Professional Documents
Culture Documents
Semimetallicity?: Department of Chemistry, Oregon State University, Corvallis, OR 97331-4003 Stephen - Hawkes@orst - Edu
Semimetallicity?: Department of Chemistry, Oregon State University, Corvallis, OR 97331-4003 Stephen - Hawkes@orst - Edu
Uploaded by
Aitor PastorOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Semimetallicity?: Department of Chemistry, Oregon State University, Corvallis, OR 97331-4003 Stephen - Hawkes@orst - Edu
Semimetallicity?: Department of Chemistry, Oregon State University, Corvallis, OR 97331-4003 Stephen - Hawkes@orst - Edu
Uploaded by
Aitor PastorCopyright:
Available Formats
Research: Science and Education
Semimetallicity?
Stephen J. Hawkes
Department of Chemistry, Oregon State University, Corvallis, OR 97331-4003; stephen.hawkes@orst.edu
Lists of “semimetallic” elements differ. Most include all
8-
the elements (other than aluminum and beryllium) that are Ag Cu
K Sn
next to the zigzag line separating metals from nonmetals on As Sb Po
Fe
Conductors
6- Bi
the periodic table. Most exclude bismuth and selenium, and Mn
most exclude either polonium or astatine or neither but not 4-
C (graphite)
both. At one time I taught from a text that asserted that semi-
Te
metals are semiconductors, and I wondered how true that 2 - Se (gray)
was. This paper investigates the listing of semimetals. S emiconductors
Silicon and boron are often listed as both semiconductors 0-
Ge
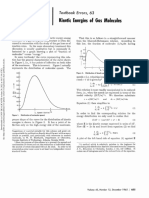
and semimetals. As shown in Figure 1, the amorphous form
Log(Conductivity / (S/m))
Si (microcryst)
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
of silicon is a nonconductor rather than a semiconductor, -2 -
whereas the microcrystalline form has a conductivity in the Si (monocryst)
ambiguous range that is neither clearly semimetallic nor
Downloaded via UNIV DE ALICANTE on September 23, 2022 at 17:38:59 (UTC).
-4 -
B (β -r hombohedral)
clearly insulating. There is no value of conductivity that can be
defined as separating semiconducting elements from insulators, -6 -
so it is meaningless to assert that microcrystalline silicon is one I
Si(amorph)
or the other. Both conduct electricity by the same mechanism, -8 -
both have conductivities that increase with increasing tempera- P (yellow)
ture (unlike metals), and both can be made unambiguously -10- Insulators
semiconducting by doping with suitable additives. Silicon and C (diamond)
boron have no metallic properties, so they are not semimetals. -12-
The metallic appearance of crystalline silicon is the usual
appearance of opaque nonmetallic crystals like iron pyrite -14-
(“fool’s gold”) and iodine. If silicon and boron are classified S (rhombic)
as semiconductors rather than insulators, then they should -16-
be classified as semiconducting nonmetals.
Selenium in its usual form, on the other hand, has
conductivity well within the semiconductor range and its Figure 1. Log of electrical conductivity in S m᎑1 at 25 °C. All the
structure is one of infinite spiral chains of selenium atoms semiconducting and nonconducting elements of which the conduc-
with weak interaction of a metallic nature between the chains tivity is known are included, and sufficient conducting elements to
(1). Its compounds mostly show a nonmetallic nature for the illustrate the range. The values are the reciprocal of the resistivities
selenium. The element is therefore metallic in some of its given by Emsley (11) except for the value for polonium, which is
calculated from Maxwell’s (12 ) value 4 × 10 ᎑5 Ω cm at 0 °C
properties but a nonmetal in others. This ambiguity defines it
(correction for temperature does not change the first significant fig-
as a semimetal even though it does not adjoin the zigzag line. ure in this case) and for the three forms of Si from refs 13–16 .
