Professional Documents
Culture Documents
Nanostr
Nanostr
Uploaded by
SJOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nanostr
Nanostr
Uploaded by
SJCopyright:
Available Formats
26884011, 0, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/nano.202200081 by National Medical Library The Director, Wiley Online Library on [07/11/2022].
See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
6 PAVITHRA and DEY
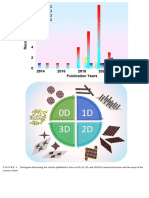
TA B L E 1 0D-HEA nanostructures
0D/Nanoparticles Application Alloys Refs.
Carbothermal shock synthesis ∙ Ammonia PtCoNiFeCu, PtPdCoNiFe, [24,27]
oxidation/decomposition PtCoNiFeCuAu,
∙ Oxygen reduction reaction PtPdCoNiFeCuAu,
(ORR) PtPdCoNiFeCuAuSn,
∙ Oxygen catalysis (Li–O2 CoMoFeNiCu, RuRhCoNiIr,
battery) PtPdRhRuIr,
∙ Energy storage PtPdRhRuIrFeCoNi,
RuIrCeNiWCuCrCo,
FeNiCoMnMg,
FeNiCoMnCu
Aerosol synthesis ∙ In situ Fe0.28 Co0.21 Ni0.20 Cu0.08 Pt0.23 , [22a,28]
oxidation/high-temperature NiCoFeCuPt, NiCoFePdPt,
reduction behavior NiCoCuFePd
[29]
Microwave heating PtPdFeCoNi
Wet chemical synthesis ∙ Ethanol oxidation reaction NiFeCrCuCo, RuRhPdIrPt [23,30]
Ultrasonication-assisted wet ∙ Hydrogen evolution reaction PtAuPdRhRu [31]
chemistry (HER)
Sol–gel autocombustion ∙ Magnetic CoCrCuNiAl [32]
[33]
Solvothermal synthesis Pt0.20 Pd0.20 Ir0.20 Rh0.20 Ru0.20,
PtIrPdRhRu
Chemical co-reduction ∙ Hydrogen evolution catalysis NiCoFePtRh [34]
One-pot polyol process ∙ HER IrPdPtRhRu [35]
Arc-discharge process ∙ Corrosion resistance AlCoCrCuFeNi, CoCrCuFeNi [36]
∙ Magnetic
[16b]
Spark discharge process CoCrFeNiPt, CoFeNiCr0.5 Pd0.8
Pyrolysis of metallocenes ∙ Magnetic CrMnFeCoNi [37]
Bed pyrolysis ∙ HER FeCoPdIrPt, [38]
∙ Water splitting Mn, Co, Ni, Cu, Rh, Pd, Sn, Ir,
Pt, and Au,
CuPdSnPtAu, NiCuPdSnPtAu,
NiCuPdSnIrPtAu,
CoNiCuPdSnIrPtAu
[39]
Scanning-probe block AuAgCuCoNi
copolymer lithography
method
Direct current sputtering ∙ Oxygen reduction reaction CrMnFeCoNi [40]
(OER)
Laser-synthesis ∙ OER CoCrFeNiMn [41]
picosecond-pulsed laser
ablation
[42]
Vapor-source technique NiCrFeAuAg, NiCrCoAuAg,
NiCrCoMoAuAg,
NiCrFeCuPd,
NiCrCoMoCuPd
immiscible/unmixable elements is not possible through droplets of <1 µm in size, which are then subjected to
conventional techniques. The steps of this process are fast heating and rapid quenching. The individual droplets
similar to those of CTS, however, this method addresses of smaller-length scales with very low thermal mass act
the challenges faced by CTS and is complimentary to as a nanoreactor, thereby affording NPs while avoiding
it. Thus, aerosol synthesis[22a,28] also involves aqueous the thermal and mass transfer gradients within the par-
solutions of metal salts containing the required alloy- ticles. Like CTS, fast heating in the aerosol process also
ing elements. These aqueous solutions are nebulized into results in decomposition of the precursor followed by rapid
You might also like
- General Purpose Steel Grade ChartDocument2 pagesGeneral Purpose Steel Grade ChartDavid D'Agostino50% (2)
- Aquilini Renewable Energy Inc.Document3 pagesAquilini Renewable Energy Inc.lpulian100% (2)
- Moor 1994 Cane ShreddingDocument4 pagesMoor 1994 Cane Shreddingamarnath jagirdar100% (1)
