Professional Documents
Culture Documents
Iron Determination Calibration Graphs
Iron Determination Calibration Graphs
Uploaded by
510418106 ARITRYAGHOSHOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Iron Determination Calibration Graphs
Iron Determination Calibration Graphs
Uploaded by
510418106 ARITRYAGHOSHCopyright:
Available Formats
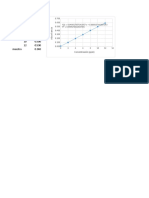
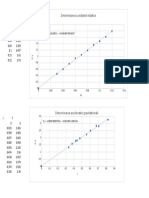
x (Sample Conc.
mg/l) y (Absorbance)
0 0
0.25 0.034 PG CALIBRATION CURVE
0.5 0.081
0.75 0.121
1 0.154
1.5 0.215
2 0.383
3 0.585
4 0.73
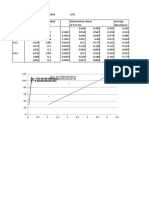
Serial No. Conical Flask No. Concentration (mg/l) Absorbance at 510 nm
1 1 0 0
2 2 0.2 0.034
3 3 0.5 0.081
4 4 0.75 0.121
5 5 1 0.154
6 6 1.5 0.215
7 7 2 0.383
8 8 3 0.585
9 9 4 0.730
10 10 stnd. soln. before aeration 0.711
11 11 stnd. soln. after aeration (4 mg/l) 0.129
12 12 - 0.057
13 13 - 0.007
14 14 - 0.015
15 15 - 0.1047
16 16 - 0.126
17 17 - 0
Calibration cu
0.8
0.7 f(x) = 0.182860377358491 x
R² = 0.994181937339136
0.6
0.5
Absorbance
0.4
0.3
Description Sample Concentration (mg/l) 0.2
50 ml distilled water -
1 ml I.S. + 49 ml d.w. - 0.1
2.5 ml I.S. + 47.5 ml d.w. -
3.75 ml I.S. + 46.25 d.w. -
0
5 ml + 45 d.w. -
0 0.5 1 1.5
7.5 ml I.S. + 42.5 d.w. -
10 ml I.S. + 40 ml d.w. - S
15 ml I.S. + 35 ml d.w. -
20 ml I.S. + 30 ml d.w. - Approx. values 2 decimal places
w/o aeration 30 mins. 3.88737014762165 3.89
After aeration 30 mins. 0.705303444505194 0.71
Dipanwita 0.311645708037179 0.31
Poonam 0.0382722799343904 0.04
A. D. 0.0820120284308365 0.08
A. M. 0.572443958447239 0.58
T B. 0.688901038819027 0.69
Aritrya Ghosh (Municipal Tap water) 0 0
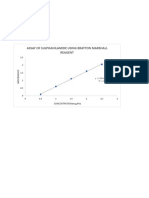
Calibration curve for total iron content
.182860377358491 x
994181937339136
0.5 1 1.5 2 2.5 3 3.5 4 4.5
Sample concentration (mg/l)
Sample description
Burdwan tap water
Birbhum tap water
Old iiest building tap water
howrah apartment tap water
point blank solution taken as distilled water
Sample description
Burdwan tap water
Birbhum tap water
Old iiest building tap water
howrah apartment tap water
point blank solution taken as distilled water
Sample description
Burdwan tap water
Birbhum tap water
Old iiest building tap water
howrah apartment tap water
point blank solution taken as distilled water
25 degrees celsius
CONTACT TIME (minutes)
5
10
15
30
60
90
120
150
180
210
240
360
Initial volume of sample (ml)
50
50
50
50
50
Initial volume of sample (ml)
50
50
50
50
50
Initial volume of sample (ml)
50
50
50
50
50
effect of initial time on adsorption on rice husk ash for iron removal
Initial volume of sample (ml)
50
50
50
50
50
50
50
50
50
50
50
50
Final volume of filtrate after adsorption (ml) Amount of adsorbent added (gm)
48 2
47 2
48 2
47 2
50 2
Final volume of filtrate after adsorption (ml) Amount of adsorbent added (gm)
48 2
47 2
48 2
47 2
50 2
Final volume of filtrate after adsorption (ml) Amount of adsorbent added (gm)
48 2
47 2
48 2
47 2
50 2
colour developed time = 10 to 15 minutes
150 to 160 rpm CALIBRATION CURVE TO CALCULATE ADS
description Amount of adsorbent added (gm)
5 min 1
10 min 1
15 min 1
30 min 1
60 min 1
equilibrium reached 1
already reached equilibrium 1
already reached equilibrium 1
already reached equilibrium 1
already reached equilibrium 1
already reached equilibrium 1
already reached equilibrium 1
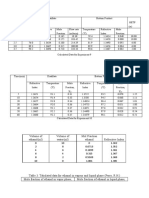
y =0.1829 x and R square = 0.9876
Absorbance at 510 nm (before adsorption) Concentration in (mg/l) before adsorption
