Citra DeviNo ratings yet
- GI History IntroductoryDocument74 pagesGI History IntroductoryNur Hamizah Md FuziNo ratings yet
- PATH-261 (SPO) L2SII Theory (Full)Document174 pagesPATH-261 (SPO) L2SII Theory (Full)Shakil MahmodNo ratings yet
- TOPNOTCH Diagnostic Exam ANSWER KEY September 2018Document20 pagesTOPNOTCH Diagnostic Exam ANSWER KEY September 2018CDNo ratings yet
- Mila Amor V. Reyes, MD, FPSP Anatomic and Clinical PathologistDocument41 pagesMila Amor V. Reyes, MD, FPSP Anatomic and Clinical PathologistFranco Rommel ReyesNo ratings yet
- BiologyDocument249 pagesBiologysagarNo ratings yet
- Black S Medical Dictionary PDFDocument2,413 pagesBlack S Medical Dictionary PDFAlexandr Trotsky100% (1)
- CorrosivesDocument25 pagesCorrosivesahmed.farag.ali2020No ratings yet
- 9 Digestive System Part 1Document14 pages9 Digestive System Part 1Fhayee Sulaik HaronNo ratings yet
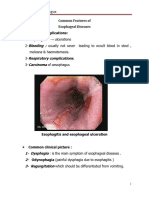
- Common Features of Diseases of EsophagusDocument4 pagesCommon Features of Diseases of EsophagusSri PoopaseNo ratings yet
- Anatomi Fisiologi Sistem PencernaanDocument38 pagesAnatomi Fisiologi Sistem Pencernaanyo2_laxanaNo ratings yet
- Gastro-Esophageal Reflux Disease (GERD) : Prof DRDocument21 pagesGastro-Esophageal Reflux Disease (GERD) : Prof DRmohammed salahNo ratings yet
- Am.J.PharmTech Res. 2020 10 (01), Formulation and Evaluation of Rabeprazole Sodium DelayedDocument14 pagesAm.J.PharmTech Res. 2020 10 (01), Formulation and Evaluation of Rabeprazole Sodium DelayedThu Huyền TrầnNo ratings yet
- نم انوسنت لا مكوجرأ ,يلهلا و يل ةيراج ةقدص مكئاعد حلا ص Please Grace us with your good prayersDocument538 pagesنم انوسنت لا مكوجرأ ,يلهلا و يل ةيراج ةقدص مكئاعد حلا ص Please Grace us with your good prayersSahan EpitawalaNo ratings yet
- Anatomy Ii: by Dr. Ziyad M. Al Zeer Orthopedic Surgeon Assistant Professor MD - PHDDocument64 pagesAnatomy Ii: by Dr. Ziyad M. Al Zeer Orthopedic Surgeon Assistant Professor MD - PHDMOHAMMAD ALSWEITYNo ratings yet
- Visceral OMT: AAO Convocation March 2018 Kenneth Lossing DODocument59 pagesVisceral OMT: AAO Convocation March 2018 Kenneth Lossing DODiana SchlittlerNo ratings yet
- Band Igation AccessoryDocument4 pagesBand Igation AccessoryFady Maher WadeaNo ratings yet
- Nausea and Vomiting: William F - MauleDocument2 pagesNausea and Vomiting: William F - Mauleडा. सत्यदेव त्यागी आर्यNo ratings yet
- Surgical Notes PDFDocument118 pagesSurgical Notes PDFRebecca Wong100% (1)
10.1055@s 0039 1693992
10.1055@s 0039 1693992
Uploaded by
Claudia Sughey Herrera PalominoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
10.1055@s 0039 1693992
10.1055@s 0039 1693992
Uploaded by
Claudia Sughey Herrera PalominoCopyright:
Available Formats
Review Article
Esophageal Atresia: State of the Art in Translating
Experimental Research to the Bedside
Nicholas E. Bruns1 Ian C. Glenn1 Todd A. Ponsky2
1 Department of General Surgery, Cleveland Clinic, Cleveland, Address for correspondence Nicholas E. Bruns, MD, Department of
Ohio, United States General Surgery, Cleveland Clinic, 9500 Euclid Avenue, Cleveland,
2 Division of Pediatric General and Thoracic Surgery, Cincinnati OH 44195-5243, United States (e-mail: nickebruns@gmail.com).
Children’s Hospital, Cincinnati, Ohio, United States
Eur J Pediatr Surg
Abstract Long-gap esophageal atresia is one of the most challenging diseases in the field of
Keywords pediatric surgery. There is no optimal therapy, and thus many potential therapies and
► esophageal atresia techniques are being actively explored, both in animal models and in neonates. This
► tracheoesophageal article will review the available experimental treatment options with a focus on novel
fistula techniques.
Downloaded by: Boston University. Copyrighted material.
► esophageal
lengthening
► esophageal
replacement
Introduction considering current mainstream therapies. This article will
focus on discussing research avenues in regard to treatment
Esophageal atresia (EA) is a congenital disorder in which the of pure EA.
esophagus is in discontinuity. EA and tracheoesophageal The American Pediatric Surgery Association released
fistula (TEF) have multiple configurations and are believed guidelines in 2019 which recommended delayed repair for
to be a spectrum of anomalies. There are multiple classifica- treatment of long-gap EA.3 Surgical treatment of pure EA
tion systems, but for the purposes of this article, descriptive usually starts with laryngoscopy to assess for a proximal TEF,
names will be used to prevent confusion. The incidence of EA gastrostomy tube placement, and delayed reconstruction.
