Professional Documents
Culture Documents
Proteins
Proteins
Uploaded by
MUHAMMAD SMITHOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Proteins
Proteins
Uploaded by
MUHAMMAD SMITHCopyright:
Available Formats
PROTEINS (1)
Dr. Aileen B. Angcajas October 2022
Department of Chemistry
College of Science and Mathematics
MSU-Iligan Institute of Technology
Iligan City
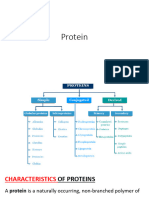
Characteristics of Proteins
A protein is a naturally-occurring, unbranched polymer in
which the monomer units are amino acids.
Specific definition: A protein is a peptide in which at least
40 amino acid residues are present:
– The terms polypeptide and protein are often used
interchangeably to describe a protein
– Several proteins with >10,000 amino acid residues are
known
– Common proteins contain 400–500 amino acid residues
– Small proteins contain 40–100 amino acid residues
BIOCHEMISTRY I ABA, Oct 2022 | Page 2
Protein Classification Based on Chemical
Composition
Simple proteins
A protein in which only amino acid residues are present:
– More than one protein subunit may be present but all
subunits contain only amino acids
Albuminoids - keratin in skin, hair, nails; collagen in
cartilage
Albumins - egg albumin, serum albumin
Globulins - antibodies
Histones - chromatin in chromosomes
BIOCHEMISTRY I ABA, Oct 2022 | Page 3
Protein Classification Based on Chemical
Composition
Conjugated (complex) proteins
A protein that has one or more non-amino acid entities
(prosthetic groups) present in its structure: Prosthetic
group may be organic or inorganic
BIOCHEMISTRY I ABA, Oct 2022 | Page 4
BIOCHEMISTRY I ABA, Oct 2022 | Page 5
Protein Classification Based on Shape
Fibrous Proteins: Alpha-Keratin & Collagen
The polypeptide chains are arranged in long strands or
sheets
• Have long, rod-shaped or string-like molecules that can
intertwine with one another and form strong fibers;
water-insoluble
• Structural functions
BIOCHEMISTRY I ABA, Oct 2022 | Page 6
Protein Classification Based on Shape
Globular Proteins: Myoglobin & Hemoglobin
The polypeptide chains are folded into spherical or globular
shapes
• Nonpolar amino acids are in the interior, polar amino
acids are on the surface
• Water-soluble which allows them to travel through the
blood and other body fluids to sites where their activity is
needed
• Dynamic functions
BIOCHEMISTRY I ABA, Oct 2022 | Page 7
BIOCHEMISTRY I ABA, Oct 2022 | Page 8
Major Categories of Proteins Based on Function
• Catalytic proteins: Enzymes are best known for their catalytic role.
– Almost every chemical reaction in the body is driven by an
enzyme
• Defense proteins: Immunoglobulins or antibodies are central to
functioning of the body’s immune system.
• Transport proteins: Bind small biomolecules, e.g., oxygen and
other ligands, and transport them to other locations in the body
and release them on demand.
• Messenger proteins: transmit signals to coordinate biochemical
processes between different cells, tissues, and organs.
– Insulin and glucagon - regulate carbohydrate metabolism
– Human growth hormone – regulate body growth
BIOCHEMISTRY I ABA, Oct 2022 | Page 9
Major Categories of Proteins Based on Function
•Contractile proteins: Necessary for all forms of movement.
– Muscles contain filament-like contractile proteins (actin and
myosin).
– Human reproduction depends on the movement of sperm –
possible because of contractile proteins.
• Structural proteins: Confer stiffness and rigidity
– Collagen is a component of cartilage
– Keratin gives mechanical strength as well as protective covering
to hair, fingernails, feathers, hooves, etc.
• Transmembrane proteins: Span a cell membrane and help control
the movement of small molecules and ions.
– Have channels – help molecules to enter and exist the cell.
– Transport is very selective - allow passage of one type of
molecule or ion.
BIOCHEMISTRY I ABA, Oct 2022 | Page 10
Major Categories of Proteins Based on Function
• Storage proteins: Bind (and store) small molecules.
– Ferritin - an iron-storage protein - saves iron for use in the
biosynthesis of new hemoglobin molecules.
– Myoglobin - an oxygen-storage protein present in muscle
• Regulatory proteins: Often found “embedded” in the exterior surface
of cell membranes - act as sites for receptor molecules
– Often the molecules that bind to enzymes (catalytic proteins),
thereby turning them “on” and “off,” and thus controlling
enzymatic action.
• Nutrient proteins: Particularly important in the early stages of life -
from embryo to infant.
– Casein (milk) and ovalalbumin (egg white) are nutrient proteins
– Milk also provide immunological protection for mammalian young.
BIOCHEMISTRY I ABA, Oct 2022 | Page 11
Amino Acid: The Building Blocks for Proteins
• Amino acid: a compound that
contains both an amino group
and a carboxyl group
• -Amino acid has an amino
group attached to the carbon
adjacent to the carboxyl group
• -carbon also bound to side
chain group, R
• R gives identity to amino acid
• Two steroisomers of amino acids
are designated L- or D-. Based on
similarity to glyceraldehdye
BIOCHEMISTRY I ABA, Oct 2022 | Page 12
Amino Acid: The Building Blocks for Proteins
– R = side chain –vary in size, shape, charge, acidity,
functional groups present, hydrogen-bonding ability, and
chemical reactivity.
– >700 amino acids are known
– Based on common “R” groups, there are 20 standard
amino acids
BIOCHEMISTRY I ABA, Oct 2022 | Page 13
BIOCHEMISTRY I ABA, Oct 2022 | Page 14
BIOCHEMISTRY I ABA, Oct 2022 | Page 15
BIOCHEMISTRY I ABA, Oct 2022 | Page 16
BIOCHEMISTRY I ABA, Oct 2022 | Page 17
BIOCHEMISTRY I ABA, Oct 2022 | Page 18
Amino Acid: The Building Blocks for Proteins
• All amino acids differ from one another by their R-groups
– There are 20 common (standard) amino acids
• Standard amino acids are divided into four groups based
on the properties of R-groups
• Non-polar amino acids: R-groups are non-polar
– Such amino acids are hydrophobic-water fearing
(insoluble in water)
– 8 of the 20 standard amino acids are non-polar
– When present in proteins, they are located in the
interior of protein where there is no polarity
BIOCHEMISTRY I ABA, Oct 2022 | Page 19
Amino Acid Structure and Properties
• With the exception of glycine, all protein-derived amino
acids have at least one stereocenter (the α-carbon) and
are chiral (stereoisomers)
• The vast majority of α -amino acids have the L-
configuration at the α -carbon (Proline is usually D)
• Side-chain carbons in other amino acids designated with
Greek symbols, starting at a carbon (…etc)
• Amino acids can be referred to by three-letter or one-
letter codes.