0.127 0.694368507381083
0.185 1.01148168398032
0.151 0.825587752870421
0.139 0.759978130125752
0 0
y =0.1829 x and R square = 0.9876
Absorbance at 510 nm (before adsorption) Concentration in (mg/l) before adsorption
0.127 0.694368507381083
0.185 1.01148168398032
0.151 0.825587752870421
0.139 0.759978130125752
0 0
y =0.1829 x and R square = 0.9876
Absorbance at 510 nm (before adsorption) Concentration in (mg/l) before adsorption
0.127 0.694368507381083
0.185 1.01148168398032
0.151 0.825587752870421
0.139 0.759978130125752
0 0
IBRATION CURVE TO CALCULATE ADSORPTION CAPACITY OF RICE HUSK ASH FOR IRON REMOVAL BY USING KNOWN CONCENTRATIONS O
Concentration in (mg/l) before adsorption (Co)
3
3
3
3
3
3
3
3
3
3
3
3
Absorbance at 510 nm (after adsorption) Concentration in (mg/l) after adsorption (Ce)
0.068 0.371787862219792
0.046 0.251503553854565
0.063 0.344450519409513
0.055 0.300710770913067
0 0
Absorbance at 510 nm (after adsorption) Concentration in (mg/l) after adsorption
0.053 0.289775833788956
0.077 0.420995079278294
0.063 0.344450519409513
0.058 0.317113176599235
0 0
Absorbance at 510 nm (after adsorption) Concentration in (mg/l) after adsorption
0.025 0.136686714051394
0.036 0.196828868234008
0.030 0.164024056861673
0.027 0.147621651175506
0 0
y =0.1829 x and R square = 0.9876
USING KNOWN CONCENTRATIONS OF STOCK STANDARD SOLUTION OF IRON
Absorbance at 510 nm (after adsorption) Concentration in (mg/l) after adsorption (Ce)
0.487 2.66265718972116
0.445 2.43302351011482
0.411 2.24712957900492
0.335 1.83160196828868
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
0.221 1.20831055221432
Difference in Concentration = initial conc. - final conc.
0.32258064516129
0.759978130125752
0.481137233460908
0.459267359212685
0
Difference in Concentration = initial conc. - final conc.
0.404592673592127
0.590486604702026
0.481137233460908
0.442864953526517
0
Difference in Concentration = initial conc. - final conc.
0.557681793329689
0.814652815746312
0.661563696008748
0.612356478950246
0
Difference in Concentration = initial conc. - final conc.
0.337342810278841
0.566976489885183
0.752870420995079
1.16839803171132
1.79168944778568
1.79168944778568
1.79168944778568
1.79168944778568
1.79168944778568
1.79168944778568
1.79168944778568
1.79168944778568
% removal of iron = (diff. in conc./initial conc.) x 100
46.4566929133858
75.1351351351351
58.2781456953642
60.431654676259
0
Average = (46+75+58+60) / 4 = 59.75 %
% removal of iron = (diff. in conc./initial conc.) x 100
58.2677165354331
58.3783783783785
58.2781456953643
58.273381294964
0
% removal of iron = (diff. in conc./initial conc.) x 100
80.3149606299213
80.5405405405406
80.1324503311258
80.5755395683453
0
% removal of iron = (diff. in conc./initial conc.) x 100
11.244760342628
18.8992163295061
25.095680699836
38.9466010570439
59.7229815928558
59.7229815928558
59.7229815928558
59.7229815928558
59.7229815928558
59.7229815928558
59.7229815928558
59.7229815928558
You might also like
- Lab Report (Atomic Absorption Spectroscopy)Document8 pagesLab Report (Atomic Absorption Spectroscopy)Shirley Cheong67% (6)
- Iron Calibration Curve For PGDocument6 pagesIron Calibration Curve For PG510418106 ARITRYAGHOSHNo ratings yet
- NLSJNDKLSNKLSJDocument2 pagesNLSJNDKLSNKLSJPutri Pah Kumala DewiNo ratings yet
- A F (Concentratie) : 0.6 F (X) 0.107621917808219 X + 0.007476712328767 R 0.984180901225002Document2 pagesA F (Concentratie) : 0.6 F (X) 0.107621917808219 X + 0.007476712328767 R 0.984180901225002Nastase DamianNo ratings yet