Arsenic is rightly classified as a semimetal in all tables
that I have seen. Its appearance is not clearly metallic or non-
metallic, it is an electrical conductor (not a semiconductor),
and its chemistry resembles that of nonmetals. Antimony is a silvery-white metal (3, 4), bismuth has a less clearly metallic
also classified as a semimetal but the case is less compelling. appearance. Aesar describes it as “gray with a reddish tinge”
It has a metallic appearance (Aesar describes it as a bluish- (2) and the CRC Handbook describes it as “a white crystalline
white metal [2]), it is more brittle than most metals, and it brittle metal with a pinkish tinge” (5). It is less electrically
is an electrical conductor outside the range of semiconduc- conducting than either antimony or arsenic (see Fig. 1), both
tors. It forms a few cationic compounds, so its chemistry is a of which are usually classified as semimetals, or than polonium,
little more metallic than that of arsenic. It is reasonable to which is a metal and a conductor, or than most other metals.
classify it as a semimetal but it would also be reasonable to With the exception of mercury, bismuth has a lower thermal
classify it as a metal. This brings us to bismuth. conductivity than all other metals (2). These properties require
The case for classifying bismuth as a semimetal is stronger bismuth to be classified as a semimetal although it is separated
than for antimony, but that makes it an exception to well- from the zigzag line by polonium, which is not a semimetal
known trends, which is probably why it is usually classified but a metal.
as a metal. Being to the left of polonium in the periodic table, Of the other elements next to the zigzag line, beryllium
bismuth would be expected to be more metallic than polonium and aluminum are metals, and it has been shown elsewhere
but is actually less so. Bismuth forms more cationic compounds (6 ) that polonium and astatine are not semimetals but are
than antimony, but fewer than polonium. It does not adjoin respectively a metal and a nonmetal. The line is therefore a
the zigzag line. While its neighbor polonium is unambiguously misleading guide to which elements are semimetals. The prac-
1686 Journal of Chemical Education • Vol. 78 No. 12 December 2001 • JChemEd.chem.wisc.edu
Research: Science and Education
tice of calling Si, B, Po, and At (and perhaps Sb) semimetals, Literature Cited
but not Se or Bi, is thus more deceptive than informative.
The most easily defensible listing of semimetallic elements 1. Cotton, F. A.; Wilkinson, G. Advanced Inorganic Chemistry,
is Ge, As, Se, Te, Sb, and Bi. If Bi is omitted, as is traditional, 3rd ed.; Interscience: New York, 1972; p 427.
then logic requires that Sb also be omitted. 2. Alfa Aesar Catalog of Research Chemicals, Metals and Materials;
Alfa Aesar: Ward Hill, MA, 1997.
Definition of “Semimetal” 3. Gmelin Handbook of Inorganic and Organometallic Chemistry.
System Number 12. Po—Polonium, 8th ed.; Springer: New
Several scales have been proposed which give values for York, 1990; p 275.
semimetals that are intermediate between those for metals 4. Bagnall, K. W. Chemistry of the Rare Radioelements Polonium–
and nonmetals, suggesting that semimetals may be recognized Actinium; Academic: New York, 1957; p 45.
from their position on the scale. Electronegativity (7), average 5. CRC Handbook of Chemistry and Physics, 71st ed.; Lide, D. R.,
valence electron energy or an electronegativity derived from Ed.; CRC Press: Boca Raton, FL, 1990; pp 4–6.
it (8, 9), and the ratio of molar refractivity to molar volume 6. Hawkes, S. J. CHEM13 News 1999, 273 (Feb), 14.
(10) have been proposed. Anomalies can be found for each 7. Pauling, L. Nature 1992, 357, 26–27.
of these scales and they do not identify the same elements as 8. Allen, L. C. J. Am. Chem. Soc. 1989, 111, 9003–9014.
semimetals. Metallic character is a combination of several 9. Periodic Table Including Configuration Energies; Gelest Inc.:
properties, so it is more useful to judge semimetallicity sepa- Tullytown, PA, 1995.
rately for each element. 10. Edwards, P. P.; Sienko, M. J. J. Chem. Educ. 1983, 60, 691–
696.
Periodic Table 11. Emsley, J. The Elements, 3rd ed.; Clarendon: Oxford, 1998.