is estimated to be 3.5 in 10,000 live births.1 EA without TEF With delay of up to several months, the gap size has been
(pure EA, gross type A) accounts for 7% of EA.2 Typically, the shown to decrease.5 Numerous techniques have been de-
upper (proximal) esophageal pouch is dilated and terminates scribed for lengthening, anastomosing, or reconstructing the
at the level of the azygous vein and the distal pouch is esophagus (►Table 1). This article will discuss novel techni-
frequently short, making primary anastomosis difficult. ques for dealing with long-gap EA that have been employed
There is no consensus for the definition of a long gap, but in animals and/or humans, with an emphasis on techniques
criteria have been proposed using measurements in centi- that have evolved in the last 10 to 20 years. Techniques such
meters, number of vertebral bodies, or merely the inability to as bougienage, electromagnetic bougienage, myotomy,
perform an anastomosis.3 Surgical repair has been associated thread-and-olive technique, and extrathoracic esophageal
with a leak rate of 16% and a stricture rate of 35%.4 As long- elongation (EEE) will not be discussed in great detail in
gap EA is the most challenging subtype of EA, it represents this article.6–9
the subtype with the most room for improvement when
Animal Model
Nicholas E. Bruns's ORCID is https://orcid.org/0000-0001-9607- Numerous animal models have been used to study the
0633. esophagus for the purpose of investigating novel techniques
received © Georg Thieme Verlag KG DOI https://doi.org/
June 20, 2019 Stuttgart · New York 10.1055/s-0039-1693992.
accepted after revision ISSN 0939-7248.
June 25, 2019
Esophageal Atresia Bruns et al.
Table 1 Nonoperative and operative techniques for long-gap esophageal atresia
Technique Study population Author
Lengthening for delayed anastomosis
Botulinum toxin A injection Pig,23 human,24 rabbit15 Larsen et al,23 Ellebæk et al,24 Usui and Ono15
Bougienage Human Hays et al,20 Mahour et al,21 de Lorimier and Harrison6
Magnetic bougienage Human Hendren and Hale7,49
Traction sutures Human Foker et al,25,26 van der Zee et al28
Extrathoracic elongation Human Kimura and Soper29
Lower pouch hydrostatic distention Human Vogel et al31
Bioabsorbable spring Pig Sullins et al17
Lengthening and immediate anastomosis
Transluminal thread with olives Human Rehbein and Schweder,33 Sauer and Kurz8
Magnetic compression anastomosis Human,22,34,35 Pig10 Zaritzky et al,22,34 Ellebaek et al,35 Bruns et al10
Esophageal replacement
Colon Human German and Waterston,42 Esteves et al43
Stomach Human Heimlich and Winfield,40 Anderson and Randolph,41
Spitz,37 Spitz et al,38 Ure et al39
Downloaded by: Boston University. Copyrighted material.
Small bowel Human Ring et al,44 Bax and Van Renterghem,45
Bax and van der Zee,46 Saitua et al47
Scaffold Rat,16 pig11 Urbani et al,16 Jensen et al11
for EA. These include pig,10–14 rabbit,12,15 and rat.16 In our gienage in which over a 2- to 10-week period combined with
experience, we found the pig to be an optimal model due to intraoperative circular myotomy, a successful anastomosis,
the ease of handling, anatomic similarity to humans, and was achieved.6
downward facing snout to reduce aspiration risk.12 As aspi-
ration can be a major issue impacting the survival models Electromagnetic Bougienage
when facing esophageal discontinuity, there have been mul- In 1975, Hendren and Hale reported the use of electromag-
tiple attempts at maintaining a form of esophageal continui- netic bougienage in two infants to obtain esophageal length-
ty. These include a bifurcated esophageal model,13 ening and delayed primary anastomosis.7 The concept of
esophageal bypass loop,14 and creation of a distal esophageal combining this technique with magnetic compression anas-
pouch in parallel to a esophagogastric anastomosis.17 tomosis was not performed until Zaritzky et al did so in 2009,
which will be discussed later in this article.22
Mechanical Lengthening Techniques
With the exception of the bioengineered scaffolds and inter- Botulinum Toxin A (BoTox) Injection
position grafts, all techniques rely on esophageal stretch. The It has been postulated that by inducing muscle relaxation
effect of stretch at the microscopic and/or molecular level is with intramural botulinum toxin A (BoTox) injection, esoph-
poorly understood. When evaluated in rats, increased esoph- ageal tissue could significantly elongate with stretch. Larsen
ageal tension led to increased cholinergic responses in et al studied the effect of on esophageal lengthening as a
smooth muscle as well as increased electrical response in potential adjunct for treatment of long-gap EA.23 Twenty-
skeletal muscle and decreased relaxation to serotonin, indi- four piglets were randomized to intramural esophageal
cating impaired motility.18 In a separate study of esophageal BoTox injection or saline injection. One hour later, the
stretching in rats, histology showed thinning of the mucosa esophagus was removed en bloc and analyzed in a stretch-
and muscle when compared with the control.19 There was tension device. There was significantly more esophageal
uniform elongation and increased cell proliferation as indi- elongation in the test group (84%) than in the control group
cated by an increased Ki-67 positive ratio. (65%).
Usui and Ono published the use of a series of intramural
Bougienage esophageal BoTox injection to promote decreased tension on
Preoperative bougienage has been described by several rabbits.15 Twenty rabbits had a 1.5-cm cervical esophageal
authors.6,20,21 Proximal bougienage was described by Hays resection followed by immediate esophageal anastomosis
et al and Mahour et al. De Lorimier and Harrison are credited with injection of BoTox (experimental group) or saline
with the first description of both proximal and distal bou- (control group). Of the six rabbits in each group that
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
ness of the muscularis propria, mucosa, and submucosa in
both the upper and lower pouches.27
More recently, van der Zee et al applied thoracoscopy to
this technique in 10 children with successful anastomosis in
eight children in 2015.28 Specifically, the technique included
thoracoscopic mobilization of both esophageal pouches and
placement of transthoracic traction sutures on both esoph-
ageal ends (►Fig. 2). In the two failures, one patient had no
esophageal growth initially, and the traction sutures pulled
through the distal esophagus. They ultimately underwent
jejunal interposition. The other patient had two perforations
related to Replogle tube insertion and underwent gastric
pull-up. Beyond the introduction of thoracoscopy, there has
Fig. 1 Traction suture technique. (Reproduced with permission from been no further revision to this technique. As indicated by
Foker et al. 26)
this later experience, there may be issues with controlling
tension and prevention of sutures pulling through resulting
survived, there was a lower rate of anastomotic stricture and in esophageal perforation.
fibrosis in the BoTox group. There was no difference in wall
thickness and no muscle fracturing. The authors concluded Extrathoracic Esophageal Elongation
that BoTox may have contributed to better anastomotic In 1994, Kimura and Soper described a single case of multi-
stage EEE.29 The procedure consisted of proximal esopha-
Downloaded by: Boston University. Copyrighted material.
healing due to less tension.
