Professional Documents
Culture Documents
Reg Flowchart HumanPharma
Reg Flowchart HumanPharma
Uploaded by
Sughra ChandioOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reg Flowchart HumanPharma
Reg Flowchart HumanPharma
Uploaded by
Sughra ChandioCopyright:
Available Formats
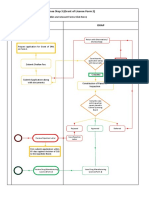
Grant of Drug Registration (Pharmaceutical drug for human use)
(For complete Process Description, Checklist and relevant Forms Click Here)
Manufacturer / Applicant DRAP
Shortcoming letter issued
Fill Drug (Pharmaceutical)
Registration Application Non-
Form 5F Receive Application Dossier Compliant
Submit Fee Challan
Technical Evaluation and Verification by
Pharmaceutical Evaluation Cell
Submit Compliant
Application
Dossier
New Drug/Subsequent Generic
(product Specific Inspection if Generic Drugs
required)
Registration
Not approved Deferred
Board Meeting
Receive Rejection Expert opinion (if
Rejection letter required)
letter
Approved
Firm submits application within 60
days against decision of DRB to the
Appellate Board. Locally Manufactured Imported Drugs
Not Dosage form specific
Recommended inspection (if required)
Recommended
Issue Registration Letter (Subject
Receive Registration
to price fixation by Federal
Letter
Government)
You might also like
- FDA Circular 2019-001Document4 pagesFDA Circular 2019-001Apple Mae Princess RacpanNo ratings yet
- Joint InfectionsDocument10 pagesJoint InfectionsJPNo ratings yet
- Overview Process Map On The Healthcare Practitioners Registration-Licensing ProcessDocument1 pageOverview Process Map On The Healthcare Practitioners Registration-Licensing ProcesssaaidNo ratings yet
- Grant of DML Step 3 PDFDocument1 pageGrant of DML Step 3 PDFAfrazNo ratings yet
- FC For Grant of Drug Import LicenseDocument1 pageFC For Grant of Drug Import LicenseAwais PanhwarNo ratings yet
- Guidelines For NursesDocument21 pagesGuidelines For NursesSumayya AhmedNo ratings yet
- Pe 10Document6 pagesPe 10KARTHIKNo ratings yet
- Flow Chart For Application Ap Type (Inq) - Other Vehicle Permanent Import - Special Purpose Vehicle (SPV)Document1 pageFlow Chart For Application Ap Type (Inq) - Other Vehicle Permanent Import - Special Purpose Vehicle (SPV)I.W.R Gaming 2.0No ratings yet
- Trademark Registration Flow ChartDocument1 pageTrademark Registration Flow ChartgautamNo ratings yet
- Oecd Recommendation Governance of Clinical Trials PDFDocument51 pagesOecd Recommendation Governance of Clinical Trials PDFMarianaSoaresNo ratings yet
- Guidelines For DentistsDocument42 pagesGuidelines For DentistsNadine El Jundi OuaidaNo ratings yet
- Submission GuidanceDocument10 pagesSubmission Guidancemayar.mo7med.876No ratings yet
- Guidelines For DentistsDocument41 pagesGuidelines For Dentistspradeep samuelNo ratings yet
- Guidelines For PhysiciansDocument34 pagesGuidelines For PhysiciansSajid KhanNo ratings yet
- Screenshot 2023-05-14 at 12.44.49 PMDocument43 pagesScreenshot 2023-05-14 at 12.44.49 PMrq678phccNo ratings yet
- OPPE Indicator List by DeparDocument11 pagesOPPE Indicator List by DeparYandiNo ratings yet
- Repair Maintenance Labor FinalDocument21 pagesRepair Maintenance Labor FinalJaneDandanNo ratings yet
- DD Process FlowDocument1 pageDD Process FlowshamsolNo ratings yet
- Guidelines For PharmacistsDocument14 pagesGuidelines For PharmacistsHUMANITARIAN Sajjad Ahmad KhanNo ratings yet
- Renewal Application of LTO 1.2Document2 pagesRenewal Application of LTO 1.2Hazel BisaNo ratings yet
- Purchase Process Flow Chart: (Along With All Specification, Quality and Estimated Cost of Procurement.)Document1 pagePurchase Process Flow Chart: (Along With All Specification, Quality and Estimated Cost of Procurement.)SadanandNo ratings yet
- Notices For The Application of Plant Master File Form A PDFDocument5 pagesNotices For The Application of Plant Master File Form A PDFAnandharaj AsaithambiNo ratings yet