12. Maxwell, C. R. J. Chem. Phys. 1949, 17, 1288; quoted from ref 4.
Rather than defining a list of “semimetals”, it may be 13. Veprek, S. In Properties of Amorphous Silicon, 2nd ed.; EMIS
more useful to print three-dimensional periodic tables or Data Reviews, Series 1; Information Services Division, Institute
diagrams similar to Figure 1, showing the variation in prop- of Electrical Engineers: London & New York, 1989; p 185.
erties that are associated with semimetallicity. These may be 14. Neimark, K. N.; Trubitsyn, Yu. V.; Fal’kevich, É. S.;
electrical conductivity, hardness, average valence electron energy, Chervonyi, I. F. Inorg. Mater. (translation of Neorg. Mater.)
reflectivity, the energy gap between the conductance and valence 1992, 28, 866–868.
bands, or any other relevant property. This will be more 15. Pavlov, A.; Khoklov, A. F.; Kudryavtseva, R. V.; Ershov, A. V.
informative than using the undefined (and possibly unde- Phys. Status Solidi A 1989, 116, 697.
finable) term “semimetal”. 16. Mittas, A.; Georgoulas, N.; Girginoudi, D.; Thanalaikis, A.
A diagram similar to Figure 1 appears in Edwards’s Phys. Status Solidi A 1989, 116, 725.
paper (17 ). The conductivity shown there for diamond is a 17. Edwards, P. P. In The New Chemistry; Hall, N., Ed.; Cambridge
millionfold lower than Emsley’s value shown here. University Press: Cambridge, 2000; p 89.
JChemEd.chem.wisc.edu • Vol. 78 No. 12 December 2001 • Journal of Chemical Education 1687
You might also like
- FumingationDocument8 pagesFumingationLeslie MasiyandimaNo ratings yet
- SNC 1D1 Exam Review Chemistry Review: Name: - DateDocument10 pagesSNC 1D1 Exam Review Chemistry Review: Name: - DateMarileth CoNo ratings yet
- PTGNI - FTTM ITB - Nikel - PendahuluanDocument45 pagesPTGNI - FTTM ITB - Nikel - PendahuluanBudiawan DatunsolangNo ratings yet
- Specac Useful Spectroscopy PosterDocument1 pageSpecac Useful Spectroscopy PosterBechir ChammemNo ratings yet
- Classification of Elements (Javed)Document37 pagesClassification of Elements (Javed)Asim AliNo ratings yet
- Periodic TableDocument1 pagePeriodic TablevxlrNo ratings yet
- 2 25 Intermediate BondingDocument6 pages2 25 Intermediate BondingAliya RahmanNo ratings yet
- Covalent Bonding and HybridizationDocument1 pageCovalent Bonding and Hybridizationdomcruz0308No ratings yet
- Atomic Structure and The Periodic Table Revision Activity MatDocument1 pageAtomic Structure and The Periodic Table Revision Activity Matkareem el barbaryNo ratings yet
- Scheme of Work - Cambridge IGCSE® Chemistry (0620)Document7 pagesScheme of Work - Cambridge IGCSE® Chemistry (0620)Delta-007No ratings yet
- Topic 1 Key Concepts in Chemistry Revision 1Document1 pageTopic 1 Key Concepts in Chemistry Revision 1trishthamaheshwari01No ratings yet
- Class 8 2020Document4 pagesClass 8 2020Ahnaf FarhanNo ratings yet
- A New Look at The Periodic TableDocument11 pagesA New Look at The Periodic TableJ. GirotoNo ratings yet
- Agenda: Review ReviewDocument9 pagesAgenda: Review ReviewSara Al SaeedNo ratings yet
- Heat Treatment Lecture 1Document19 pagesHeat Treatment Lecture 1Solcastic SoulNo ratings yet
- Chemestry Honors ReferencesDocument4 pagesChemestry Honors ReferencesAna MorenoNo ratings yet
- Helpful For CAPE U1 Chemistry - Transition ElementsDocument30 pagesHelpful For CAPE U1 Chemistry - Transition ElementsDenison Dwarkah100% (1)