Transmural BoTox injection has been applied to a single gostomy and gastrostomy tube followed by serial elongations
child with long-gap EA with success.24 These above studies of the esophagostomy down the chest wall until, ultimately, a
suggest that there is need for ongoing clinical trial in humans mediastinal esophago-esophageal anastomosis could be per-
to elicit if these findings in animal models will pan out to formed. In the first patient, there were satisfactory results. In
measurable outcomes in neonates. the largest published series of EEE which included 15
patients, 12 were successfully completed. Of those, two
Traction Sutures developed esophageal dysmotility and five developed gas-
In 1997, Foker et al described a technique of using internal troesophageal reflux.30 Since the original description, there
and/or external traction sutures through a thoracotomy to has been little modification to the technique.
obtain primary anastomosis in four patients (►Fig. 1).25 They
subsequently published a series of 63 consecutive children Lower Pouch Hydrostatic Distention
with long-gap EA over a 20-year period.26 The results were Mechanical elongation of the distal pouch has been explored
promising but have been difficult to reproduce. High-reso- as a potential area of intervention. Vogel et al in 2006
lution ultrasound was later used to compare the effects of described a technique to stimulate distal pouch growth via
traction on esophageal structure in EA. By comparing 15 intermittent pressurization by means of a surgically placed
children who required traction to obtain primary anastomo- indwelling balloon catheter in a neonate (►Fig. 3).31 The
sis versus eight children who did not require traction, it was patient ultimately had a successful anastomosis and good
found that there was no significant difference in the thick- long-term outcome. There have been no further published
Fig. 2 Thoracoscopic traction suture technique. (Reproduced with permission from van der Zee et al. 28)
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
device was secured inside the distal pouch (►Fig. 4). After
4 weeks, the distal pouch length increased from 1.9 to
4.5 cm. When comparing lengthened to native esophagus,
there was no difference in thickness of muscularis mucosa or
muscularis propria, or in the number of myenteric or sub-
mucosal ganglia. Lengthened esophagus showed mild to
moderate superficial inflammation and fibrosis. Remaining
unanswered questions include the function the of the
stretched distal pouch and whether the histological findings
will have any clinical significance.
Combined Lengthening and Anastomotic Techniques
Transluminal Thread and Olive
First described by Rehbein and Schweder, this technique
used tension from a thread and olive to stretch the esophagus
together and ultimately perform pressure-induced anasto-
mosis.33 The thread was passed endoscopically (anterograde
through to oropharynx and retrograde from a gastrostomy),
which had potential as a minimally invasive technique.
Downloaded by: Boston University. Copyrighted material.
Fig. 3 Lower pouch hydrostatic distention technique for elongation However, issues included pulling through of the device and
of the distal pouch. (Reproduced with permission from Vogel et al. 31) bronchial injury during endoscopic insertion of the thread,
and this technique has been largely abandoned.8
trials with this technique. It would be valuable to compare
against a control group to elicit the efficacy of this therapy. Magnetic Compression Anastomosis
Magnetic techniques have been proposed because the mag-
Spring nets can achieve esophageal elongation as well as anastomo-
Bioabsorbable springs have been implemented in experi- sis formation. A theoretical benefit of a sutureless, magnetic
ments to lengthen the bowel as a potential therapy for short compression anastomosis is that since it leaves no foreign
bowel syndrome through the process of distraction entero- material, it could have an improved leak or stricture rate
genesis.32 Sullins et al described a technique of attaching a compared with conventional sutured anastomosis. Other
biodegradable polycaprolactone spring device in the distal than the need for gastrostomy tube placement, these tech-
pouch as a potential therapy for long-gap EA.17 In six mini- niques can avoid the morbidity of thoracic surgery.
pigs, the distal esophagus was transected 2 cm proximal to Zaritzky et al described 9 children, 6 with pure EA and 3
the gastro-esophageal junction and the proximal pouch was with EA with distal TEF with prior surgical ligation of the
anastomosed to the stomach to restore continuity. The spring TEF, who were treated with magnetic compression
Fig. 4 Bioabsorbable spring technique for elongation of the distal pouch with pre- (A) and postprocedural (B) images. (Reproduced with
permission from Sullins et al. 17)
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
Downloaded by: Boston University. Copyrighted material.
Fig. 5 Magnetic compression anastomosis technique using catheter-based magnets (A) inserted anterograde and retrograde (B and C).
(Reproduced with permission from Zaritzky et al. 34)
anastomosis.34 Catheter-based bullet-shaped magnets with The Magnamosis device consists of two donut-shaped rings
5-mm diameter (Cook Medical, Winston-Salem, North Car- (Harrison rings) constructed with rare earth neodymium–
olina, United States) were used with the proximal catheter iron–boron ring magnets encased in medical grade polycar-
introduced through the oropharynx and the distal catheter bonate (►Fig. 6). In this study, three anastomoses were
through a gastrostomy (►Fig. 5). Eight of these patients formed in five pigs. The study served as proof of concept of
developed strictures requiring endoscopic dilations, one of forming an esophageal anastomosis and did not use a true EA
whom required stenting and another required surgical model with a long gap, removing the potential lengthening
revision. Of the six patients with long-term follow-up, ability from that trial. More investigation is required to
two have gastroesophageal reflux and three have esoph- determine the elongation ability of the device.
ageal dysmotility. Ellebaek et al provided a description of a For magnetic techniques to advance as a more popular
similar technique in a 2-month-old child with pure EA who therapy, a design must be developed that leads to less
had already undergone gastrostomy tube and waited until stricturing than currently available devices.
the gap was 5 mm.35 Magnetic compression anastomosis
was performed with similar 5-mm cylindrical magnets
Esophageal Replacement Techniques
attached to catheters that were aligned under fluoroscopy.