BIOCHEMISTRY I ABA, Oct 2022 | Page 20
Amino Acid: The Building Blocks for Proteins
Nomenclature
Common names assigned to the amino acids are currently
used.
• Three letter abbreviations - widely used for naming:
– First letter of amino acid name is compulsory and
capitalized followed by next two letters not capitalized
except in the case of Asparagine (Asn), Glutamine (Gln)
and tryptophan (Trp).
• One-letter symbols - commonly used for comparing amino
acid sequences of proteins:
– Usually the first letter of the name
– When more than one amino acid has the same letter the
most abundant amino acid gets the 1st letter.
BIOCHEMISTRY I ABA, Oct 2022 | Page 21
Amino Acid Abbreviations and Codes
BIOCHEMISTRY I ABA, Oct 2022 | Page 22
Learning the Names and Codes
BIOCHEMISTRY I ABA, Oct 2022 | Page 23
Essential Amino Acids
Essential Amino acid: A standard amino acid needed for protein
synthesis that must be obtained from dietary sources – adequate
amounts cannot be synthesized in human body.
Nine of the 20 standard amino acids are considered essential
*Required for growth
in children and is not
essential for adults.
BIOCHEMISTRY I ABA, Oct 2022 | Page 24
Essential Amino Acids
A complete protein contains all the essential amino acids in
the proper amounts.
An incomplete protein is low in one or more of the essential
amino acids, usually lysine, tryptophan, or methionine.
Except for gelatin, proteins from animal sources are complete,
whereas proteins from vegetable sources are incomplete except soy
protein.
Complementary proteins are incomplete proteins which
when served together, complement each other and provide
all the essential amino acids
BIOCHEMISTRY I ABA, Oct 2022 | Page 25
Examples of Complete and Incomplete Protein Sources
BIOCHEMISTRY I ABA, Oct 2022 | Page 26
All the essential amino acids are provided in a meal that consists of
a complete protein of animal origin or complementary proteins of
vegetable origin.
BIOCHEMISTRY I ABA, Oct 2022 | Page 27
Nonstandard/Uncommon Amino Acids
Each derived from a
common amino acid by
a modification
• Hydroxylysine and
hydroxyproline are
found only in a few
connective tissues
such as collagen
• Thyroxine is found only
in the thyroid gland
BIOCHEMISTRY I ABA, Oct 2022 | Page 28
Ionization of Amino
Acids
• In amino acid,
carboxyl group (-) and
amino group (+) are
charged at neutral
pH.
• In free amino acids
-carboxyl, and a-
amino groups have
titratable protons.
Some side chains do
as well
BIOCHEMISTRY I ABA, Oct 2022 | Page 29
Ionization of Amino Acids
In neutral solution, the COOH is ionized and the NH2 is
protonated
The resulting structures have “+” and “-” charges (a dipolar
ion, or zwitterion)
BIOCHEMISTRY I ABA, Oct 2022 | Page 30
Ionization vs pH
The net charge on an amino acid depends on the
pH of the solution in which it is dissolved.
If we dissolve an amino acid in water, it is present in the
aqueous solute as its zwitterion.
If we now add a strong acid such as HCl to bring the pH of
the solution to 2.0 or lower, the strong acid
-
donates a proton to the -COO of the amino acid turning
the zwitterion into a positive ion.
BIOCHEMISTRY I ABA, Oct 2022 | Page 31
Ionization vs pH
If we now add a strong acid such as HCl to bring the pH of
the solution to 2.0 or lower, the strong acid
-
donates a proton to the -COO of the amino acid turning
the zwitterion into a positive ion.
BIOCHEMISTRY I ABA, Oct 2022 | Page 32
Ionization vs pH
If we add a strong base such as NaOH to the solution and
bring its pH to 10.0 or higher, a proton is transferred from
the NH3+ group to the base turning the
zwitterion into a negative ion.
BIOCHEMISTRY I ABA, Oct 2022 | Page 33
Ionization vs pH
to summarize:
BIOCHEMISTRY I ABA, Oct 2022 | Page 34
Isoelectric Point (pI)
The pH at which the majority of molecules of a compound
in solution have no net charge.
The pH at which the concentration of Zwitterion is
maximum -- net charge is zero
– Different amino acids have different isoelectric points
– At isoelectric point - amino acids are NOT attracted
towards an applied electric field because they carry net
zero charge
BIOCHEMISTRY I ABA, Oct 2022 | Page 35
Isoelectric Point (pI)
To calculate the pI:
1. Draw out the complete ionization of amino acid
2. Determine net charge on each ionized form
3. Find the structure that has no net charge
4. Take the average of the pKa’s that are around the
structure
5. Note do NOT just take the average of all pKa’s.
BIOCHEMISTRY I ABA, Oct 2022 | Page 36
* pKa1 = COOH
* pKa2 = NH3+
* pKaR = R group H+
BIOCHEMISTRY I ABA, Oct 2022 | Page 37
Isoelectric Point (pI)
The pI for glycine, for example, falls midway between
the pKa values for the carboxyl and amino groups
2.4+9.8
pI = = 6.1
2
BIOCHEMISTRY I ABA, Oct 2022 | Page 38
BIOCHEMISTRY I ABA, Oct 2022 | Page 39
BIOCHEMISTRY I ABA, Oct 2022 | Page 40
Summary of the changes in ionic charges when acid
or base is added to a solution of amino acid at
isoelectric point
BIOCHEMISTRY I ABA, Oct 2022 | Page 41
Isoelectric Point (pI)
Isoelectric pH values for the 20
protein-derived amino acids
BIOCHEMISTRY I ABA, Oct 2022 | Page 42
Titration of Amino Acids
When an amino acid is
titrated, the titration curve
represents the reaction of
each functional group with
the hydroxide ion
BIOCHEMISTRY I ABA, Oct 2022 | Page 43
Titration of Alanine with NaOH
+1 0 -1
pI = 2.3 + 9.7
2
pI = 6.0
BIOCHEMISTRY I ABA, Oct 2022 | Page 44
Titration of Histidine with NaOH
BIOCHEMISTRY I ABA, Oct 2022 | Page 45
PRACTICE
Consider the following amino acid and its pKa values:
A. Draw the structure of the amino acid as the pH of the
solution changes from highly acidic to strongly basic.
BIOCHEMISTRY I ABA, Oct 2022 | Page 46
A. Draw the structure of the amino acid as the pH of the
solution changes from highly acidic to strongly basic.