- Niobium Carbide From OxideDocument15 pagesNiobium Carbide From OxideHygaNo ratings yet
- Shuai 2020Document5 pagesShuai 2020Virat KohNo ratings yet
- Chem 1140 Ring-Closing Metathesis (RCM) and Ring-Opening Metathesis (ROMP)Document26 pagesChem 1140 Ring-Closing Metathesis (RCM) and Ring-Opening Metathesis (ROMP)Abhishek GuddadNo ratings yet
- Church 1994Document7 pagesChurch 1994Sebastian Ortiz BuitragoNo ratings yet
- Catalysts: Adipic Acid Route: Oxidation of Cyclohexene vs. CyclohexaneDocument7 pagesCatalysts: Adipic Acid Route: Oxidation of Cyclohexene vs. CyclohexaneLuiz Rodrigo AssisNo ratings yet
- 00400019b5 Papers 00400019b5 00400019b5 Co-Zn PDFDocument4 pages00400019b5 Papers 00400019b5 00400019b5 Co-Zn PDFShreshta JainNo ratings yet
- 9f 20100076 Rishi Biswas Chemistry ProjectDocument17 pages9f 20100076 Rishi Biswas Chemistry ProjectRishi BiswasNo ratings yet
- Ammonium Sulfate: Physical PropertiesDocument2 pagesAmmonium Sulfate: Physical PropertiesHafiz Rama DevaraNo ratings yet
- Chapter 12 TNDocument18 pagesChapter 12 TNambreenlateef814No ratings yet
- Selective Oxidation of Styrene To Benzaldehyde byDocument7 pagesSelective Oxidation of Styrene To Benzaldehyde bywiam wiamNo ratings yet
- Monte-Carlo Methods For Simulating The Catalytic Oxidative Dehydrogenation of Propane Over Vmgo CatalystDocument10 pagesMonte-Carlo Methods For Simulating The Catalytic Oxidative Dehydrogenation of Propane Over Vmgo CatalystMayteNo ratings yet
- RDRP of VC - Perspective2023Document35 pagesRDRP of VC - Perspective2023Jorge CoelhoNo ratings yet
- Electroreduction of Carbon Dioxide by Heterogenized Cofacial PorphyrinsDocument7 pagesElectroreduction of Carbon Dioxide by Heterogenized Cofacial Porphyrinsbin caiNo ratings yet
- Artigo Controle Cinetico e TermodinamicoDocument6 pagesArtigo Controle Cinetico e TermodinamicoPatricia Amaral CeldeiraNo ratings yet
- Ammonium Sulfate PDFDocument2 pagesAmmonium Sulfate PDFSetya SandyNo ratings yet
- Organic SynthesisDocument7 pagesOrganic SynthesisBalogunNo ratings yet
- Direct Nitration of Five Membered HeteroDocument13 pagesDirect Nitration of Five Membered HeteroJose David Gomez ColinNo ratings yet
- Coordination Compound CHEMHACKDocument3 pagesCoordination Compound CHEMHACKakashhk1001No ratings yet
- Selective Extraction of Cobalt From Nickel Sulphate Solutions by CyanexDocument6 pagesSelective Extraction of Cobalt From Nickel Sulphate Solutions by CyanexArifo Gunawan CahyanegoroNo ratings yet
- Thermochemistry of A Biomimetic and Rubisco-Inspired CO Capture System From AirDocument14 pagesThermochemistry of A Biomimetic and Rubisco-Inspired CO Capture System From AirAhdanNo ratings yet
- Preparation of Nanocrystalline Bifeo Via A Simple and Novel Method and Its Kinetics of CrystallizationDocument8 pagesPreparation of Nanocrystalline Bifeo Via A Simple and Novel Method and Its Kinetics of CrystallizationBojan StojadinovićNo ratings yet
- Important Questions 2022Document3 pagesImportant Questions 2022Ammi KhanNo ratings yet
- Assignment - 1 - FuransDocument2 pagesAssignment - 1 - FuransJitendra Kumawat 17115No ratings yet
- Tellier 2007Document14 pagesTellier 2007Venu CharyNo ratings yet