The anastomosis was formed in 5 days. The patient devel- Stomach
oped a stricture requiring balloon dilation. Gastric transposition is currently the most common tech-
Our group described an experience using the Magnamosis nique for esophageal replacement, likely due to its technical
device to form an anastomosis in a live porcine esophagus.10 ease, single anastomosis, and robust vascular supply.36 Spitz
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
Small Bowel
Jejunal and ileal interposition grafts are the most technically
difficult procedures but may have the best long-term func-
tional outcomes.36 Ring et al described a series of staged
jejunal interpositions performed in 32 children in 1982.44
There were no reported failures of the graft to reach the
proximal pouch, no graft loss, and no mortalities. In 2007,
Bax et al published a series of 24 children who underwent
jejunal pedicle grafts as well as a case report of ileal pedicle
graft.45,46 Free jejunal graft has also been described47 in a
single patient.
Bioengineered Scaffolds
Tissue engineering is a novel method that may show promise
in EA management by stimulating the body’s intrinsic ability
to regenerate esophagus. Urbani et al described a layered
esophagus graft derived from acellular rat esophagus.16 By
combining a decellularized scaffold with patient-derived
cells, there is the potential to regenerate tissue defects.
The scaffold is bioengineered in a two-stage process that
recreates a muscularis externa with smooth muscle, fibro-
Downloaded by: Boston University. Copyrighted material.
Fig. 6 Magnamosis rings with donut of tissue after completion of a blasts, and enteric nervous system followed by a multilay-
magnetic compression anastomosis. ered mucosa derived from epithelial precursors. The result is
a structurally organized and vascularized esophageal substi-
described gastric transposition in four infants with pure EA tute which may be an alternative to esophageal replacement
in 1984.37 Since, Spitz published a series of 173 children who grafts.
underwent gastric transposition, majority for EA.38 There Jensen et al developed a graft from a polyurethane scaffold
was a 5.2% mortality rate, a 12% anastomotic leak rate, and an that was seeded with autologous cells.11 Porcine esophageal
anastomotic stricture rate of 19%. Laparoscopic gastric trans- epithelial cells or porcine amniotic fluid was cultured on the
position was subsequently described by Ure et al in a single graft in a bioreactor and then implanted in piglets as a 5-cm
patient with success.39 esophageal interposition graft (►Fig. 7). By 21 days, the
Gastric tube was first described by Heimlich and Winfield scaffolds were extruded and demonstrated regeneration of
in 1955, and Anderson and Randolph later applied gastric a complete tube. By 6 months, the tissue appeared as
tube for EA in children.40,41 It is less popular due to the need complete epithelium. Grafts seeded with either cell type
for a large suture line and with an increased leak and demonstrated epithelial and muscle regeneration, and the
stricture rate.36 pigs were ultimately able to eat with the bioengineered
segment of esophagus. Application in neonates appears to
Colon be the next step.
German and Waterston published a series of 32 patients who
underwent transverse colon interposition with long-term What Is on the Horizon?
follow-up with positive results.42 It is a technically more There are several unanswered questions in the current under-
challenging procedure and requires three anastomoses. In standing of EA and its existing therapies. The role of molecular
2010, Esteves et al described a laparoscopic-assisted ap- mechanisms is largely unknown when it comes to mechanical
proach in a series of five children.43 lengthening.48 Furthermore, it is unclear whether mechanical
Fig. 7 Polyurethane scaffold seeded with autologous cells on initial esophageal replacement (A) and at 3 weeks postprocedure on endoscopy
(B) and after extrusion (C). (Reproduced from Jensen et al.11 )
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
lengthening leads to new growth rather than stretch alone, and 13 Glenn IC, Bruns NE, Schomisch SJ, Ponsky TA. Creation of an
how this potentially impacts inflammation, stricture forma- esophageal atresia animal model using a bifurcated esophagus to
tion, and motility. Finally, as tissue engineering is coming maintain digestive tract continuity. J Laparoendosc Adv Surg Tech
A 2017;27(10):1079–1084
closer to reality in neonates, questions regarding muscle
14 Oetzmann von Sochaczewski C, Lindner A, Heimann A, et al.
formation and function need to be addressed. Beyond magnamosis: a method to test sutureless esophageal
anastomotic devices in living swine by creating an esophageal
bypass loop for natural oral nutrition. J Laparoendosc Adv Surg
Conclusion Tech A 2019;29(06):852–855
15 Usui Y, Ono S. Impact of botulinum toxin A injection on esoph-
In the last decade, strides have been made in the develop-
ageal anastomosis in a rabbit model. Pediatr Surg Int 2016;32
ment of novel techniques for long-gap EA. BoTox injection
(09):881–886
may prove to be a useful adjunct during anastomosis once 16 Urbani L, Camilli C, Phylactopoulos D-E, et al. Multi-stage
adequately studied in neonates. Magnetic compression anas- bioengineering of a layered oesophagus with in vitro expanded
tomosis has the potential to be a minimally invasive tech- muscle and epithelial adult progenitors. Nat Commun 2018;9
nique for long-gap EA if the current devices can be revised to (01):4286
17 Sullins VF, Traum PK, French SW, Wu BM, Dunn JCY, Lee SL. A novel
improve stricture rates. Among therapies untested in
method of esophageal lengthening in a large animal model of long
humans, bioengineered scaffolds show the most promise, gap esophageal atresia. J Pediatr Surg 2015;50(06):928–932
although there is still the question of muscle formation and 18 Soyer T, Kalkışım S, Yalcin S, et al. The effects of acute tension
function. increase on rat esophageal muscle contractions: an in vitro study.
J Pediatr Surg 2015;50(10):1691–1694
Conflict of Interest 19 Inoue S, Kosaka T, Takatsuki M, Kuroki T, Eguchi S. Histological
study of the elongated esophagus in a rat model. J Surg Res 2015;
Downloaded by: Boston University. Copyrighted material.
