Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
37 viewsFood Microbiology Laboratory Reviewer
Food Microbiology Laboratory Reviewer
Uploaded by
Charlene MartinezCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Cultural Characteristics of MicroorganismsDocument5 pagesCultural Characteristics of MicroorganismsCharlene MartinezNo ratings yet
- Micro 12 - Lesson 4 - Food SpoilageDocument39 pagesMicro 12 - Lesson 4 - Food SpoilageCharlene MartinezNo ratings yet
- Lab Activity No. 4Document2 pagesLab Activity No. 4Charlene MartinezNo ratings yet
- Entrep Module 12 ReportDocument20 pagesEntrep Module 12 ReportCharlene MartinezNo ratings yet
- D ValueDocument2 pagesD ValueCharlene MartinezNo ratings yet
- Laundry Business PlanDocument29 pagesLaundry Business PlanCharlene MartinezNo ratings yet
- RotcDocument51 pagesRotcCharlene MartinezNo ratings yet
- DEPAC CleanerDocument9 pagesDEPAC CleanerArdiansyah Oktavianus WahyudiNo ratings yet
- Data Sheets Ball Valves Material Compatibility KTM en en 5197068 PDFDocument6 pagesData Sheets Ball Valves Material Compatibility KTM en en 5197068 PDFMAHESH CHANDNo ratings yet
- QB ChemistryDocument69 pagesQB ChemistryLeeladhar SharmaNo ratings yet
- CH 10 SlidesDocument10 pagesCH 10 SlidesKnightdale RauschenbergNo ratings yet
- Mole Concept: Relative Atomic, Molecular and Formula Masses Relative Atomic Mass (RAM)Document7 pagesMole Concept: Relative Atomic, Molecular and Formula Masses Relative Atomic Mass (RAM)Aria PersaudNo ratings yet
- Product: Poly Vinyl Chloride (PVC) Trade Name: PVC K67Document2 pagesProduct: Poly Vinyl Chloride (PVC) Trade Name: PVC K67RoohullahParvezNo ratings yet
- CHE3703-Exam OctNov 2023Document15 pagesCHE3703-Exam OctNov 2023leratobaloyi386No ratings yet
- Advanced Chemical Escape Hood: Oil & Gas - Petrochemical and Chemical Processing - Mining - DefenceDocument4 pagesAdvanced Chemical Escape Hood: Oil & Gas - Petrochemical and Chemical Processing - Mining - DefenceJan VenterNo ratings yet
- Basicity and AcidityDocument20 pagesBasicity and AcidityshruthiNo ratings yet
- Physical Chemistry - Redox ReactionDocument4 pagesPhysical Chemistry - Redox ReactionDivyanshuMittalNo ratings yet
- 2021 Sepulveda Microarqueology and Archaeology of Color QIDocument19 pages2021 Sepulveda Microarqueology and Archaeology of Color QIMarcela SepulvedaNo ratings yet
- Amorphous SiO2C Composite As Anode Material For Lithium-Ion BatteriesDocument7 pagesAmorphous SiO2C Composite As Anode Material For Lithium-Ion BatteriesSabrina BrittoNo ratings yet
- Premier Supplier of Escitalopram API & Reference Standards - SynZeal ResearchDocument5 pagesPremier Supplier of Escitalopram API & Reference Standards - SynZeal ResearchsynzealNo ratings yet
- DFF 3Document9 pagesDFF 3Umar SiddiqueNo ratings yet
- Product Temperature: Specific GravityDocument8 pagesProduct Temperature: Specific GravityRyan BacalaNo ratings yet
- 0620 m19 QP 42 PDFDocument16 pages0620 m19 QP 42 PDFAlmeeraNo ratings yet
- Lecture Structure Of-A TerpineolDocument12 pagesLecture Structure Of-A TerpineolRk ShituNo ratings yet
- Bacterial Transformation: 1-Calcium Ion TreatmentDocument3 pagesBacterial Transformation: 1-Calcium Ion TreatmentE MeerNo ratings yet
- Surfactants For Use As CodispersantsDocument10 pagesSurfactants For Use As Codispersantsramitkatyal21881No ratings yet
- The Effect of Regrind Mills On The Separation of Chalcopyrite From Pyrite in Cleaner Flotation 2015 Minerals EngineeringDocument11 pagesThe Effect of Regrind Mills On The Separation of Chalcopyrite From Pyrite in Cleaner Flotation 2015 Minerals EngineeringW ZuoNo ratings yet
- Energy Recovery - TspaceDocument26 pagesEnergy Recovery - TspaceAlfonso Blanco100% (1)
- ChE Basic QuestionsDocument114 pagesChE Basic QuestionsKrizzete HernandezNo ratings yet
- Be Summer 2023Document2 pagesBe Summer 2023Abc 194748No ratings yet
- Preinsem PaperDocument1 pagePreinsem PaperDev PratapNo ratings yet
- Membrane Transport (Module 3)Document20 pagesMembrane Transport (Module 3)Aldine MabulacNo ratings yet
- Articles: Optimization of Chitin Extraction From Shrimp ShellsDocument7 pagesArticles: Optimization of Chitin Extraction From Shrimp ShellsJuan Pablo EspinosaNo ratings yet
- ASTM D5402-93 R99 Assessing The Solvent Resistance of Organic CoatingsDocument3 pagesASTM D5402-93 R99 Assessing The Solvent Resistance of Organic CoatingsJuan Carlos Contreras CherresNo ratings yet
- FDC FlexDuctConn SubmittalDocument2 pagesFDC FlexDuctConn SubmittalRaj SekharNo ratings yet
- Extraction of Active Principles From Natural SourcesDocument9 pagesExtraction of Active Principles From Natural SourcesSiddarth PalletiNo ratings yet
- Shrimp FinaleDocument19 pagesShrimp FinaleGrace Saagundo DubdubanNo ratings yet
Food Microbiology Laboratory Reviewer
Food Microbiology Laboratory Reviewer
Uploaded by
Charlene Martinez0 ratings0% found this document useful (0 votes)
37 views11 pagesCopyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
37 views11 pagesFood Microbiology Laboratory Reviewer
Food Microbiology Laboratory Reviewer
Uploaded by
Charlene MartinezCopyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 11
FOOD MICROBIOLOGY LABORATORY 5.