BIOCHEMISTRY I ABA, Oct 2022 | Page 47
B. Which form of the amino acid is present at the
isoelectric point
BIOCHEMISTRY I ABA, Oct 2022 | Page 48
C. Calculate the isoelectric point (pI).
BIOCHEMISTRY I ABA, Oct 2022 | Page 49
PROTEINS (2)
Dr. Aileen B. Angcajas November 2022
Department of Chemistry
College of Science and Mathematics
MSU-Iligan Institute of Technology
Iligan City
Peptides and Peptide Bonds
Under proper conditions, amino acids can bond together to
produce an unbranched chain of amino acids.
– The reactions is between amino group of one amino
acid and carboxyl group of another amino acid.
• The length of the amino acid chain can vary from a few
amino acids to hundreds of amino acids.
• Such a chain of covalently-linked amino acids is called a
peptide.
• The covalent bonds between amino acids in a peptide are
called peptide bonds (amide).
ABA, Nov 2022 | Page 2
Peptides and Peptide Bonds
ABA, Nov 2022 | Page 3
Peptides and Peptide Bonds
ABA, Nov 2022 | Page 4
Nature of Peptide Bond
The C – N bond in the peptide linkage has partial double
bond character that makes it rigid and prevents the
adjacent groups from rotating freely; the peptide bond is
planar, and the two adjacent a-carbons lie trans to it.
The H of the amide nitrogen is also trans to the O of the
carbonyl group. Almost all the peptide bonds in proteins
are planar and have a trans configuration.
ABA, Nov 2022 | Page 5
ABA, Nov 2022 | Page 6
Polypeptide Structure and Trans Amide
ABA, Nov 2022 | Page 7
Peptide Nomenclature
• Structure left (N-term) to right (C-term)
• Structure is based on the repeating sequence
N-C-C-N-C-C-N-C-C
N is the a-amino group; red is the α-carbon; and blue is the
carbonyl carbon.
• All the other amino acid residues have names that end in
-yl. The -yl suffix replaces the -ine or -ic acid ending of
the amino acid name, except for tryptophan, for which -
yl is added to the name.
ABA, Nov 2022 | Page 8
Ala-leu-gly has the IUPAC name of alanylleucylglycine
ABA, Nov 2022 | Page 9
• Peptide: the name given to a short polymer of amino
acids joined by peptide bonds; they are
classified by the number of amino acids in the
chain
• Dipeptide: a molecule containing two amino acids
joined by a peptide bond
• Tripeptide: a molecule containing three amino acids
joined by peptide bonds
• Polypeptide: a macromolecule containing many amino
acids joined by peptide bonds
• Protein: a biological macromolecule of molecular
weight 5000 g/mol or greater, consisting of
one or more polypeptide chains
ABA, Nov 2022 | Page 10
PRACTICE
Given the (a) three-letter abbreviation ; (b) one-
letter symbol, and (c) name of the following
tripeptide:
BIOCHEMISTRY I ABA, Nov 2022 | Page 11
PRACTICE
Write the structure of the ff. peptides at pH 7
a) threonylcysteine
b) Isoleucylmethionylaspartate
BIOCHEMISTRY I ABA, Nov 2022 | Page 12
Isomeric Peptides
Peptides that contain the same amino acids but present in
different order are different molecules (constitutional
isomers) with different properties
Two different dipeptides can be formed between alanine
and glycine
The number of isomeric peptides possible increases rapidly
as the length of the peptide chain increases
ABA, Nov 2022 | Page 13
Biologically Important Peptides
Glutathione (GSH)
• Protects cells from the destructive effects of oxidation by reacting
with substances such as peroxides.
• In red blood cells: glutathione protects against formation of
methemoglobin by reducing H2O2 in a reaction catalyzed by the
enzyme glutathione peroxidase
BIOCHEMISTRY I ABA, Nov 2022 | Page 14
Biologically Important Peptides
Met-enkephalin and Leu-enkephalin
Met-enkephalin Leu-enkephalin
• Pentapeptide neurotransmitters produced by the brain
and bind receptors within the brain
• Help reduce pain
BIOCHEMISTRY I ABA, Nov 2022 | Page 15
Biologically Important Peptides
Oxytocin and Vasopressin
– Produced by the pituitary gland
– Nonapeptide (nine amino acid residues) with six of the residues held
in the form of a loop by a disulfide bond formed between two
cysteine residues
BIOCHEMISTRY I ABA, Nov 2022 | Page 16
Biologically Important Peptides
Aspartame
Dipeptide sold under trade names Equal and Nutrasweet;
~180x as sweet as sucrose
BIOCHEMISTRY I ABA, Nov 2022 | Page 17
Levels of Protein Structure
1° structure: the sequence of amino acids in a polypeptide
chain, read from the N-terminal end to the C-
terminal end
2° structure: the ordered 3-dimensional arrangements
(conformations) in localized regions of a
polypeptide chain; refers only to interactions
of the peptide backbone
e. g., α-helix and β-pleated sheet
3˚ structure: 3-D arrangement of all atoms
4˚ structure: arrangement of monomer subunits with
respect to each other
BIOCHEMISTRY I ABA, Nov 2022 | Page 18
Levels of Protein Structure
BIOCHEMISTRY I ABA, Oct 2022 | Page 19
PROTEINS (3)
Dr. Aileen B. Angcajas November 2022
Department of Chemistry
College of Science and Mathematics
MSU-Iligan Institute of Technology
Iligan City
Primary Structure (1o) of Proteins
The order in which the amino acid residues of a peptide
molecule are linked is the amino acid sequence of the
molecule.
Differences in the chemical and physiologic properties of
peptides result from differences in the amino acid
sequence.
Bradykinin vs Boguskinin
(Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg) (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe)
partly responsible for triggering pain, - completely inactive
welt formation (as in scratches),
movement of smooth muscle, and
lowering of blood pressure
BIOCHEMISTRY I ABA, Nov 2022 | Page 2
Primary Structure (1o) of Proteins
Changes in just one amino acid in sequence can alter biological
function, e.g. hemoglobin associated with sickle-cell anemia
Normal 𝛽 𝑐ℎ𝑎𝑖𝑛: Val-His-Leu-Thr-Pro-Glu-Glu-Lys-….
Sickled 𝛽 𝑐ℎ𝑎𝑖𝑛 : Val-His-Leu-Thr-Pro-Val-Glu-Lys-….
BIOCHEMISTRY I ABA, Nov 2022 | Page 3
Amino Acid Sequencing Steps
Determination of 1˚ sequence of protein is routine biochemistry
lab work
1. Cleavage of all disulfide bonds–oxidation with
performic acid is commonly used.
2. Determination of the N–terminal amino acid.
a. Sanger’s method - the polypeptide chain is reacted
with 1–fluoro–2,4–dinitrobenzene (DNFB).