- Aryl Halides As Precursors of Electrogenerated Bases. Utilization in Coupling Reactions of Acetonitriie With Various Electrophilic CompoundsDocument8 pagesAryl Halides As Precursors of Electrogenerated Bases. Utilization in Coupling Reactions of Acetonitriie With Various Electrophilic CompoundsWalid Ebid ElgammalNo ratings yet
- Hasmizam 2008Document6 pagesHasmizam 2008Ananda Vallezi PaladinoNo ratings yet
- 1group and 2 Group Disconnections 04-Mar-2021Document10 pages1group and 2 Group Disconnections 04-Mar-2021Sowmya N DNo ratings yet
- Micro Nano Letters - 2020 - Kuntyi - Electrochemical Synthesis of Silver Nanoparticles in Solutions of RhamnolipidDocument6 pagesMicro Nano Letters - 2020 - Kuntyi - Electrochemical Synthesis of Silver Nanoparticles in Solutions of Rhamnolipidnoura farahNo ratings yet
- Kinetic Model Development For Steam PyroDocument8 pagesKinetic Model Development For Steam PyroAyuanda PutriNo ratings yet
- Living Polymerization: ý ổng hợp & Biến tính poloymerDocument50 pagesLiving Polymerization: ý ổng hợp & Biến tính poloymernhathangbinhduongNo ratings yet
- Monatshefte Fuer Chemie 2020, 151, 213-222Document10 pagesMonatshefte Fuer Chemie 2020, 151, 213-222RohanNo ratings yet
- Niobium: Niobium, Also Known As Columbium, Is A Chemical Element With TheDocument15 pagesNiobium: Niobium, Also Known As Columbium, Is A Chemical Element With TheVysakh VasudevanNo ratings yet
- Salicylic Acid, Ethyl Ether, Ethyl Ester: Physical PropertiesDocument2 pagesSalicylic Acid, Ethyl Ether, Ethyl Ester: Physical PropertiesLuthfa Umi AzizahNo ratings yet
- Lec 10Document11 pagesLec 10DechenPemaNo ratings yet
- Phase Diagram LiCl+MgCl2+KCl+H2O at 323.15K PDFDocument8 pagesPhase Diagram LiCl+MgCl2+KCl+H2O at 323.15K PDFJhos Micky Condori AndiaNo ratings yet
- FYP Material Balance Rev.11Document128 pagesFYP Material Balance Rev.11Hassaan AhmadNo ratings yet
- 2 Teaching CHM614 Jobin 2021 UV VisDocument44 pages2 Teaching CHM614 Jobin 2021 UV VisSoumyajyoti DeyNo ratings yet
- Preparation of The Cop Catalysts: ( (S) - Cop-Oac), ( (S) - Cop-Cl), and (S) - Cop-HfacacDocument12 pagesPreparation of The Cop Catalysts: ( (S) - Cop-Oac), ( (S) - Cop-Cl), and (S) - Cop-HfacacHemin H. MuhammadNo ratings yet
- CO Separation With Molecular Gate Membrane: Shingo KAZAMADocument27 pagesCO Separation With Molecular Gate Membrane: Shingo KAZAMAzubin OchasNo ratings yet
- SR Chem Ipe Imp Questions 60/60Document6 pagesSR Chem Ipe Imp Questions 60/60KARTHIK BALAJINo ratings yet
- Presentation 2023 06 02Document74 pagesPresentation 2023 06 02iammouliNo ratings yet
- 2-23 C460 Organocopper and Cuprate Mini-LectureDocument5 pages2-23 C460 Organocopper and Cuprate Mini-LecturetangduchoangminhNo ratings yet
- Sahodaya Pre Board Examination - 2021-22: Class-XIIDocument6 pagesSahodaya Pre Board Examination - 2021-22: Class-XIIAmitNo ratings yet
- Tydhegn: FollovingDocument4 pagesTydhegn: FollovingVineet SierraNo ratings yet
- Synergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionDocument7 pagesSynergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionRiza SaidNo ratings yet
- Plutonium Metallurgy in IndiaDocument36 pagesPlutonium Metallurgy in IndiaMiloš BulajićNo ratings yet
- Synthesis and Transformation of Pyrrole C-GlycoconjugatesDocument10 pagesSynthesis and Transformation of Pyrrole C-GlycoconjugatesViviana TorresNo ratings yet