- Experiment 3 Bradford AssayDocument12 pagesExperiment 3 Bradford AssaySayre BongoNo ratings yet
- Data Total Polifenol Batch3Document5 pagesData Total Polifenol Batch3RIZAAANo ratings yet
- Analisa Air LautDocument8 pagesAnalisa Air LautUfafa AnggariniNo ratings yet
- Clase 13-05Document4 pagesClase 13-05Juliana Suárez OlayaNo ratings yet
- Kurva Kalibrasi Fe Absorban Vs KonsentrasiDocument1 pageKurva Kalibrasi Fe Absorban Vs KonsentrasiKinanthi SekarNo ratings yet
- Pengamatan Prak FarmakokinetikDocument5 pagesPengamatan Prak FarmakokinetikDalvaMNo ratings yet
- Recta de Calibrado A (595nm)Document4 pagesRecta de Calibrado A (595nm)AlanaNo ratings yet
- Recta de Calibrado A (595nm)Document4 pagesRecta de Calibrado A (595nm)AlanaNo ratings yet
- Exp 3 GraphsDocument1 pageExp 3 Graphsankit.alawlaNo ratings yet
- Kurva Kalibrasi Larutan Standar C-Organik: 1. Penentuan LinieritasDocument4 pagesKurva Kalibrasi Larutan Standar C-Organik: 1. Penentuan Linieritasaprilia kurnia putriNo ratings yet
- (Glu) Absorbancia 0.1 0.055 Tstigo 0.1633279483 0.08 0.2 0.087 0.3 0.159 0.4 0.228 Prob. 0.463812601 0.266 0.5 0.294Document3 pages(Glu) Absorbancia 0.1 0.055 Tstigo 0.1633279483 0.08 0.2 0.087 0.3 0.159 0.4 0.228 Prob. 0.463812601 0.266 0.5 0.294dev3mondrag3nNo ratings yet
- Grafik FisikaDocument2 pagesGrafik FisikaGrace PaulaNo ratings yet
- Chart Title: FD Absorbancia Preparación Muestra (G/ML)Document4 pagesChart Title: FD Absorbancia Preparación Muestra (G/ML)Tatiana GamarraNo ratings yet
- Standard GraphDocument7 pagesStandard GraphVAISHNAVI JADHAVNo ratings yet
- Courbe D'etalonageDocument2 pagesCourbe D'etalonagenourbouchradjirouniNo ratings yet
- FMO4 5 MinDocument6 pagesFMO4 5 MinKatharina SchwallerNo ratings yet
- BCA Standard CurveDocument4 pagesBCA Standard CurveJavier AlemanNo ratings yet
- G Plant Extracts and DPPHDocument12 pagesG Plant Extracts and DPPHKevin YongNo ratings yet
- Wastewater Dilution Ratio Wastewater Dilution RatioDocument1 pageWastewater Dilution Ratio Wastewater Dilution RatioYeni Khairina AzkNo ratings yet
- Data EksperimenDocument1 pageData EksperimenAn Nur Zajar BmtNo ratings yet
- Kurva Parasetamol BiofarmasDocument5 pagesKurva Parasetamol BiofarmasSahda Sabilah luhtansaNo ratings yet
- Graphs - 1Document11 pagesGraphs - 1mr copy xeroxNo ratings yet
- X Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaDocument4 pagesX Vs Y at 1315.45 MMHG Etanol-Agua X, Y Vs T at 1315.45 MMHG Etanol-AguaCarlos FloresNo ratings yet
- CV Vs Nre Co Vs Nre: Axis TitleDocument3 pagesCV Vs Nre Co Vs Nre: Axis TitleCrezpo YzNo ratings yet
- Kurva Standar Metode Biuret Dengan Panjang Gelombang 520 NM: Konsentrasi Albumin (MG/ ML)Document1 pageKurva Standar Metode Biuret Dengan Panjang Gelombang 520 NM: Konsentrasi Albumin (MG/ ML)Teti HungkulNo ratings yet
- Glucose StandardDocument3 pagesGlucose StandardRajesh RaviNo ratings yet
- Kurva Kalibrasi Parasetamol λ maks 243 nmDocument2 pagesKurva Kalibrasi Parasetamol λ maks 243 nmGelisaNo ratings yet
- Trabalho Cinética - Grupo 1 - Relatório FinalDocument13 pagesTrabalho Cinética - Grupo 1 - Relatório FinalMariana AssadeNo ratings yet
- Excel 2Document52 pagesExcel 2Indah SuardhyNo ratings yet
- Book 1Document5 pagesBook 1Dwi WahyudiNo ratings yet
- Experiment Eight: Spectrophotometric Analysis of A Complex MixtureDocument12 pagesExperiment Eight: Spectrophotometric Analysis of A Complex MixtureMark AwNo ratings yet