Dr. Ponsky is the owner of Globalcast MD and consultant
195(02):495–501
for Conmed, outside the submitted work. 20 Hays DM, Woolley MM, Snyder WH Jr. Changing techniques in the
management of esophageal atresia. Arch Surg 1966;92(04):
611–616
References 21 Mahour GH, Woolley MM, Gwinn JL. Elongation of the upper
1 Depaepe A, Dolk H, Lechat MF; EUROCAT Working Group. The pouch and delayed anatomic reconstruction in esophageal atre-
epidemiology of tracheo-oesophageal fistula and oesophageal sia. J Pediatr Surg 1974;9(03):373–383
atresia in Europe. Arch Dis Child 1993;68(06):743–748 22 Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compres-
2 Spitz L. Oesophageal atresia. Orphanet J Rare Dis 2007;2(01):24 sion anastomosis as a nonsurgical treatment for esophageal
3 Baird R, Lal DR, Ricca RL, et al. Management of long gap esoph- atresia. Pediatr Radiol 2009;39(09):945–949
ageal atresia: a systematic review and evidence-based guidelines 23 Larsen HF, Jensen TSR, Rasmussen L, Ellebæk M, Qvist N. Intramu-
from the APSA Outcomes and Evidence Based Practice Committee. ral injection with botulinum toxin significantly elongates the pig
J Pediatr Surg 2019;54(04):675–687 esophagus. J Pediatr Surg 2013;48(10):2032–2035
4 Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR III. 24 Ellebæk M, Qvist N, Rasmussen L. Secondary anastomosis after
Analysis of morbidity and mortality in 227 cases of esophageal preoperative botulinum type A toxin injection in a case with long
atresia and/or tracheoesophageal fistula over two decades. Arch gap oesophageal atresia. Eur J Pediatr Surg 2013;23(04):325–326
Surg 1995;130(05):502–508 25 Foker JE, Linden BC, Boyle EM Jr, Marquardt C. Development of a
5 Friedmacher F, Puri P. Delayed primary anastomosis for management true primary repair for the full spectrum of esophageal atresia.
of long-gap esophageal atresia: a meta-analysis of complications Ann Surg 1997;226(04):533–541
and long-term outcome. Pediatr Surg Int 2012;28(09):899–906 26 Foker JE, Kendall TC, Catton K, Khan KM. A flexible approach to
6 de Lorimier AA, Harrison MR. Long gap esophageal atresia: achieve a true primary repair for all infants with esophageal
primary anastomosis after esophageal elongation by bougienage atresia. Semin Pediatr Surg 2005;14(01):8–15
and esophagomyotomy. J Thorac Cardiovasc Surg 1980;79(01): 27 Khan KM, Sabati AA, Kendall T, Foker JE. The effect of traction on
138–141 esophageal structure in children with long-gap esophageal atre-
7 Hendren WH, Hale JR. Electromagnetic bougienage to lengthen sia. Dig Dis Sci 2006;51(11):1917–1921
esophageal segments in congenital esophageal atresia. N Engl J 28 van der Zee DC, Gallo G, Tytgat SHA. Thoracoscopic traction
Med 1975;293(09):428–432 technique in long gap esophageal atresia: entering a new era.
8 Sauer H, Kurz R. Experiences in the treatment of esophageal Surg Endosc 2015;29(11):3324–3330
atresia with Rehbein’s olive technique. Prog Pediatr Surg 1986; 29 Kimura K, Soper RT. Multistaged extrathoracic esophageal elon-
19:93–102 gation for long gap esophageal atresia. J Pediatr Surg 1994;29(04):
9 von Allmen D, Wijnen RM; Allmen von D. Bridging the gap in the 566–568
repair of long-gap esophageal atresia: still questions on diagnos- 30 Tamburri N, Laje P, Boglione M, Martinez-Ferro M. Extrathoracic
tics and treatment. Eur J Pediatr Surg 2015;25(04):312–317 esophageal elongation (Kimura’s technique): a feasible option for
10 Bruns NE, Glenn IC, Craner DR, Schomisch SJ, Harrison MR, Ponsky the treatment of patients with complex esophageal atresia.
TA. Magnetic compression anastomosis (magnamosis) in a por- J Pediatr Surg 2009;44(12):2420–2425
cine esophagus: proof of concept for potential application in 31 Vogel AM, Yang EY, Fishman SJ. Hydrostatic stretch-induced
esophageal atresia. J Pediatr Surg 2019;54(03):429–433 growth facilitating primary anastomosis in long-gap esophageal
11 Jensen T, Wanczyk H, Sharma I, Mitchell A, Sayej WN, Finck C. atresia. J Pediatr Surg 2006;41(06):1170–1172
Polyurethane scaffolds seeded with autologous cells can regener- 32 Scott A, Sullins VF, Steinberger D, et al. Repeated mechanical
ate long esophageal gaps: an esophageal atresia treatment model. lengthening of intestinal segments in a novel model. J Pediatr Surg
J Pediatr Surg 2018. Doi: 10.1016/j.jpedsurg.2018.09.024 2015;50(06):954–957
12 Glenn IC, Bruns NE, Gabarain G, Craner DR, Schomisch SJ, Ponsky 33 Rehbein F, Schweder N. Reconstruction of the esophagus without
TA. Creation of an animal model for long gap pure esophageal colon transplantation in cases of atresia. J Pediatr Surg 1971;6
atresia. Pediatr Surg Int 2017;33(02):197–201 (06):746–752
European Journal of Pediatric Surgery
Esophageal Atresia Bruns et al.
34 Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anasto- 43 Esteves E, Sousa-Filho HB, Watanabe S, Silva JF, Neto EC, da Costa
mosis in pediatric patients. J Pediatr Surg 2014;49(07): AL. Laparoscopically assisted esophagectomy and colon interpo-
1131–1137 sition for esophageal replacement in children: preliminary results
35 Ellebaek MBB, Qvist N, Rasmussen L. Magnetic compression of a novel technique. J Pediatr Surg 2010;45(05):1053–1060
anastomosis in long-gap esophageal atresia gross type A: a case 44 Ring WS, Varco RL, L’Heureux PR, Foker JE. Esophageal replace-
report. European J Pediatr Surg Rep 2018;6(01):e37–e39 ment with jejunum in children: an 18 to 33 year follow-up.