- Enthalpies of Solution of ElectrolytesDocument1 pageEnthalpies of Solution of ElectrolytesFavio Andrés ChavezNo ratings yet
- AbstractDocument8 pagesAbstractfaramarzkazemiNo ratings yet
- CitronellalDocument2 pagesCitronellalAminatu JuriahNo ratings yet
- Mirkhani2009 Article PhotocatalyticDegradationOfAzoDocument10 pagesMirkhani2009 Article PhotocatalyticDegradationOfAzoAjit Kumar DhankaNo ratings yet
- Wastewater CharacterisationDocument36 pagesWastewater Characterisationrokojeg956No ratings yet
- (1996) Placid-A Clean Process For Recycling Lead From BaterriesDocument3 pages(1996) Placid-A Clean Process For Recycling Lead From BaterriesYeimy Vivar LobosNo ratings yet
- Molecules: Eco-Friendly Synthesis of A New Class of Pyridinium-Based Ionic Liquids With Attractive Antimicrobial ActivityDocument14 pagesMolecules: Eco-Friendly Synthesis of A New Class of Pyridinium-Based Ionic Liquids With Attractive Antimicrobial ActivityBilal KazmiNo ratings yet
- Metallurgy - Short Notes (Tomorrow IITians)Document6 pagesMetallurgy - Short Notes (Tomorrow IITians)SatyamNo ratings yet
- Reaction With Miscellaneous-NPTEL PDFDocument25 pagesReaction With Miscellaneous-NPTEL PDFRathinNo ratings yet
- Chandramouli ReddyDocument70 pagesChandramouli ReddyiammouliNo ratings yet
- The Extraction of Curium and Americium by Tri N Octyl Phosphine OxideDocument3 pagesThe Extraction of Curium and Americium by Tri N Octyl Phosphine OxideHamdi Zae malikNo ratings yet
- Novel Highly Active Carbon Supported Ternary Pdnibi Nanoparticles As Anode Catalyst For The Alkaline Direct Ethanol Fuel CellDocument11 pagesNovel Highly Active Carbon Supported Ternary Pdnibi Nanoparticles As Anode Catalyst For The Alkaline Direct Ethanol Fuel CellFabricio CarrilloNo ratings yet
- Graphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1From EverandGraphene-based Carbocatalysis: Synthesis, Properties and Applications: Volume 1No ratings yet
- Odisha Chemistry 11 Syllabus For IscDocument5 pagesOdisha Chemistry 11 Syllabus For IscSJNo ratings yet
- Syllabus ICSE Chemistry-10Document4 pagesSyllabus ICSE Chemistry-10SJNo ratings yet
- CVR CBSE-Physics 9thDocument1 pageCVR CBSE-Physics 9thSJNo ratings yet
- Clock PuzzlesDocument5 pagesClock PuzzlesSJ100% (1)
- Electronic Layers in Copper Oxide SuperconductorsDocument1 pageElectronic Layers in Copper Oxide SuperconductorsSJNo ratings yet
- CVR Conceptual Chemistry 11 Vol 1Document1 pageCVR Conceptual Chemistry 11 Vol 1SJNo ratings yet
- CVR CBSE Chemistry Class-9Document1 pageCVR CBSE Chemistry Class-9SJNo ratings yet
- CVR CBSE - Physics10Document1 pageCVR CBSE - Physics10SJNo ratings yet
- Chemists Control The HurricaneDocument1 pageChemists Control The HurricaneSJNo ratings yet
- Arithmetic & Algebraic Problems - pt-1Document4 pagesArithmetic & Algebraic Problems - pt-1SJ100% (1)
- Physical Processes of Luminescence On Isolated CentresDocument1 pagePhysical Processes of Luminescence On Isolated CentresSJNo ratings yet
- Speed and Distance PuzzlesDocument7 pagesSpeed and Distance PuzzlesSJNo ratings yet
- Life Cycle Framework For Clean Manufacturing TechnologyDocument1 pageLife Cycle Framework For Clean Manufacturing TechnologySJNo ratings yet