- Izin Edar - IPAKDocument6 pagesIzin Edar - IPAKChitra TjahjonoNo ratings yet
- Form 5 Form 5A Form 5D Form 5EDocument2 pagesForm 5 Form 5A Form 5D Form 5Eمحمد ہاشمNo ratings yet
- Reg PER1Document2 pagesReg PER1Neneng Aini KaruniawanNo ratings yet
- 2 - DS SATK Form - Renewal Application of LTO 1.2Document2 pages2 - DS SATK Form - Renewal Application of LTO 1.2Lexi MontanteNo ratings yet
- Section 47 1 F Website GuidanceDocument1 pageSection 47 1 F Website Guidancehammad123456789No ratings yet
- Guidelines For AHPDocument19 pagesGuidelines For AHPHatem MetahNo ratings yet
- Check List For Scrutinization of Registration Application DossiersDocument10 pagesCheck List For Scrutinization of Registration Application DossiersTayyab Tahir100% (1)
- HtahaadDocument8 pagesHtahaadmahmoudessa3444No ratings yet
- Dr. Ramadan IbrahimDocument6 pagesDr. Ramadan IbrahimShorbanNo ratings yet
- Overview of New OTC Application Process 6-23-15Document1 pageOverview of New OTC Application Process 6-23-15dragonboyy3kNo ratings yet
- TFA Mechanisms Table - RevDocument14 pagesTFA Mechanisms Table - RevLaurence MillanNo ratings yet
- Quality LEGAL AND OTHER REQUIREMENTS REGISTERDocument8 pagesQuality LEGAL AND OTHER REQUIREMENTS REGISTERanoushia alviNo ratings yet
- 2020 ChecklistDocument17 pages2020 ChecklistPhoeza Espinosa Villanueva100% (1)
- (TEMPLATE) ESH Compliance Obligations RegisterDocument8 pages(TEMPLATE) ESH Compliance Obligations RegisterAlexis AlbosNo ratings yet
- Canada LicenseDocument6 pagesCanada LicenseLucy BrittainNo ratings yet
- Flow Chart For General PurchasingDocument1 pageFlow Chart For General PurchasingVinod SaleNo ratings yet
- Infante Patent Flowchart IPLDocument5 pagesInfante Patent Flowchart IPLJanileahJudetteInfanteNo ratings yet
- Alchemy Medicine Private LTD.: Annexure-1Document4 pagesAlchemy Medicine Private LTD.: Annexure-1kardam.bajajfNo ratings yet
- Workflow Process For New Drug Request Form PDFDocument1 pageWorkflow Process For New Drug Request Form PDFIchigo ShoonNo ratings yet
- Guidelines For PharmacistsDocument19 pagesGuidelines For PharmacistsebayNo ratings yet
- SUGAMDocument22 pagesSUGAMSreedhar TirunagariNo ratings yet
- Dao 99-53Document1 pageDao 99-53Ronalyn OrpianoNo ratings yet
- S010 - D002 - A0038-PROCEDUREDETAIL-Application For SellStorage For WholesellerRetailer (Service Under Fertilizer)Document1 pageS010 - D002 - A0038-PROCEDUREDETAIL-Application For SellStorage For WholesellerRetailer (Service Under Fertilizer)animeshmoh1No ratings yet
- Section 3 Evaluation For New SMEDocument7 pagesSection 3 Evaluation For New SMEDereje AberaNo ratings yet
- Documents ListDocument1 pageDocuments ListsdNo ratings yet
- IPS UM 161115 - Thesis or Dissertation Correction Report Form - EmelDocument4 pagesIPS UM 161115 - Thesis or Dissertation Correction Report Form - EmelNazalia KiprawiNo ratings yet
- CTD EDA Submission Guidance Version 3 - March 2024Document24 pagesCTD EDA Submission Guidance Version 3 - March 2024dra.orchidiaNo ratings yet
- Guidelines For Allied Healthcare PractitionersDocument21 pagesGuidelines For Allied Healthcare PractitionersSohl SantosNo ratings yet
- BPLO - Unified Application Form For Business Permit NEW BUSINESS PERMIT 2023Document3 pagesBPLO - Unified Application Form For Business Permit NEW BUSINESS PERMIT 2023Jlj ChuaNo ratings yet
- SRF-Serviced Plots-Manual Submission ONLYDocument1 pageSRF-Serviced Plots-Manual Submission ONLYARUL SANKARANNo ratings yet
- Revised Corporation Code Table of ApprovalDocument9 pagesRevised Corporation Code Table of ApprovalYannah HidalgoNo ratings yet
- Tesda Op Ias 01 F04 DDocument15 pagesTesda Op Ias 01 F04 DJan Peter PiliNo ratings yet
- 21 CFR Part 814 Premarket Approval of Medical DevicesDocument24 pages21 CFR Part 814 Premarket Approval of Medical DevicesrajivheroNo ratings yet
- FVC Latest FormatDocument3 pagesFVC Latest Formatrohan.rishi01No ratings yet