- Chapter 5 Chemical BondDocument5 pagesChapter 5 Chemical BondPuteri Alya maisarahNo ratings yet
- 1 BhattiAcademy - Com Chemistry 3. Azeem Acadmy (Subjective)Document32 pages1 BhattiAcademy - Com Chemistry 3. Azeem Acadmy (Subjective)hba sportsNo ratings yet
- Iron CarbonDocument18 pagesIron CarbonAshish AgarwalNo ratings yet
- Intro To Organic ChemistryDocument14 pagesIntro To Organic ChemistrylivvyridpNo ratings yet
- t4 SC 568 Aqa Chemistry Gcse Unit 41 Atomic Structure and The Periodic Table Highe Ver 3Document4 pagest4 SC 568 Aqa Chemistry Gcse Unit 41 Atomic Structure and The Periodic Table Highe Ver 3Karolina GawlakNo ratings yet
- Padhle 10th - Metals & Non-Metals + Integrated PYQsDocument23 pagesPadhle 10th - Metals & Non-Metals + Integrated PYQsDhruv SariaNo ratings yet
- Edexcel A P1 18 Q8-1Document4 pagesEdexcel A P1 18 Q8-1maryamnoonari21No ratings yet
- Prof. Dr.-Ing. Bambang Suharno Dr.-Ing. Reza M. Ulum: 1. Alasan Penggunaan 2. Klasifikasi 3. PenggunaanDocument31 pagesProf. Dr.-Ing. Bambang Suharno Dr.-Ing. Reza M. Ulum: 1. Alasan Penggunaan 2. Klasifikasi 3. Penggunaanraihan dzakyNo ratings yet
- 2015 May 20 Q9 (Edexcel 4PH0 FBI)Document2 pages2015 May 20 Q9 (Edexcel 4PH0 FBI)superpooh-1No ratings yet
- Wafer-Scale Production of Patterned Transition Metal Ditelluride Layers For Two-Dimensional Metal-Semiconductor Contacts at The Schottky-Mott LimitDocument9 pagesWafer-Scale Production of Patterned Transition Metal Ditelluride Layers For Two-Dimensional Metal-Semiconductor Contacts at The Schottky-Mott Limitseo minseongNo ratings yet
- 12 Physics Revision Notes Chapter 14Document20 pages12 Physics Revision Notes Chapter 14Shahbaz KhanNo ratings yet
- The Periodic TableDocument2 pagesThe Periodic TableJulieta FelliniNo ratings yet
- May 1 Lab - Lewis StructuresDocument21 pagesMay 1 Lab - Lewis Structuress76wz579ryNo ratings yet
- Application Note CORR-4 PDFDocument15 pagesApplication Note CORR-4 PDFaneesh19inNo ratings yet
- Artículo EDTADocument10 pagesArtículo EDTAALDAIR COSSIO POLONo ratings yet
- Atomic Structure and The Periodic Table Foundation Revision Activity MatDocument4 pagesAtomic Structure and The Periodic Table Foundation Revision Activity MatHồng Ngọc VõNo ratings yet
- CDSC: Checking Concurrent Data Structures Written With C/C++ AtomicsDocument19 pagesCDSC: Checking Concurrent Data Structures Written With C/C++ AtomicsbobNo ratings yet
- The Origin and Evolution of Saturn: A Post-Cassini PerspectiveDocument32 pagesThe Origin and Evolution of Saturn: A Post-Cassini PerspectivepadrblanNo ratings yet
- Handwritten - Metals and Non - Metals - Metals and Non-Metals-CompressedDocument15 pagesHandwritten - Metals and Non - Metals - Metals and Non-Metals-CompressedMack Tripathi100% (1)
- 08-Metals and Non-Metals Theory PDFDocument51 pages08-Metals and Non-Metals Theory PDFvikash singh rajpurohitNo ratings yet
- Shiny Periodic TableDocument1 pageShiny Periodic Tablefffake2010No ratings yet
- Electron Arrangement Y10Document10 pagesElectron Arrangement Y10Iftitahur Rohmah -No ratings yet
- Chapter 11: Applications and Processing of Metal Alloys Classification of Metal AlloysDocument8 pagesChapter 11: Applications and Processing of Metal Alloys Classification of Metal AlloysChelseyNo ratings yet