- Curva Cromonte Linealidad 1Document5 pagesCurva Cromonte Linealidad 1Shirley Margarita Martinez PadillaNo ratings yet
- Proiect FizicaDocument1 pageProiect FizicamaxNo ratings yet
- X Avance Del FrenteDocument6 pagesX Avance Del FrenteDARWIN ALBERTO QUEVEDO ALBURQUEQUENo ratings yet
- Break Even Stripping Ratio Short TonneDocument19 pagesBreak Even Stripping Ratio Short TonneLMNo ratings yet
- Grafik Instrumen Metode Penambahan Standar Kelompok 2Document4 pagesGrafik Instrumen Metode Penambahan Standar Kelompok 2Rahul AlfariziNo ratings yet
- V V C C: Mass Ion ConcentratDocument2 pagesV V C C: Mass Ion ConcentratJoshua RuizNo ratings yet
- Result and CalculationDocument13 pagesResult and CalculationgerrykhangNo ratings yet
- Sampel Daun StroberiDocument6 pagesSampel Daun StroberiI Made Astawa Ari Putra 245No ratings yet
- Calibration Curve SampleDocument2 pagesCalibration Curve SampleleyNo ratings yet
- Calibration Curve For Uv Visible SpectrosDocument4 pagesCalibration Curve For Uv Visible SpectrosShreya IndurkarNo ratings yet
- TP Ambiental Resultados Alumnos 2024 1cDocument2 pagesTP Ambiental Resultados Alumnos 2024 1cdaianapassarinoNo ratings yet
- Kurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Document6 pagesKurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Fajar RamadhanNo ratings yet
- Kurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Document6 pagesKurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Fajar RamadhanNo ratings yet
- Kurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Document6 pagesKurva Kalibrasi Paracetamol: Konsentrasi ( G/ML)Fajar RamadhanNo ratings yet
- KurvakalibrasiiDocument2 pagesKurvakalibrasiiDiva DenitaNo ratings yet
- Absorbanta 510 NM Concentratia (MG Cumarine/50 ML) 0.211 0.1 0.324 0.2 0.438 0.4 0.551 0.6 0.679 0.8 0.801 1 0.573 0.5362Document2 pagesAbsorbanta 510 NM Concentratia (MG Cumarine/50 ML) 0.211 0.1 0.324 0.2 0.438 0.4 0.551 0.6 0.679 0.8 0.801 1 0.573 0.5362MihaNo ratings yet
- Graph of Refractive Index Against Composition of EthanolDocument11 pagesGraph of Refractive Index Against Composition of EthanolchaitanyaNo ratings yet
- Perhiungan NstrumenDocument2 pagesPerhiungan NstrumenBellaNo ratings yet
- FE (Besi)Document2 pagesFE (Besi)Widya kalimatusyahdiahNo ratings yet
- Thermofluids Lab Report Centrifugal PumpDocument3 pagesThermofluids Lab Report Centrifugal PumpMohamedNo ratings yet
- Data Prak Kimling 4Document4 pagesData Prak Kimling 4Putri Nur AngelinaNo ratings yet
- Concentración de Equilibrio de Adsorbato Cantidad Específica de Equilibrio de AdsorbatoDocument5 pagesConcentración de Equilibrio de Adsorbato Cantidad Específica de Equilibrio de AdsorbatoDayNo ratings yet
- Kurva Baku TeofilinDocument3 pagesKurva Baku Teofilinrima rasidaNo ratings yet
- Exp 4Document2 pagesExp 4liew_yen_3No ratings yet
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- M.tech. 3rd Semester Progress Report - Notification - 01-12-2022Document8 pagesM.tech. 3rd Semester Progress Report - Notification - 01-12-2022510418106 ARITRYAGHOSHNo ratings yet
- Adsorption Isotherm Fitting Spreadsheet v01Document19 pagesAdsorption Isotherm Fitting Spreadsheet v01510418106 ARITRYAGHOSHNo ratings yet
- Iron Calibration Curve For PGDocument6 pagesIron Calibration Curve For PG510418106 ARITRYAGHOSHNo ratings yet
- Areas of Law What and What Not To StudyDocument7 pagesAreas of Law What and What Not To Study510418106 ARITRYAGHOSHNo ratings yet