36 Shieh HF, Jennings RW. Long-gap esophageal atresia. Semin J Thorac Cardiovasc Surg 1982;83(06):918–927
Pediatr Surg 2017;26(02):72–77 45 Bax NMA, Van Renterghem KM. Ileal pedicle grafting for esoph-
37 Spitz L. Gastric transposition via the mediastinal route for infants ageal replacement in children. Pediatr Surg Int 2005;21(05):
with long-gap esophageal atresia. J Pediatr Surg 1984;19(02): 369–372
149–154 46 Bax NMA, van der Zee DC. Jejunal pedicle grafts for reconstruc-
38 Spitz L, Kiely E, Pierro A. Gastric transposition in children–a 21- tion of the esophagus in children. J Pediatr Surg 2007;42(02):
year experience. J Pediatr Surg 2004;39(03):276–281 363–369
39 Ure BM, Jesch NK, Sümpelmann R, Nustede R. Laparoscopically 47 Saitua F, Madrid A, Capdeville F, Ferrada C, Herrera P. Pharyngo-
assisted gastric pull-up for long gap esophageal atresia. J Pediatr esophageal reconstruction by free jejunal graft and microvascular
Surg 2003;38(11):1661–1662 anastomosis in a 10-year-old girl. J Pediatr Surg 2004;39(07):
40 Heimlich HJ, Winfield JM. The use of a gastric tube to replace or e10–e12
by-pass the esophagus. Surgery 1955;37(04):549–559 48 Rayyan M, Rommel N, Tack J, Deprest J, Allegaert K. Esophageal
41 Anderson KD, Randolph JG. The gastric tube for esophageal atresia: future directions for research on the digestive tract. Eur J
replacement in children. J Thorac Cardiovasc Surg 1973;66(03): Pediatr Surg 2017;27(04):306–312
333–342 49 Hendren WH, Hale JR. Esophageal atresia treated by electromag-
42 German JC, Waterston DJ. Colon interposition for the replacement netic bougienage and subsequent repair. J Pediatr Surg 1976;11
of the esophagus in children. J Pediatr Surg 1976;11(02):227–234 (05):713–722
Downloaded by: Boston University. Copyrighted material.
European Journal of Pediatric Surgery
You might also like
- Gerd, Peptic Ulcer, Ulcerative Colitis, Crohn's DiseaseDocument11 pagesGerd, Peptic Ulcer, Ulcerative Colitis, Crohn's DiseasekamipimaNo ratings yet
- Surgery OsceDocument69 pagesSurgery OsceRebecca BrandonNo ratings yet
- Jung 2014Document8 pagesJung 2014Fabrizzio BardalesNo ratings yet
- Annrcse01521 0053Document1 pageAnnrcse01521 0053Muhammad Fiqhi HardiansyahNo ratings yet
- Alargamiento Del Complejo Gastronemio SoleoDocument8 pagesAlargamiento Del Complejo Gastronemio SoleoC Martin TraumatoNo ratings yet
- Usg 17050Document7 pagesUsg 17050Amit Kumar RanoNo ratings yet
- JTD 06 S3Document8 pagesJTD 06 S3DH SiriruiNo ratings yet
- A Rare Variant Case of Pure Esophageal Atresia With An Atretic SegmentDocument3 pagesA Rare Variant Case of Pure Esophageal Atresia With An Atretic SegmentDetry Kala'lembangNo ratings yet
- Kunio Et Al. - 2015 - Short EsophagusDocument12 pagesKunio Et Al. - 2015 - Short EsophagusDaniel PredaNo ratings yet
- Imaging LGEADocument10 pagesImaging LGEANuanSyafrinaNo ratings yet
- Dysphagia Due To Upper Esophageal Sphincter DisordDocument3 pagesDysphagia Due To Upper Esophageal Sphincter DisordEustakia Rini Kartika DewiNo ratings yet
- 1 s2.0 S0016510711024308 MainDocument6 pages1 s2.0 S0016510711024308 Mainpainkiller78No ratings yet
- Cutaneous Needle Aspirations in Liver DiseaseDocument6 pagesCutaneous Needle Aspirations in Liver DiseaseRhian BrimbleNo ratings yet
- Clinical Anatomy - 2020 - Le Saint Grant - Arterial Anatomy of The Anterior Abdominal Wall Ultrasound Evaluation As ADocument6 pagesClinical Anatomy - 2020 - Le Saint Grant - Arterial Anatomy of The Anterior Abdominal Wall Ultrasound Evaluation As AarifNo ratings yet
- Role of Open Surgical Drainage or Aspiration in Management of Amoebic Liver AbscessDocument9 pagesRole of Open Surgical Drainage or Aspiration in Management of Amoebic Liver AbscessIJAR JOURNALNo ratings yet
- Update On Foregut Molecular Embryology and Role of Regenerative Medicine TherapiesDocument9 pagesUpdate On Foregut Molecular Embryology and Role of Regenerative Medicine TherapiesmikeNo ratings yet
- Poh 2011Document8 pagesPoh 2011DH SiriruiNo ratings yet
- What Proportion of Children With Complex Oesophageal Atresia Require Oesophageal Lengthening ProceduresDocument5 pagesWhat Proportion of Children With Complex Oesophageal Atresia Require Oesophageal Lengthening ProceduresGunduz AgaNo ratings yet
- PuIpal and Periapical Tissue Reactions AfterDocument4 pagesPuIpal and Periapical Tissue Reactions AfterANGEL ADALBERTO GALVAN GARCIANo ratings yet
- Ultrasonographic Atlas of Splenic LesionsDocument14 pagesUltrasonographic Atlas of Splenic LesionsFlavio RojasNo ratings yet
- 1 s2.0 S0039610903001191 MainDocument15 pages1 s2.0 S0039610903001191 MainJuan Jos� Beltr�n Hern�ndezNo ratings yet
- Proposal New - 060952Document33 pagesProposal New - 060952sommylydiiNo ratings yet