- Periodic Table ElectronegativityDocument1 pagePeriodic Table ElectronegativityddddNo ratings yet
- Periodic Table of ElementsDocument1 pagePeriodic Table of Elementsteknologipangan bthNo ratings yet
- History - Development of The Periodic TableDocument5 pagesHistory - Development of The Periodic TableChonama FetalcoNo ratings yet
- 3A Metals Edrolo Study NotesDocument22 pages3A Metals Edrolo Study NotesMr FiddleNo ratings yet
- Chapter 01Document16 pagesChapter 01LegiyanaNo ratings yet
- Notas de Clase 0 EnlacesDocument4 pagesNotas de Clase 0 EnlacesLEANDRO ORTIZ TABARESNo ratings yet
- English-Chinese Periodic Table of ElementsDocument2 pagesEnglish-Chinese Periodic Table of Elementsdavelo99100% (1)
- Periodic Table - Google SearchDocument1 pagePeriodic Table - Google Searchemmepatt7No ratings yet
- Electrode Potentials - FactRecallDocument3 pagesElectrode Potentials - FactRecallmalshiNo ratings yet
- Topic 1 Atomic Structure Revision MatDocument6 pagesTopic 1 Atomic Structure Revision MatMireiaNo ratings yet
- MetalsDocument6 pagesMetalsSean MarananNo ratings yet
- CH 18B - Acid BaseDocument2 pagesCH 18B - Acid BaseElle QuizonNo ratings yet
- Chemical Resistance enDocument22 pagesChemical Resistance enJose Manuel MorancieNo ratings yet
- Minn Kota Digital Onboard ChargersDocument64 pagesMinn Kota Digital Onboard ChargersGarrick BarberNo ratings yet
- Chemical Compatibility TableDocument18 pagesChemical Compatibility TableSiddhesh BagaveNo ratings yet
- 6 Lecture 21Document6 pages6 Lecture 21Muthu PalanisamyNo ratings yet
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- Ed066p853 2Document1 pageEd066p853 2Aitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- For Conceptualization The Franck-Condon PrincipleDocument1 pageFor Conceptualization The Franck-Condon PrincipleAitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Appendix A - Conversion From Molar To Molal: PL PLDocument57 pagesAppendix A - Conversion From Molar To Molal: PL PLAitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Acs Joc 8b00707Document14 pagesAcs Joc 8b00707Aitor PastorNo ratings yet
- Microscale Preparation of Alcl3 Journal of ChemicaDocument2 pagesMicroscale Preparation of Alcl3 Journal of ChemicaAitor PastorNo ratings yet
- Kinetics: Electrode ProcessesDocument8 pagesKinetics: Electrode ProcessesAitor PastorNo ratings yet
- The Multistep: Is Rate-Limiting ofDocument5 pagesThe Multistep: Is Rate-Limiting ofAitor PastorNo ratings yet
- Textbook: ForumDocument5 pagesTextbook: ForumAitor PastorNo ratings yet
- Malam Doc Chemistry Form 5: Chapter 2: Name: . ClassDocument7 pagesMalam Doc Chemistry Form 5: Chapter 2: Name: . ClassAzie Nurul AkhtarNo ratings yet
- TDS VISCOATEX 730 en WW 2020 09 16Document1 pageTDS VISCOATEX 730 en WW 2020 09 16umar buttNo ratings yet
- Chemistry Investigatory ProjectDocument18 pagesChemistry Investigatory ProjectSubhikshaNo ratings yet
- Space Charge and Dielectric Behavior of Epoxy Composite With SiO2-Al2O3 Nano-Micro Fillers at Varied TemperaturesDocument28 pagesSpace Charge and Dielectric Behavior of Epoxy Composite With SiO2-Al2O3 Nano-Micro Fillers at Varied TemperaturesOussama El BouadiNo ratings yet
- Kusadasi ACEMPDocument5 pagesKusadasi ACEMPapacheNo ratings yet