- Comparative Adsorption Study On Rice Husk and Rice Husk Ash by Using Amaranthus Gangeticus Pigments As DYEDocument12 pagesComparative Adsorption Study On Rice Husk and Rice Husk Ash by Using Amaranthus Gangeticus Pigments As DYE510418106 ARITRYAGHOSHNo ratings yet
- Bresol008 AR HimalayaDocument6 pagesBresol008 AR HimalayaShilpa SharmaNo ratings yet
- Sci 9-DLP 2Document5 pagesSci 9-DLP 2elsie tequinNo ratings yet
- GPSDocument35 pagesGPSak721No ratings yet
- RPT Sains DLP t1 2023-2024Document17 pagesRPT Sains DLP t1 2023-2024raudhatulasnidaNo ratings yet
- Gy4006 L6 Gis 2023Document39 pagesGy4006 L6 Gis 2023Karrie ChambersNo ratings yet
- Finding Inverse of A Matrix PDFDocument1 pageFinding Inverse of A Matrix PDFPritish SinglaNo ratings yet
- Jeremy Bentham: Which Promotes The Greatest Good For The Greatest Number of People". Unlike Egoism in WhichDocument3 pagesJeremy Bentham: Which Promotes The Greatest Good For The Greatest Number of People". Unlike Egoism in WhichAnna Michelle MagbanuaNo ratings yet
- Smart Test Series: 1-Write Short Answers To Any 5 QuestionsDocument1 pageSmart Test Series: 1-Write Short Answers To Any 5 QuestionsAbdul WahabNo ratings yet
- Consumer Buying BehaviorDocument15 pagesConsumer Buying BehaviorpogsNo ratings yet
- Ant 101: Introduction To AnthropologyDocument20 pagesAnt 101: Introduction To AnthropologyAmina MatinNo ratings yet
- Analysing Data Using MEGASTATDocument33 pagesAnalysing Data Using MEGASTATMary Monique Llacuna LaganNo ratings yet
- En DVC 2018 35Document18 pagesEn DVC 2018 35Lina María CañónNo ratings yet
- The Chi-Square Test: Statistics and Research DesignDocument2 pagesThe Chi-Square Test: Statistics and Research DesignTommy Winahyu PuriNo ratings yet
- State Bank of Pakistan (2015) Concept Paper On Green BankingDocument41 pagesState Bank of Pakistan (2015) Concept Paper On Green BankingMeeroButtNo ratings yet
- UntitledDocument489 pagesUntitledEnginNo ratings yet
- Lecture Notes - Sedimentation TankDocument45 pagesLecture Notes - Sedimentation TankJomer Levi PortuguezNo ratings yet
- Aclm ReportDocument3 pagesAclm Reportabdulhadee naimNo ratings yet
- ACJC 2012 Prelim P2 PDFDocument8 pagesACJC 2012 Prelim P2 PDFSabitendra TelukulaNo ratings yet
- MATH 117 Syllabus Fall 2019Document2 pagesMATH 117 Syllabus Fall 2019PerzivalNo ratings yet
- Shell Rimula R3 Turbo 15W-40Document2 pagesShell Rimula R3 Turbo 15W-40HUM CIREBON DFLTSNo ratings yet
- Cambridge Checkpoint Science English Language Skills Teachers SupportDocument14 pagesCambridge Checkpoint Science English Language Skills Teachers SupportDanilo Pereira GonçalvesNo ratings yet
- NEEDLE ROLLER BEARINGS SKF CatalogDocument84 pagesNEEDLE ROLLER BEARINGS SKF CatalogashtariNo ratings yet
- 1 Place ValueDocument4 pages1 Place ValueKNAH TutoringNo ratings yet
- Astak Verga - ManishDocument74 pagesAstak Verga - ManishManishNo ratings yet
- Math A Module18Document18 pagesMath A Module18Zen Zhyree CorpuzNo ratings yet
- My Little Island L3 U2 - WorkbookDocument16 pagesMy Little Island L3 U2 - WorkbookMaría Fernanda López AvilezNo ratings yet
- ACOMD2H16Document3 pagesACOMD2H16boss100% (1)
- GCP CarbonBudget 2023 Slides v1.0-2-1Document94 pagesGCP CarbonBudget 2023 Slides v1.0-2-1YULIANTONo ratings yet
- Cu THHNDocument2 pagesCu THHNdekadimahendra03No ratings yet
- 4 DSKP KSSR Science Year 6 - 210927 - 165154Document88 pages4 DSKP KSSR Science Year 6 - 210927 - 165154MADLANE AK ASSING MoeNo ratings yet