- Synthetic Versus Biological Mesh in Laparoscopic and Open Ventral Hérnia RepairDocument9 pagesSynthetic Versus Biological Mesh in Laparoscopic and Open Ventral Hérnia RepairRhuan AntonioNo ratings yet
- Ureteral ReconstructionDocument6 pagesUreteral ReconstructiongumNo ratings yet
- Leiomyoma of The Esophagus: Open Versus Thoracoscopic EnucleationDocument5 pagesLeiomyoma of The Esophagus: Open Versus Thoracoscopic EnucleationYacine Tarik AizelNo ratings yet
- Clinical Study of Bowel ObstructionDocument6 pagesClinical Study of Bowel ObstructionhorzhanNo ratings yet
- Liver Abscess: Catheter Drainage V/s Needle AspirationDocument6 pagesLiver Abscess: Catheter Drainage V/s Needle AspirationMishel Rodriguez GuzmanNo ratings yet
- KumarasingheDocument4 pagesKumarasingheVitta Kusma WijayaNo ratings yet
- Artigo 5Document8 pagesArtigo 5doctorbanNo ratings yet
- Scholars Journal of Medical Case Reports: ISSN 2347-6559 (Online) ISSN 2347-9507 (Print)Document2 pagesScholars Journal of Medical Case Reports: ISSN 2347-6559 (Online) ISSN 2347-9507 (Print)galihmuchlishermawanNo ratings yet
- Ureteric-Urethral Engraftment As A New Surgical Technique For Management of Incontinence in Bladder Exstrophy Complex A Retrospective CohortDocument6 pagesUreteric-Urethral Engraftment As A New Surgical Technique For Management of Incontinence in Bladder Exstrophy Complex A Retrospective CohortJad DegheiliNo ratings yet
- Abdalla 2017Document3 pagesAbdalla 2017dewaprasatyaNo ratings yet
- Abdominal Wall Hernia ThesisDocument7 pagesAbdominal Wall Hernia Thesisaflpaftaofqtoa100% (2)
- Testicular BloodDocument6 pagesTesticular Bloodsalcor00No ratings yet
- (2020) Colonography Evaluation of The Relationship Between Colon Anatomy and DiverticulaDocument7 pages(2020) Colonography Evaluation of The Relationship Between Colon Anatomy and DiverticulaChristian ToalongoNo ratings yet
- A New Method For The Removal of Safety Pins Ingested by ChildrenDocument2 pagesA New Method For The Removal of Safety Pins Ingested by ChildrenAnusha SainiNo ratings yet
- Elipse Balloon The Pitfalls of Excessive SimplicityDocument3 pagesElipse Balloon The Pitfalls of Excessive SimplicityTony Miguel Saba SabaNo ratings yet
- Manavella 2018Document10 pagesManavella 2018dimiz77No ratings yet
- Comparison of Flank and Midline Approaches To OvarDocument6 pagesComparison of Flank and Midline Approaches To Ovartrisnaput3No ratings yet
- Bove-Chapelle ViscMobilizAdhesionRatDocument7 pagesBove-Chapelle ViscMobilizAdhesionRatQuiroprácticaParaTodosNo ratings yet
- Anatomy. Eight Edition. Saint Louis: Mosby.: Ped PDFDocument10 pagesAnatomy. Eight Edition. Saint Louis: Mosby.: Ped PDFannisa ussalihahNo ratings yet
- A Dental Nightmare, Resolved - What A Radiologist Needs To Know When Consulted About Ingestion of Dental Foreign Body Material - PMCDocument17 pagesA Dental Nightmare, Resolved - What A Radiologist Needs To Know When Consulted About Ingestion of Dental Foreign Body Material - PMCsj5h4vwxxfNo ratings yet
- Treatment For Failed Epispadias Repair Presenting in AdultsDocument6 pagesTreatment For Failed Epispadias Repair Presenting in AdultsDhonz R AdiwaramanNo ratings yet
- Figures 1Document8 pagesFigures 1Arman KozhakhmetNo ratings yet
- Gastrointestinal Emergency in NeonatesDocument15 pagesGastrointestinal Emergency in NeonatesGuillermo Andrés AriasNo ratings yet
- Effects of Tracheostomy Tube On SwallowingDocument21 pagesEffects of Tracheostomy Tube On SwallowingPaola A. MartinezNo ratings yet
- Role of Endoscopic Stent Insertion On Managmeent of Gastric Twist After Sleeve GastrectomyDocument6 pagesRole of Endoscopic Stent Insertion On Managmeent of Gastric Twist After Sleeve GastrectomyJorge SalazarNo ratings yet
- Undescended TestisDocument3 pagesUndescended TestisIoannis ValioulisNo ratings yet
- Managemen Esofagitis Corosive 1Document6 pagesManagemen Esofagitis Corosive 1yunia chairunnisaNo ratings yet
- Journal of Pediatric Surgery: Shabnam Sabetkish, Nastaran Sabetkish, Abdol-Mohammad KajbafzadehDocument7 pagesJournal of Pediatric Surgery: Shabnam Sabetkish, Nastaran Sabetkish, Abdol-Mohammad KajbafzadehAmatystNo ratings yet
- Hum. Reprod.-2005-Remorgida-2317-20Document4 pagesHum. Reprod.-2005-Remorgida-2317-20lediayukimeNo ratings yet
- Massive Congenital Lymphatic Malformation of The SM - 2013 - Journal of PediatriDocument3 pagesMassive Congenital Lymphatic Malformation of The SM - 2013 - Journal of PediatriGunduz AgaNo ratings yet
- Pi Is 0022534714035575Document2 pagesPi Is 0022534714035575Andri Feisal NasutionNo ratings yet
- Complete Penile DisassemblyDocument2 pagesComplete Penile DisassemblyGunduz AgaNo ratings yet
- ABRAVAS 5 de 9 - Hernandez Divers 2007 Liver BXDocument5 pagesABRAVAS 5 de 9 - Hernandez Divers 2007 Liver BXCamilo SantanderNo ratings yet