- 2015 12 Heat-loss-from-Insulated-pipeDocument7 pages2015 12 Heat-loss-from-Insulated-pipeRamu NallathambiNo ratings yet
- Ionic Bonding Vs Metallic BondingDocument2 pagesIonic Bonding Vs Metallic BondingsakuraleeshaoranNo ratings yet
- Introductionto Geomorphic ProcessDocument43 pagesIntroductionto Geomorphic ProcessSai PraveenyaNo ratings yet
- Electrical Conductivity of Carbon Blacks Under CompressionDocument7 pagesElectrical Conductivity of Carbon Blacks Under CompressionМирослав Кузишин100% (1)
- Cosmic Luminous Shock WavesDocument9 pagesCosmic Luminous Shock WavesRussell BagdooNo ratings yet
- 7075 Extrusion PDFDocument6 pages7075 Extrusion PDFJoselo HRNo ratings yet
- Proposed Plant For Citric Acid Production From Banana Peels Through Solid State FermentationDocument10 pagesProposed Plant For Citric Acid Production From Banana Peels Through Solid State FermentationCiara DevelosNo ratings yet
- ESI - Mesoporous Titania Nanofibers by Solution Blow Spinning PDFDocument6 pagesESI - Mesoporous Titania Nanofibers by Solution Blow Spinning PDFalkimiaNo ratings yet
- Chemical Incompatibility - tcm17-6734 PDFDocument1 pageChemical Incompatibility - tcm17-6734 PDFBasha Yazn AnjakNo ratings yet
- Fusion - PPTDocument10 pagesFusion - PPTZhijie TangNo ratings yet
- PW-SAT - Exam SyllabusDocument1 pagePW-SAT - Exam SyllabusHimanshu PalNo ratings yet
- Unisim Design Tutorial For Chee470: Queen'S University Department of Chemical EngineeringDocument75 pagesUnisim Design Tutorial For Chee470: Queen'S University Department of Chemical EngineeringMurrali Raj JeyagapalNo ratings yet
- MSDS of Hypochlorous AcidDocument4 pagesMSDS of Hypochlorous AcidHervian LanangNo ratings yet
- Organicchem ProbsetsDocument132 pagesOrganicchem ProbsetskimyNo ratings yet
- 2014-Preparation of The Graphene Oxide (GO) - Nafion Composite Membrane For The Vanadium Redox Flow Battery (VRB) SystemDocument8 pages2014-Preparation of The Graphene Oxide (GO) - Nafion Composite Membrane For The Vanadium Redox Flow Battery (VRB) SystemsomethingNo ratings yet
- CHE 322 - Gaseous Fuel Problems PDFDocument26 pagesCHE 322 - Gaseous Fuel Problems PDFDanice LunaNo ratings yet
- Chem 17 - DETERMINATION OF THE SOLUBILITY PRODUCT CONSTANT OF CALCIUM HYDROXIDE PDFDocument10 pagesChem 17 - DETERMINATION OF THE SOLUBILITY PRODUCT CONSTANT OF CALCIUM HYDROXIDE PDFWilfredo LlanaNo ratings yet
- 18.04.2019 Type of Fire ExtinguisherDocument1 page18.04.2019 Type of Fire ExtinguisherVaibhav Vithoba Naik100% (2)
- CHEM 17.1 Full ReportDocument6 pagesCHEM 17.1 Full ReportAaron MejiaNo ratings yet
- How To Interpret A Soil Test ReportDocument4 pagesHow To Interpret A Soil Test ReportKentChonNo ratings yet
- Full TextDocument8 pagesFull Textonlymusic16No ratings yet
- Hapter: Occurrence of Noble GasesDocument10 pagesHapter: Occurrence of Noble GasesSandipan SahaNo ratings yet
- Tugas Operasi Teknik Kimia 1 TranslateDocument12 pagesTugas Operasi Teknik Kimia 1 Translategira daraNo ratings yet
- Zeeco Incinerators & Thermal OxidizersDocument3 pagesZeeco Incinerators & Thermal OxidizersKR PANo ratings yet
- S.N. Sapali - Refrigeration and Air Conditioning-PHI Learning Private Limited (2014)Document593 pagesS.N. Sapali - Refrigeration and Air Conditioning-PHI Learning Private Limited (2014)kkmsNo ratings yet