BIOCHEMISTRY I ABA, Nov 2022 | Page 4
Sangers Method
BIOCHEMISTRY I ABA, Nov 2022 | Page 5
b. Edman degradation – uses phenylisothiocyanate (PITC),
often referred to as Edman’s reagent
BIOCHEMISTRY I ABA, Nov 2022 | Page 6
3. Determination of the C–terminal amino acid
A group of enzymes called the carboxypeptidases are used to identify
the C – terminal residue.
Caboxypeptidases A and B, both secreted by the pancreas, hydrolyze
peptides one residue at a time from the C–terminal end.
a. Carboxypeptidase A – preferentially cleaves peptide bonds when
an aromatic amino acid is the C–terminal residue.
b. Carboxypeptidase B – cleaves basic amino acid residues.
Because these enzymes sequentially cleave peptide bonds starting at
the C–terminal residue, the first amino acid liberated is the C–
terminal residue.
BIOCHEMISTRY I ABA, Nov 2022 | Page 7
4. Cleavage of the polypeptide into fragments
a. Trypsin -cleaves peptide bonds on the carboxyl side of the two
strongly basic amino acids Arg and Lys.
b. Chymotrypsin-cleaves peptide bonds on the carboxyl side of the
three aromatic amino acids (Phe, Tyr, and Trp).
c. Pepsin - cleaves peptide bonds at the amino end of aromatic
amino acids (Phe, Trp, Tyr), acidic amino acids (Asp, Glu) and Ile.
d. Cyanogen Bromide (CNBr) - specifically cleaves peptide bonds on
the carboxyl side of methionine residues
BIOCHEMISTRY I ABA, Nov 2022 | Page 8
BIOCHEMISTRY I ABA, Nov 2022 | Page 9
5. Ordering the peptide fragments
The amino acid sequence information derived from two or
more sets of polypeptide fragments are next examined
for overlapping segments. Such segments make it possible
to piece together the overall sequence.
BIOCHEMISTRY I ABA, Nov 2022 | Page 10
Sample Problem 1
1. Consider the following peptide:
Gly – Ile – Glu – Trp – Thr – Pro – Tyr – Gln – Phe – Arg – Lys
What amino acids and peptides are produced when the
above peptide is treated with each of the following
reagents?
a. Carboxypeptidase c. Trypsin
b. Chymotrypsin d. DNFB
BIOCHEMISTRY I ABA, Nov 2022 | Page 11
Solution
a. Because carboxypeptidase cleaves at the carboxyl end of peptides,
the products are
Gly – Ile – Glu – Trp – Thr – Pro – Tyr – Gln – Phe – Arg
and
Lys
b. Because chymotrypsin cleaves peptide bonds in which aromatic
amino acids (i.e., Phe, Tyr, and Trp) contribute a carboxyl group,
the products are
Gly – Ile – Glu – Trp, Thr – Pro – Tyr,
Gln – Phe and Arg – Lys
BIOCHEMISTRY I ABA, Nov 2022 | Page 12
c. Trypsin cleaves at the carboxyl end of lysine and
arginine. The products are
Gly – Ile – Glu – Tyr – Thr – Pro – Tyr – Gln – Phe – Arg
and
Lys
d. DNFB tags the amino terminal amino acid. The products:
DNP – Gly and
Ile – Glu – Trp – Thr – Pro -Tyr – Gln – Phe – Arg – Lys
BIOCHEMISTRY I ABA, Nov 2022 | Page 13
Sample Problem 2
BIOCHEMISTRY I ABA, Nov 2022 | Page 14
Sample Problem 2
BIOCHEMISTRY I ABA, Nov 2022 | Page 15
Sample Problem 3
BIOCHEMISTRY I ABA, Nov 2022 | Page 16
Sample Problem 3
BIOCHEMISTRY I ABA, Nov 2022 | Page 17
Secondary (2o) Structures of Proteins
Arrangement of atoms of peptide backbone in space.
BIOCHEMISTRY I ABA, Nov 2022 | Page 18
Secondary Structures of Proteins
The peptide linkages are essentially planar thus allows only two
possible arrangements for the peptide backbone for the following
reasons:
– For two amino acids linked through a peptide bond six atoms lie in
the same plane
– The planar peptide linkage structure has considerable rigidity,
therefore rotation of groups about the C–N bond is hindered
– Cis–trans isomerism is possible about C–N bond.
– The trans isomer is the preferred orientation
BIOCHEMISTRY I ABA, Nov 2022 | Page 19
BIOCHEMISTRY I ABA, Nov 2022 | Page 20
Secondary Structures of Proteins
The only bond responsible for the 2o structure of proteins
is H-bonding between peptide bonds, the – C = O of one
peptide group and the – N – H of another peptide linkage
farther along the backbone.
The two most common types :
alpha-helix (α-helix)
beta-pleated sheet (β-pleated sheet).
BIOCHEMISTRY I ABA, Nov 2022 | Page 21
Alpha-helix (α-helix)
– H-bonding between amino acids
within the same chain –
intramolecular H-bonding
– Coiled helical spring
– R-groups stay outside of the
helix -- not enough room for
them to stay inside
– The helix is so tightly wound
that the space in the center is
too small for solvent molecules
to enter
BIOCHEMISTRY I ABA, Nov 2022 | Page 22
Alpha-helix (α-helix)
• Coil of the helix is clockwise or right-
handed
• There are 3.6 amino acids per turn
• Repeat distance is 5.4Å
• Each peptide bond is s-trans and planar
• C=O of each peptide bond is hydrogen
bonded to the N-H of the fourth amino
acid away
• C=O----H-N hydrogen bonds are parallel
to helical axis
• All R groups point outward from helix
BIOCHEMISTRY I ABA, Nov 2022 | Page 23
Several factors can disrupt an α -helix
• Proline creates a bend because of (1) the restricted
rotation due to its cyclic structure and (2) its α-amino
group has no N-H for hydrogen bonding
• Strong electrostatic repulsion caused by the proximity
of several side chains of like charge, e.g., Lys and Arg
or Glu and Asp
• Steric crowding caused by the proximity of bulky side
chains, e.g., Val, Ile, Thr
BIOCHEMISTRY I ABA, Nov 2022 | Page 24
Beta-Pleated Sheets (β-pleated sheet)
Completely extended protein chain
segments in same or different
molecules governed by intermolecular
or intramolecular H-bonding
H-bonding between different parts of
a single chain – intramolecular H-
bonding
Side chains below or above the axis
“U-turn” structure – most frequently
encountered
BIOCHEMISTRY I ABA, Nov 2022 | Page 25
Beta-Pleated Sheets (β-pleated sheet)
• Polypeptide chains lie adjacent to one another; may be
parallel or antiparallel
• R groups alternate, first above and then below plane
• Each peptide bond is s-trans and planar
• C=O and N-H groups of each peptide bond are
perpendicular to axis of the sheet
• C=O---H-N hydrogen bonds are between adjacent sheets
and perpendicular to the direction of the sheet
BIOCHEMISTRY I ABA, Nov 2022 | Page 26
Antiparallel β-pleated sheet
Chains run in opposite direction; more stable because of fully
collinear H- bonds
N-terminus of one chain is aligned with the C-terminus of a second
chain (head to tail)
BIOCHEMISTRY I ABA, Nov 2022 | Page 27
Parallel β-pleated sheet
Chains run in the same direction
N-termini are head-to-head
BIOCHEMISTRY I ABA, Nov 2022 | Page 28
Some proteins contain bot α-helices and β-sheets
• α -helices can gradually curve,
and are not exactly straight,
and that in the β -sheet the
strands are not the same in
length, nor is each perfectly
'flat’
• some parts that do not have
either α-helical or β-sheet
character—these regions may
be 'disordered’, or may
represent a type of turn
structure
BIOCHEMISTRY I ABA, Nov 2022 | Page 29
Structures of Reverse Turns
• Glycine found in reverse turns
• Spatial (steric) reasons
• Polypeptide changes direction
• Proline also encountered in reverse turns.