- Ofnz Off Farm Inputs ScheduleDocument1 pageOfnz Off Farm Inputs Scheduleapi-285921977No ratings yet
- Chinese Taipei UpdatesDocument11 pagesChinese Taipei UpdatesShankar BNo ratings yet
- Trademark Registration Procedure Flow ChartDocument1 pageTrademark Registration Procedure Flow ChartIzzyMaxinoNo ratings yet
- RF FVDB-05 Application For Veterinary Drug and Product Registration (CPR)Document5 pagesRF FVDB-05 Application For Veterinary Drug and Product Registration (CPR)jeffrey ignacioNo ratings yet
- A Study On Customers Perception and Satisfaction Towards Green Tea With Special Reference To Coimbotore City"Document4 pagesA Study On Customers Perception and Satisfaction Towards Green Tea With Special Reference To Coimbotore City"Divyam JakhmolaNo ratings yet
- PGDMHDocument79 pagesPGDMHBGangadharReddyNo ratings yet
- ReDocument4 pagesReIsabel Mamani RamirezNo ratings yet
- Food Sanitation SeminarDocument11 pagesFood Sanitation SeminarsuryafarheenNo ratings yet
- Pain Management Clinical Guidelinesv2 PDFDocument15 pagesPain Management Clinical Guidelinesv2 PDFErwin Novia Rachmawati100% (1)
- Job Hazard Analysis RiskassessmentDocument4 pagesJob Hazard Analysis RiskassessmentJunard M. Lu HapNo ratings yet
- Research Paper - Drug Addiction and AbusDocument5 pagesResearch Paper - Drug Addiction and AbusAvel BadilloNo ratings yet
- CHW Pipe Installation, Insulation & Cladding - JSA - Risk AssessmentDocument13 pagesCHW Pipe Installation, Insulation & Cladding - JSA - Risk Assessmentlike saddamNo ratings yet
- Telemedicine: Indian National & Institutional Perspective: Prof. S K Mishra, MS, FACSDocument29 pagesTelemedicine: Indian National & Institutional Perspective: Prof. S K Mishra, MS, FACSRobbin BajpaiNo ratings yet
- Case Analysis Assignment AUBF 1Document2 pagesCase Analysis Assignment AUBF 1Emma CentenoNo ratings yet
- Q1-1. Consumer HealthDocument35 pagesQ1-1. Consumer HealthMaven JadeNo ratings yet
- Junard M. Lu Hap, RN: #51 Champaca Lapu-Lapu Street Brgy. Dadiangas East, General Santos City Contact Number 050-310-9586Document6 pagesJunard M. Lu Hap, RN: #51 Champaca Lapu-Lapu Street Brgy. Dadiangas East, General Santos City Contact Number 050-310-9586Junard M. Lu HapNo ratings yet
- Wei Zheng, 2018Document8 pagesWei Zheng, 2018Hananya ManroeNo ratings yet
- Burn Project ReferencesDocument2 pagesBurn Project Referencesapi-279967709No ratings yet
- Toronto Notes - Case 4 - DiabetesDocument1 pageToronto Notes - Case 4 - DiabetesSumer ChauhanNo ratings yet
- Registered Nurse Resume Sample FormatDocument7 pagesRegistered Nurse Resume Sample Formatafllbybas100% (1)
- Unnatural CausesDocument2 pagesUnnatural CausesSovia MuspahNo ratings yet
- Presentation Masks PDFDocument42 pagesPresentation Masks PDFGrafica TurboblenderNo ratings yet
- DDX of Generalized OedemaDocument33 pagesDDX of Generalized Oedemazaw wai aungNo ratings yet
- Ascariasis Levels of PrevDocument2 pagesAscariasis Levels of PrevLeslie Noelle GarciaNo ratings yet
- OCA - MarchDocument33 pagesOCA - Marchbmc liqsNo ratings yet
- Risk Register Peralatan Medik 2022Document8 pagesRisk Register Peralatan Medik 2022maintenance anmedNo ratings yet
- Dr. Nasir: Curriculum VitaeDocument15 pagesDr. Nasir: Curriculum VitaeJoshua BoltonNo ratings yet
- Management QuotaDocument441 pagesManagement Quotaroh iniNo ratings yet
- Newborn Infant Hearing Screening DMIMSUDocument12 pagesNewborn Infant Hearing Screening DMIMSUSharnie JoNo ratings yet
- Fostering Students CreativityDocument43 pagesFostering Students CreativityPaulo GomesNo ratings yet
- Explosive RegDocument26 pagesExplosive RegvictorNo ratings yet
- Health Trends, Issues, and Concerns Part 2Document30 pagesHealth Trends, Issues, and Concerns Part 2D AngelaNo ratings yet
- Preparation No. "04" "Castor Oil Capsule" A. Wrap-Up Guide QuestionsDocument4 pagesPreparation No. "04" "Castor Oil Capsule" A. Wrap-Up Guide QuestionsJames AzurinNo ratings yet