Stage The platform on which
REVIEWER slides and specimens are
placed for viewing.
6.Diaphragm The diaphragm or iris is
PART 1: MICROSCOPE located under the stage and
is an apparatus that can be
adjusted to vary the
intensity, and size, of the
cone of light that is
projected through the slide
7. Illuminator A steady light source (110
volts in the US) that shines
up through the slide.
Mirrors are sometimes used
in lieu of a built-in light. If
your microscope has a
mirror, it is used to reflect
light from an external light
source up through the
bottom of the stage
8.Eye Piece/ Otherwise referred to as an
Ocular Lense ocular, the eyepiece is the
lens nearest to your eye.
Total magnification of a
microscope is determined
by the sum of the eyepiece
PART OF FUNCTIONS magnification multiplied by
MICROSCOPE that of the objective lens.
1. Body Tube Where the eyepieces are 9. Arm The part of the microscope
dropped in. Also, it that connects the eyepiece
connects the eyepieces to tube to the base.
the objective lenses. 10.Coarse This is the knob on the side
2.Nosepiece his circular structure is Focus of the microscope that
where the different moves the objective lens up
objective lenses are and down. It is used in
screwed in. To change the conjunction with the fine
magnification power, simply focus.
rotate the turret 11. Fine Focus A knob used to fine-tune
3. Objective The lens closest to the the focus of a specimen in
Lenses specimen that first receives conjunction with the coarse
the rays from the specimen focus.
(the object) and forms the 12. Base A microscope is typically
image in the focal plane of composed of a head or
the eyepiece body and a base. The base is
4.Stage Clips Clips that are attached to the support mechanism.
the stage and retain the
slide.
PART 2: MATERIALS USED IN microbiological cultures can be used in
MICROBIOLOGY determination, egg incubation, heated storage,
food and beverage testing, and pharmaceutical
Apparatus and Equipment Commonly Used
testing
in the Microbiological Laboratory
Laboratory Drying Oven- evaporation,
Test tubes- Rimless tubes 160 to 180 mm in
sterilization, temperature testing, and for
length and 16 to 18 mm in diameter are
incubating temperature sensitive experiments.
preferred.
Triple beam balance - is generally used to weigh
Erlenmeyer flasks- The culture medium
media, for amounts up to 15 grams, glazed
incubated in an Erlenmeyer flask is in contact
weighing paper can be used to hold the powder
with the air over a large surface area. This
medium. For bigger amounts, a small beaker
favors growth in aerobic cultivation.
may be used.
Roux bottles- quadrangular or rounded in
Equipment of the Food Microbiology
profile, suitable for incubation in a horizontal
Research Laboratory
position.
Bioscreen-C Automated Growth Curve Analysis
Petri dishes- each 1 cm in height forms a
System- for measurement of growth of
culture vessel. The medium is poured into the
bacteria, molds, yeasts, or spores (it can create
smaller dish, which is about 9 cm in diameter,
up to 200 growth curves from 200 samples at
the larger one being used as a loosely-fitting lid.
one time)
Beakers- used for mixing, stirring and heating
miniVIDAS® instrument (multiparametric
liquids.
immunoassay system-Biomerieux) for food
Durham tubes- small glass tubes sealed at one pathogen testing and measurement of
end and open at the other. To indicate gas Staphylococcal enterotoxin
formation in a culture, it is placed with the open
A Thermo Scientific Forma 902 -86°C upright
end facing downwards in a test tube containing
Freezer for long-term storage of bacteria and
liquid medium.
samples
Pipettes- commonly used pipettes are
Microscopes: Zeiss Axion Star (with camera)
graduated ones, 1 mL full scale.
and Axion Star Plus.