BIOCHEMISTRY I ABA, Nov 2022 | Page 30
β Turns
- carbonyl C of one residue is H-bonded to the amide proton of a
residue three residues away
- proline and glycine are prevalent in beta turns
BIOCHEMISTRY I ABA, Nov 2022 | Page 31
BIOCHEMISTRY I ABA, Nov 2022 | Page 32
Not all amino acids are equally likely to be found in each
type of secondary structure
BIOCHEMISTRY I ABA, Nov 2022 | Page 33
Fibrous Proteins
• Fibrous proteins: contain polypeptide chains
organized approximately parallel along a single axis.
They
• consist of long fibers or large sheets
• tend to be mechanically strong
• are insoluble in water and dilute salt solutions
• play important structural roles in nature
• Examples are
• keratin of hair and wool
• collagen of connective tissue of animals including cartilage,
bones, teeth, skin, and blood vessels
BIOCHEMISTRY I ABA, Nov 2022 | Page 34
Fibrous Proteins and Secondary Structures
BIOCHEMISTRY I ABA, Nov 2022 | Page 35
Fibrous Protein: Alpha-Keratin (α-Keratin)
A fibrous structural
protein consists of
~300 residues coiled
into a right-handed
helical secondary
structure, α-helix
stabilized by H-bonds
between amide N–H
groups and C=O
groups four residues
away.
BIOCHEMISTRY I ABA, Nov 2022 | Page 36
Alpha-Keratin (α-Keratin)
BIOCHEMISTRY I ABA, Nov 2022 | Page 37
Alpha-Keratin (α-Keratin)
• Provide protective coating for organs
• Major protein constituent of hair, feather, nails, horns
and turtle shells
• Mainly made of hydrophobic amino acid residues
• Hardness of keratin depends upon -S-S- bonds
– More –S-S– bonds make nail and bones hard and
hair brittle
BIOCHEMISTRY I ABA, Nov 2022 | Page 38
Alpha-Keratin (α-Keratin)
BIOCHEMISTRY I ABA, Nov 2022 | Page 39
Fibrous Protein: Collagen
- helical ("α-chain")
- left-handed, 3.3-residues per turn
- repeating tripeptide sequence
Gly-X-Pro (or Gly-X-HyPro)
- three helical strands wrap
around each other in a right-hand
sense to form a 'superhelix‘, with
H bonds extending between the
chains
BIOCHEMISTRY I ABA, Nov 2022 | Page 40
Fibrous Protein: Collagen
• Most abundant proteins in
humans (30% of total body
protein)
• Major structural material in
tendons, ligaments, blood
vessels, and skin
• Organic component of bones
and teeth
• Predominant structure - triple
helix
• Rich in proline (up to 20%) –
important to maintain struct
BIOCHEMISTRY I ABA, Nov 2022 | Page 41
Collagen
BIOCHEMISTRY I ABA, Nov 2022 | Page 42
Fibrous Protein: Silk Fibroin
• Fibroin is a protein produced by spiders & insects
• Spider dragline silk is both flexible and strong (tensile
strength of 200,000 psi
• Structure is largely "β-sheet”
BIOCHEMISTRY I ABA, Nov 2022 | Page 43
PROTEINS (4)
Dr. Aileen B. Angcajas November 2022
Department of Chemistry
College of Science and Mathematics
MSU-Iligan Institute of Technology
Iligan City
Globular Proteins
• Globular proteins: proteins which are folded
to a more or less spherical shape
• they tend to be soluble in water and salt solutions
• most of their polar side chains are on the outside
and interact with the aqueous environment by
hydrogen bonding and ion-dipole interactions
• most of their nonpolar side chains are buried
inside
• nearly all have substantial sections of α-helix and
β-sheet
BIOCHEMISTRY I ABA, Nov 2022 | Page 44
Comparison of Shapes of Fibrous and Globular Proteins
BIOCHEMISTRY I ABA, Nov 2022 | Page 45
Tertiary (3o) Structures of Proteins
Three-dimensional arrangements of all the atoms in
the molecule.
Polypeptide chain with its regions of secondary
structure, α-helix, and β-pleated sheet, further folds
on itself.
Tertiary structure forms spontaneously and is
maintained as a result of interactions among the R groups
of the amino acids.
BIOCHEMISTRY I ABA, Nov 2022 | Page 46
Forces in 3o Structure
• Noncovalent interactions, including
• Hydrogen bonding between polar side chains, e.g., Ser and Thr
• Hydrophobic interaction between nonpolar side chains, e.g., Val
and Ile
• Electrostatic attraction between side chains of opposite charge,
e.g., Lys and Glu
• Electrostatic repulsion between side chains of like charge, e.g.,
Lys and Arg, Glu and Asp
• Covalent interactions: Disulfide (-S-S-) bonds between
side chains of cysteines
BIOCHEMISTRY I ABA, Nov 2022 | Page 47
Forces in 3o Structure
BIOCHEMISTRY I ABA, Nov 2022 | Page 48
Globular Proteins: Myoglobin
A single polypeptide chain of 153 amino
acids
A single heme group in a hydrophobic
8 regions of α-helix; no regions of β-sheet
Most polar side chains are on the surface
Nonpolar side chains are folded to the
interior
BIOCHEMISTRY I ABA, Nov 2022 | Page 49
Heme: an oxygen-binding cofactor
BIOCHEMISTRY I ABA, Nov 2022 | Page 50
Heme-Iron-Protein Interaction
Fe2+ is octahedrally coordinated. Four binding groups from the
porphyrin ring are attached to it. The remaining coordination sites
lie along an axis perpendicular to the ring. One coordination of
these sites is occupied by the nitrogen of His 93 (proximal histidine).