- OHT Flank ApproachDocument6 pagesOHT Flank ApproachMarinaJiménezNo ratings yet
- Cannulation Attempts and The Development of Post EDocument2 pagesCannulation Attempts and The Development of Post EMarcio MullerNo ratings yet
- Ostomia Intestinal PDFDocument7 pagesOstomia Intestinal PDFDaniela Pérez LópezNo ratings yet
- Prosthetic Repair of Acutely Incarcerated Groin Hernias: A Prospective Clinical Observational Cohort StudyDocument6 pagesProsthetic Repair of Acutely Incarcerated Groin Hernias: A Prospective Clinical Observational Cohort Studynh2411No ratings yet
- Laparoscopic Extraperitoneal Resection of Urachal Cyst: Sun-Il Lee, M.D., Sung-Soo Kim, M.DDocument3 pagesLaparoscopic Extraperitoneal Resection of Urachal Cyst: Sun-Il Lee, M.D., Sung-Soo Kim, M.DNurul Rezki Fitriani AzisNo ratings yet
- Punção No Hipocôndrio Esquerdo Com Agulha de Veress para A Criação Do PneumoperitônioDocument8 pagesPunção No Hipocôndrio Esquerdo Com Agulha de Veress para A Criação Do PneumoperitônioSINAN SHAWKATNo ratings yet
- Esophageal Preservation and Replacement in ChildrenFrom EverandEsophageal Preservation and Replacement in ChildrenAshwin PimpalwarNo ratings yet
- Chapter 1Document20 pagesChapter 1luiperdvrouNo ratings yet
- 2015 Management of Ingested Foreign Bodies in Children - A Clinical Report of The NASPGHAN Endoscopy CommitteeDocument13 pages2015 Management of Ingested Foreign Bodies in Children - A Clinical Report of The NASPGHAN Endoscopy CommitteeCarlos CuadrosNo ratings yet
- PREP ICU 2013 Answers and Critiques - 1 - Jan & FebDocument47 pagesPREP ICU 2013 Answers and Critiques - 1 - Jan & FebNicholasHuffNo ratings yet
- Turtle Manual English PDFDocument25 pagesTurtle Manual English PDFDulce AmorNo ratings yet
- 00 GIT NotesDocument81 pages00 GIT NotesHythem Hashim100% (1)
- Molluscan Studies: Journal ofDocument19 pagesMolluscan Studies: Journal ofWidi SetyogatiNo ratings yet
- Vascular ImpressionsDocument3 pagesVascular ImpressionsfiameliaaNo ratings yet
- Lecture7 V SemesterDocument28 pagesLecture7 V SemestersreelekshmiNo ratings yet
- Diagnosis and Management of Functional HeartburnDocument9 pagesDiagnosis and Management of Functional Heartburnal ghiffari muhammad rayhanNo ratings yet
- Fisiologi Menelan Dan Keluarnya Suara1Document27 pagesFisiologi Menelan Dan Keluarnya Suara1Verani Citra DeviNo ratings yet
- GI History IntroductoryDocument74 pagesGI History IntroductoryNur Hamizah Md FuziNo ratings yet
- PATH-261 (SPO) L2SII Theory (Full)Document174 pagesPATH-261 (SPO) L2SII Theory (Full)Shakil MahmodNo ratings yet
- TOPNOTCH Diagnostic Exam ANSWER KEY September 2018Document20 pagesTOPNOTCH Diagnostic Exam ANSWER KEY September 2018CDNo ratings yet
- Mila Amor V. Reyes, MD, FPSP Anatomic and Clinical PathologistDocument41 pagesMila Amor V. Reyes, MD, FPSP Anatomic and Clinical PathologistFranco Rommel ReyesNo ratings yet
- BiologyDocument249 pagesBiologysagarNo ratings yet
- Black S Medical Dictionary PDFDocument2,413 pagesBlack S Medical Dictionary PDFAlexandr Trotsky100% (1)
- CorrosivesDocument25 pagesCorrosivesahmed.farag.ali2020No ratings yet
- 9 Digestive System Part 1Document14 pages9 Digestive System Part 1Fhayee Sulaik HaronNo ratings yet
- Common Features of Diseases of EsophagusDocument4 pagesCommon Features of Diseases of EsophagusSri PoopaseNo ratings yet
- Anatomi Fisiologi Sistem PencernaanDocument38 pagesAnatomi Fisiologi Sistem Pencernaanyo2_laxanaNo ratings yet
- Gastro-Esophageal Reflux Disease (GERD) : Prof DRDocument21 pagesGastro-Esophageal Reflux Disease (GERD) : Prof DRmohammed salahNo ratings yet
- Am.J.PharmTech Res. 2020 10 (01), Formulation and Evaluation of Rabeprazole Sodium DelayedDocument14 pagesAm.J.PharmTech Res. 2020 10 (01), Formulation and Evaluation of Rabeprazole Sodium DelayedThu Huyền TrầnNo ratings yet
- نم انوسنت لا مكوجرأ ,يلهلا و يل ةيراج ةقدص مكئاعد حلا ص Please Grace us with your good prayersDocument538 pagesنم انوسنت لا مكوجرأ ,يلهلا و يل ةيراج ةقدص مكئاعد حلا ص Please Grace us with your good prayersSahan EpitawalaNo ratings yet
- Anatomy Ii: by Dr. Ziyad M. Al Zeer Orthopedic Surgeon Assistant Professor MD - PHDDocument64 pagesAnatomy Ii: by Dr. Ziyad M. Al Zeer Orthopedic Surgeon Assistant Professor MD - PHDMOHAMMAD ALSWEITYNo ratings yet
- Visceral OMT: AAO Convocation March 2018 Kenneth Lossing DODocument59 pagesVisceral OMT: AAO Convocation March 2018 Kenneth Lossing DODiana SchlittlerNo ratings yet
- Band Igation AccessoryDocument4 pagesBand Igation AccessoryFady Maher WadeaNo ratings yet
- Nausea and Vomiting: William F - MauleDocument2 pagesNausea and Vomiting: William F - Mauleडा. सत्यदेव त्यागी आर्यNo ratings yet
- Surgical Notes PDFDocument118 pagesSurgical Notes PDFRebecca Wong100% (1)