- Irish JaunttDocument1 pageIrish JaunttSJNo ratings yet
- IntroDocument1 pageIntroSJNo ratings yet
- Age PuzzlesDocument1 pageAge PuzzlesSJNo ratings yet
- ScopeDocument1 pageScopeSJNo ratings yet
- Record CountDocument1 pageRecord CountSJNo ratings yet
- F899 10.1374633 2010Document7 pagesF899 10.1374633 2010Rouse ToxquiNo ratings yet
- Raw Material Acceptance CertiaDocument22 pagesRaw Material Acceptance CertiaWayneNo ratings yet
- Electronics ENGLISHDocument28 pagesElectronics ENGLISHSHRI COACHING CLASSES Competitive ExamsNo ratings yet
- ABS Coating GuideDocument192 pagesABS Coating GuideAnupam KumarNo ratings yet
- Environmental PollutionDocument54 pagesEnvironmental PollutionAman ShrivastavaNo ratings yet
- 2005 Chem.-Eng.-Sci. Cents-A.H.G 1 PDFDocument6 pages2005 Chem.-Eng.-Sci. Cents-A.H.G 1 PDFFarah Talib Al-sudaniNo ratings yet
- Cold Room and Storage CatalogueDocument22 pagesCold Room and Storage CatalogueTarani AttorneyNo ratings yet
- Odic Table Periodicity NeetDocument59 pagesOdic Table Periodicity NeetAnubhav KohliNo ratings yet
- Introduction of WasteDocument15 pagesIntroduction of Wastezainabshittu45No ratings yet
- HARDNESSDocument18 pagesHARDNESSK33Prathvi S KundarNo ratings yet
- MODULE 4: Introduction To Pavement Design: Requirements of A PavementDocument9 pagesMODULE 4: Introduction To Pavement Design: Requirements of A PavementRAMESH WALAKENo ratings yet
- Polyflex UltrachemDocument2 pagesPolyflex UltrachemBraulio Candela NoriegaNo ratings yet
- ECT ECL ECS NewDocument2 pagesECT ECL ECS NewToph TophNo ratings yet
- W6 - Distillation UnitDocument36 pagesW6 - Distillation UnitRoza SavitriNo ratings yet
- Mapewood Gel 120 For CrackDocument4 pagesMapewood Gel 120 For CrackSufen ZhangNo ratings yet
- Combined Use of Calcium Chloride and Fly Ash in Road Base StabilizationDocument10 pagesCombined Use of Calcium Chloride and Fly Ash in Road Base StabilizationGeorge SorosNo ratings yet
- SIA 269-6-1 - en - 160114Document40 pagesSIA 269-6-1 - en - 160114Ricardo PimentelNo ratings yet
- Nilos MatDocument8 pagesNilos MatMohd NazriNo ratings yet
- Acid-Base Indicators For Use in TitrationDocument7 pagesAcid-Base Indicators For Use in TitrationMaxNo ratings yet
- Questions of CEM PDFDocument2 pagesQuestions of CEM PDFAnonymous Anzt6fAeM100% (1)
- Introductiontoprestressedconcrete 111211203113 Phpapp02Document17 pagesIntroductiontoprestressedconcrete 111211203113 Phpapp02kiet20002000No ratings yet
- Semiconductor Basics & Semiconductor Physics Tutorial 1Document5 pagesSemiconductor Basics & Semiconductor Physics Tutorial 1Srimonta RoyNo ratings yet
- Polywithe® - 8000 CLDocument1 pagePolywithe® - 8000 CLsébastien cardinaleNo ratings yet
- Influence of Powder Porous Structure On The Deposition Behavior of Cold-Sprayed WC-12Co CoatingsDocument8 pagesInfluence of Powder Porous Structure On The Deposition Behavior of Cold-Sprayed WC-12Co CoatingsPhung Tuan AnhNo ratings yet
- ADDMIX 215 - v2Document4 pagesADDMIX 215 - v2Ankita Baban GavadeNo ratings yet
- 4-CE Thermodynamics Properties of FluidsDocument70 pages4-CE Thermodynamics Properties of FluidsApple EmiratessNo ratings yet
- Fac 7044 Acfa 4 F 0 e 6Document13 pagesFac 7044 Acfa 4 F 0 e 6Ali AliNo ratings yet