BIOCHEMISTRY I ABA, Nov 2022 | Page 51
Myoglobin:
– An oxygen storage molecule in muscles.
– Monomer - single peptide chain with one heme unit
– Binds one O2 molecule
– Has a higher affinity for oxygen than hemoglobin.
– Oxygen stored in myoglobin molecules serves as a
reserve oxygen source for working muscles
Without the oxygen (deoxymyoglobin), the remaining
coordination site on the other side of the iron is occupied by a
water molecule. In the presence of oxygen (oxymyoglobin),
this site is occupied by oxygen.
BIOCHEMISTRY I ABA, Nov 2022 | Page 52
Quaternary (4o) Structures of Proteins
Quaternary structure is the result of noncovalent
interactions between two or more protein chains.
In some cases, the quaternary structure involves binding to
a nonprotein group called a prosthetic group.
e.g. Hemoglobin has four protein chains and the
heme prosthetic group.
The chains in a quaternary structure may be identical or
different.
dimer = 2 polypeptide chains
trimer = 3 polypeptide chains
tetramer = 4 polypeptide chains
BIOCHEMISTRY I ABA, Nov 2022 | Page 53
Nomenclature for Quaternary Structure
BIOCHEMISTRY I ABA, Nov 2022 | Page 54
Nomenclature for Quaternary Structure
BIOCHEMISTRY I ABA, Nov 2022 | Page 55
Oxygen-Binding Proteins
• Hemoglobin and myoglobin are heme-containing
oxygen transport and storage proteins (not enzymes)
– Hemoglobin (Hb) in blood
– Myoglobin (Mb) in muscle
• Hemoglobin and myoglobin have different oxygen
binding curves
• Mb is monomeric
– 153 aa, 16.7 kDa
• Hb is tetrameric (α2 β2) 64.5 kDa
– 2 α-subunits of 141 residues
– 2 β-subunits of 146 residues
BIOCHEMISTRY I ABA, Nov 2022 | Page 56
Structures of Myoglobin and Hemoglobin
Each of the four chains in in
hemoglobin has a folded structure
similar to that of myoglobin and
each carries a heme.
BIOCHEMISTRY I ABA, Nov 2022 | Page 57
Globular Proteins: Hemoglobin (Hb)
• Hb is a tetrameric heme protein
• The basic physiological function
of Hb is to bind oxygen in lungs
and release it in capillaries
• When a first oxygen binds to Fe
in heme of Hb, the heme Fe is
drawn into the plane of the
porphyrin ring
– Hb without a bound O2
molecule is called
deoxyhemoglobin
BIOCHEMISTRY I ABA, Nov 2022 | Page 58
Oxygen Binding of Hemoglobin
• Oxygen binds to heme in hemoglobin cooperatively:
as one O2 is bound, it becomes easier for the next
to bind.
• The first oxygen causes 2,3-bisphospho-glycerate (BPG)
to leave deoxyhemoglobin. This causes shape changes
which favor more reaction with oxygen.
• H+ produced by metabolizing cells (low pH) favors
oxygen release from Hb and higher pH in the lungs
favors binding of oxygen to Hb.
BIOCHEMISTRY I ABA, Nov 2022 | Page 59
pH/[CO2] Variation and CO2-binding to Hemoglobin
• Hb can bind H+ and CO2 and serves to carry
them from the tissues where they are
generated to the lungs & kidneys where
they are excreted
• At low pH (high [H+]) and high [CO2] Hb has
reduced O2 affinity
O2 & H+ have inverse binding affinities to Hb
at [O2] - releases H+ from Hb
(as in lung)
at [O2] - H+ binds Hb, O2 released
(as in peripheral tissue)
BIOCHEMISTRY I ABA, Nov 2022 | Page 60
Themes in Protein Function
• Structure dictates function
– primary structure → tertiary structure → protein function
• Proteins are often flexible & dynamic
– proteins have a unique shape & architecture, but many
(aside from certain fibrous/structural proteins) are not
rigid & immobile
BIOCHEMISTRY I ABA, Nov 2022 | Page 61
PROTEIN DENATURATION
Denaturation of a protein occurs when there is a change that
disrupts the interactions between R groups that stabilize the
secondary, tertiary, or quaternary structure. However, the
covalent amide bonds of the primary structure are not affected.
BIOCHEMISTRY I ABA, Nov 2022 | Page 62
PROTEIN DENATURATION
• Partial or complete disorganization of protein’s tertiary
structure
• Cooking food denatures the protein but does not change
protein nutritional value
• Coagulation: Precipitation (denaturation of proteins)
– Egg white - a concentrated solution of protein albumin -
forms a jelly when heated because the albumin is
denatured
• Cooking:
– Denatures proteins – Makes it easy for enzymes in our
body to hydrolyze/digest protein
– Fever: >104ºF – the critical enzymes of the body start
getting denatured
BIOCHEMISTRY I ABA, Nov 2022 | Page 63
PROTEIN DENATURATION
Temperature
As the temperature continues to increase, the bonds
within the proteins begin to vibrate more violently and
eventually, the weak interactions, like hydrogen bonds
and hydrophobic interactions, that maintain the
protein structure are disrupted.
BIOCHEMISTRY I ABA, Nov 2022 | Page 64
PROTEIN DENATURATION
Denaturation of egg protein occurs when the bonds of the tertiary
structure are disrupted.
BIOCHEMISTRY I ABA, Nov 2022 | Page 65
PROTEIN DENATURATION
BIOCHEMISTRY I ABA, Nov 2022 | Page 66
BIOCHEMISTRY I ABA, Nov 2022 | Page 67
You might also like
- Song Analysis of Rina Sawayama's XSDocument2 pagesSong Analysis of Rina Sawayama's XSMUHAMMAD SMITHNo ratings yet
- AS4041 ASME B31 - 3 Pipe Wall ThicknessDocument11 pagesAS4041 ASME B31 - 3 Pipe Wall Thicknessamini_mohiNo ratings yet
- Okuma - Osp - E100m - E10m - Alarm Erros List - ME37005R4E100MAlarm883220150816 PDFDocument588 pagesOkuma - Osp - E100m - E10m - Alarm Erros List - ME37005R4E100MAlarm883220150816 PDFAntonioCanestriJúnior100% (3)
- Item Supplier Equipment Brand: Miraco CarrierDocument55 pagesItem Supplier Equipment Brand: Miraco Carriermostafaabdelrazik100% (1)
- ProteinsDocument70 pagesProteinsDianne Joy67% (3)
- Protein Intake PTC8 PDFDocument110 pagesProtein Intake PTC8 PDFvNo ratings yet
- 6-7 ProteinDocument17 pages6-7 Proteinyr44grf94kNo ratings yet
- Amino Acids, Proteins, and EnzymesDocument62 pagesAmino Acids, Proteins, and Enzymesendale gebregzabherNo ratings yet
- Amino AcidDocument24 pagesAmino AcidAdisa YellowNo ratings yet
- ProteinDocument251 pagesProteinJohn BrandonNo ratings yet
- ProteinDocument39 pagesProteinNICHOLE MOJELLO100% (2)
- Amino AcidsDocument10 pagesAmino Acidszubairahmed27272No ratings yet
- Lecture 1 Biochemistry of Amino Acids and Protein Structure FunctionDocument23 pagesLecture 1 Biochemistry of Amino Acids and Protein Structure Functionbeneficialboxer9237No ratings yet
- PROTEINSDocument10 pagesPROTEINSHarry PotterNo ratings yet
- Protein N.2023Document35 pagesProtein N.2023ea4184386No ratings yet
- ProteinDocument31 pagesProteinShiela Marie BanggotNo ratings yet
- Lecture1 - Introduction To BiomoleculesDocument25 pagesLecture1 - Introduction To BiomoleculesKrisan Mallion LuisNo ratings yet
- ProteinDocument23 pagesProteinsamantha garciaNo ratings yet
- Final ProteinDocument57 pagesFinal ProteinmfernandezNo ratings yet
- Amino Acids BiochemistryDocument21 pagesAmino Acids BiochemistryAhmed Talal100% (1)
- Biochemistry Chapter 6Document51 pagesBiochemistry Chapter 6gus peepNo ratings yet
- ASci Grade 11 T1 W6 - 2021Document6 pagesASci Grade 11 T1 W6 - 2021ntandoblaai77No ratings yet
- Proteins-Miss QudsiaDocument76 pagesProteins-Miss QudsiaAbrar KhanNo ratings yet
- Proteins: Sheila Mai S. Rocabo, RMT, MSMTDocument37 pagesProteins: Sheila Mai S. Rocabo, RMT, MSMTBlexx LagrimasNo ratings yet
- Chapter TwoDocument43 pagesChapter TwoAlemayehu MelkamuuNo ratings yet
- Protein Notes (Dipesh Routh)Document7 pagesProtein Notes (Dipesh Routh)Dipesh RouthNo ratings yet
- Chemistry of Cell-1Document101 pagesChemistry of Cell-12022mcb1267No ratings yet
- Chapter 3 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20Document7 pagesChapter 3 Biochemistry and Clinical Pathology Complete Notes by Noteskarts Acc To ER20yplsinghNo ratings yet
- Proteins: A Protein Is A Naturally Occurring, Unbranched Polymer in Which The Monomer Units Are Amino AcidsDocument45 pagesProteins: A Protein Is A Naturally Occurring, Unbranched Polymer in Which The Monomer Units Are Amino Acidsdarleen joy dimaanoNo ratings yet
- Proteins ReviewerDocument8 pagesProteins Reviewer4th otosakaNo ratings yet
- Amino Acid1Document24 pagesAmino Acid1eshaanihsNo ratings yet
- ProteinsDocument20 pagesProteinsallisonpablo49No ratings yet
- Protein 31.01.23Document87 pagesProtein 31.01.23Gourab NandaNo ratings yet
- Proteinsoct 2016Document28 pagesProteinsoct 2016Abhimanyu BalyanNo ratings yet
- PROTEINDocument90 pagesPROTEINYo1No ratings yet
- Protiens: Yeshewas Abaynew (BSC, MPH)Document38 pagesProtiens: Yeshewas Abaynew (BSC, MPH)Abebe GedamNo ratings yet
- ProteinsDocument107 pagesProteinsuy02884No ratings yet
- Proteins and AminoacidsDocument62 pagesProteins and AminoacidsHumrazNo ratings yet
- Local Media5209454088282345Document19 pagesLocal Media5209454088282345Michaela Lyn Parilla MianaNo ratings yet
- ProteinsDocument54 pagesProteinsbombarts190No ratings yet
- 3 ProtienDocument93 pages3 ProtienHasen umerNo ratings yet
- Chemistry of Amino AcidsDocument48 pagesChemistry of Amino Acidsshadrack mungutiNo ratings yet
- ProteinDocument55 pagesProteinsanjanash466No ratings yet
- ProteinsDocument22 pagesProteinsassmmNo ratings yet
- Amino AcidsDocument5 pagesAmino AcidsAdams DeborahNo ratings yet
- Amino Acid, Peptides and ProteinsDocument94 pagesAmino Acid, Peptides and Proteinsintarebatinya32No ratings yet
- ProteinsDocument38 pagesProteinseugieniogienioNo ratings yet
- Foods and Nutrition Paper OneDocument119 pagesFoods and Nutrition Paper OneNaduku EridadiNo ratings yet
- Chemistry of Aminoacids & Proteins NursingDocument69 pagesChemistry of Aminoacids & Proteins Nursinghappy9874648No ratings yet
- BIOMOLECULES Proteins and Nucleic AcidsDocument23 pagesBIOMOLECULES Proteins and Nucleic Acidsjia aganaNo ratings yet
- Midterm (Protein, Carbs&Lipids)Document132 pagesMidterm (Protein, Carbs&Lipids)Yo1No ratings yet
- Proteins PharmacyDocument26 pagesProteins PharmacywithneyNo ratings yet
- Proteins and Amino Acid-NoteDocument13 pagesProteins and Amino Acid-NoteHABIBUR RAHAMANNo ratings yet
- Amino Acids & Proteins: C, H, O, N, SDocument12 pagesAmino Acids & Proteins: C, H, O, N, SAlexandra maeNo ratings yet
- Amino Acids and ProteinsDocument62 pagesAmino Acids and ProteinsSolar Adhikari100% (1)
- Protein PDFDocument27 pagesProtein PDFSumaiyaNo ratings yet
- Physical Science SHS 8.1 ProteinsDocument21 pagesPhysical Science SHS 8.1 ProteinsjouselleduayNo ratings yet
- PROTEINSDocument20 pagesPROTEINSYam Lehte MagallanesNo ratings yet
- Bio Chemistry Chapter # 3 ProteinsDocument11 pagesBio Chemistry Chapter # 3 ProteinsEmo tionsNo ratings yet
- LifeScience I Week 2 Topic 3Document36 pagesLifeScience I Week 2 Topic 3nsemn1234No ratings yet
- ProteinDocument33 pagesProteinIhsan Badsha100% (1)
- User's Guide to Protein and Amino Acids: Learn How Protein Foods and Their Building Blocks Can Improve Your Mood and HealthFrom EverandUser's Guide to Protein and Amino Acids: Learn How Protein Foods and Their Building Blocks Can Improve Your Mood and HealthRating: 5 out of 5 stars5/5 (2)
- Peptides: A Journey Into the World of Health Optimization (The Anti-aging Power of Peptides in Cosmetics for Skin Rejuvenation)From EverandPeptides: A Journey Into the World of Health Optimization (The Anti-aging Power of Peptides in Cosmetics for Skin Rejuvenation)No ratings yet
- "Iwas Bato at Tabako para Sa Katawang Pagbabago".Document3 pages"Iwas Bato at Tabako para Sa Katawang Pagbabago".MUHAMMAD SMITHNo ratings yet
- Sandigan Ggant ChartDocument2 pagesSandigan Ggant ChartMUHAMMAD SMITHNo ratings yet
- EEE180 Lab7 - p4Document1 pageEEE180 Lab7 - p4MUHAMMAD SMITHNo ratings yet
- 5small Signal AC Analysis of BJT Amplifiers Part2Document78 pages5small Signal AC Analysis of BJT Amplifiers Part2MUHAMMAD SMITHNo ratings yet
- Electron TubesDocument43 pagesElectron TubesMUHAMMAD SMITHNo ratings yet
- EEE180 Lab7 - p3Document1 pageEEE180 Lab7 - p3MUHAMMAD SMITHNo ratings yet
- EEE180 Lab7 - p2Document1 pageEEE180 Lab7 - p2MUHAMMAD SMITHNo ratings yet
- Small Signal AC Analysis FET Part 2Document42 pagesSmall Signal AC Analysis FET Part 2MUHAMMAD SMITHNo ratings yet
- FET Amplifiers: Small Signal ModelDocument56 pagesFET Amplifiers: Small Signal ModelMUHAMMAD SMITHNo ratings yet
- 4 Pics 1 Paragraph 1. My Own Definition of PEACEDocument2 pages4 Pics 1 Paragraph 1. My Own Definition of PEACEMUHAMMAD SMITHNo ratings yet
- Field-Effect TransistorsDocument50 pagesField-Effect TransistorsMUHAMMAD SMITHNo ratings yet
- Step by Step Documentation Lab Activity 5: Muhammad P. SmithDocument15 pagesStep by Step Documentation Lab Activity 5: Muhammad P. SmithMUHAMMAD SMITHNo ratings yet
- MAPS - Mathematics Attitudes and Perceptions SurveyDocument2 pagesMAPS - Mathematics Attitudes and Perceptions SurveyMUHAMMAD SMITH100% (1)
- Step by Step DocumentationDocument23 pagesStep by Step DocumentationMUHAMMAD SMITHNo ratings yet
- Detroit Diesel 91 A 93Document3 pagesDetroit Diesel 91 A 93JoseGarzaNo ratings yet
- FT-PT UG Academic Calendar Jan-Dec 2019Document5 pagesFT-PT UG Academic Calendar Jan-Dec 2019AlexNo ratings yet
- Bearing Failure ModesDocument18 pagesBearing Failure ModesSreekanthMylavarapuNo ratings yet
- The Rhetorical Works of George of Trebizond and Their Debt To CiceroDocument11 pagesThe Rhetorical Works of George of Trebizond and Their Debt To CicerobrysonruNo ratings yet
- Design of DamsDocument101 pagesDesign of DamsLuis Rizabal Gamarra100% (1)
- Week 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesDocument27 pagesWeek 2 Endocrine Anatomy and Physiology Review & Pituitary DisturbancesZiqri Dimas SandyNo ratings yet
- Domestic Water Booster System Sizing & SpecificationDocument76 pagesDomestic Water Booster System Sizing & SpecificationKrishnan VaidyanathanNo ratings yet
- Market Risk: You Manage What You MeasureDocument14 pagesMarket Risk: You Manage What You MeasureLuis EcheNo ratings yet
- Quote CT PT Isolator VCB AB Switch Drop Out Fuse Set TPMO SMC LT Distribution Box HT PanelDocument11 pagesQuote CT PT Isolator VCB AB Switch Drop Out Fuse Set TPMO SMC LT Distribution Box HT PanelSharafatNo ratings yet
- Nibiru Research Gerald Clark 04.28.15 PDFDocument49 pagesNibiru Research Gerald Clark 04.28.15 PDFsunsangra100% (1)
- Q4 DLL Math1 Week-3Document5 pagesQ4 DLL Math1 Week-3Rosbel SoriaNo ratings yet
- Display Visay 192g0885Document3 pagesDisplay Visay 192g0885said luyNo ratings yet
- Process Hazard Analysis Software GuideDocument6 pagesProcess Hazard Analysis Software GuideAdarsh Sv100% (1)
- Answering Exam QuestionsDocument14 pagesAnswering Exam QuestionsChreze If TorioNo ratings yet
- Activities KeyDocument7 pagesActivities KeyCassandra Dianne Ferolino MacadoNo ratings yet
- AllDocument11 pagesAllCaluian YonutzNo ratings yet
- Geomechanics Abstracts: GeneralDocument1 pageGeomechanics Abstracts: Generalbelbet79No ratings yet
- Tea Stain Decolorization by Vinegar and Bleach (With Image Analysis) (Hester 2016)Document13 pagesTea Stain Decolorization by Vinegar and Bleach (With Image Analysis) (Hester 2016)login ackyipNo ratings yet
- Electric Machinery Fundamentals-8Document20 pagesElectric Machinery Fundamentals-8AmaniDarwishNo ratings yet
- Allowable Span/Depth Ratio For High Strength Concrete BeamsDocument1 pageAllowable Span/Depth Ratio For High Strength Concrete BeamsbagmassNo ratings yet
- S4 PhyDocument4 pagesS4 PhyEMMANUEL BIRUNGINo ratings yet
- PMC 2, Input Output RobodrillDocument6 pagesPMC 2, Input Output RobodrillQuyen VuongNo ratings yet
- Dbms Imp NotesDocument5 pagesDbms Imp NotesRishi Raj K0% (1)
- Variable Volume Vane Pumps Silentvane SV 20 SV 25Document8 pagesVariable Volume Vane Pumps Silentvane SV 20 SV 25Mohamed BelallNo ratings yet
- Chemical NamesDocument4 pagesChemical NamesSomesubhraDasNo ratings yet
- M201ew01 V0 AuoDocument32 pagesM201ew01 V0 AuoBonetzNo ratings yet
- 01-Ge-Aqa-9600 Itp DMF FRP Water Trough r2Document29 pages01-Ge-Aqa-9600 Itp DMF FRP Water Trough r2Eljo AndsNo ratings yet