Glass rods- used for spreading suspensions of
Microbiological safety cabinet Top Safe class II,
microorganisms on solid media.
type1.2, BIOAIR
Straight wires (needles) and wire loops for
Cooling incubators from 0°C up to 99.9 °C with
inoculation- made of platinum or chromium
12 different programs, Friocell 111, MMM
nickel and are fixed in a holder. Depending on
group and Incubators from 5°C above ambient
the use of the loop, its diameter may vary
temperature up to 99.9°C, Incucell 111, MMM
between 2 mm and 8 mm.
group
Autoclave- to sterilize equipment, instruments,
Autoclaves (3) and drying oven
and infectious waste
Microcentrifuge eppendorf, colony counters,
Incubator-provides a temperature-controlled
hot plate-stirrers, balances, pH-meter, fridges
environment to support growth of
PART 3: MEDIA PREPARATION - more associated with the examination of
(MICROBILOGICAL PROCEDURE) food products
SAMPLE 2. Social media – prepared by the addition of
CULTURE MEDIUM solidifying agent
- simple (peptone water and nutrient
agar) - agar or agar agar is the most commonly used
-complex (blood agar - additional solidifying agent, it is not hydrolyzed by most
ingredients) bacteria and doesn’t contribute any nutritive
-synthetic (defined, research purposes) property to the medium (1-3% concentration
GLASSWARE used to make a solid agar medium) gelatin a
proteinaceous substance also used for gel-
FOOD SUPPLIED – the form of a mixture of forming with the concentration of 10-14% of
nutrient substances generally known as solution
medium.
- an unbranched polysaccharide obtained
THERE IS NO UNIVERSAL MEDIUM FOR THE from the cell membranes of some species of red
CULTIVATION OF ALL MICROORGANISM algae such as the genera gelidium
BECAUSE DIFFERENT MICROOGRANISMS
REQUIRE DIFFERENT NUTRIENTS AND - more associated with the examination of
ENVIRONMENTAL CONDITIONS. food products
MICROBIOLOGICAL CULTURE MEDIUM – a COMMON SOLID MEDIA:
substance the encourages the growth and Nutrient Agar
survival of microorganisms Potato Dextrose Agar
THE CULTURE MEDIA MAY BE EITHER SOLID, Malt Extract Agar
SEMI-LIQUID OR LIQUID based on Plate Count Agar
CONSISTENCY IN A FORM AND GENERALLY
3. Semi- solid – it is formed when the amount
CONTAIN NUTRIENTS, GROWTH PROMOTING
of agar is reduced to 0.2%- 0.5%
FACTORS, ENERGY SOURCES, BUFFER SALTS,
MINERALS, METALS, AND GELLING AGENTS. - fairly soft and used in demonstrating
bacterial motility and separating motile from
TYPES OF CULTURE MEDIA (bacterial non-motile stains. (examples: STUARTS, AMIES
culture media) MEDIA, HUGH& LEIFSONS OXIDATION
1. Liquid media – refereed as “broths” FERMENTATION TEST)
- used in test-tubes, bottles, flask BIOPHASIC MEDIUM – called when a culture
system comprises both liquid and solid medium
- bacteria grow uniformly producing generally in the same bottle
turbidity
CLASSIFICATIONS OF CULTURE MEDIA
-aerobic bacteria grow as a thin film called
surface pellicle on the surface of undisturbed 1. Basal Media (generally media solid, liquid,
broth semi-solid)- simple media that support most
non fastidious bacteria (examples: PEPTONE
-used when a large number of bacteria have to WATER, NUTRIENT BROTH and NUTRIENT
be grown (example: tryptic soy sauce) AGAR)
2. Enriched Media- additional nutrients in the Use of metal caps reduce the dust or
form of blood, serum, egg yolk and other entry of other microorganism
substances to provide more nutrients/ food for Caps should not be fit too tightly,
organisms otherwise condensation may become
trapped in the vessel.
3. Selective (agar based)/ Enrichment Media Preparation of Agar Slants/Slopes- prepared by
(liquid in consistency) – formulated to inhibit measuring approximately 5 ml of melted
unwanted contaminating bacteria and help to medium into each tube, the still liquid medium
recover pathogen from a mixture of bacteria is allowed to solidify on a horizontal surface on
which glass rods or other supports are place so
4. Differential Media/ Indicator Media - are as to raise the plugged ends of the tubes
substances used to recognize different bacteria
based on colony color (examples: MacConkey’s Preparation of Agar Stab- prepared by
agar, CLED agar and TCBS agar) measuring approximately 5ml to 7ml of melted
medium into each test tube, liquid medium is
Preparation and Dispensing of Culture allowed to solidify on an upright position by
Media allowing to stand undisturbed on a test tube
1.Clean, preferably pyrex glasses, must be used. rack.
2. Dissolve ingredients in deionized or distilled
water. MEDIUM AMOUNT AUTOCLAVING
3.The pH value must be adjusted by using dilute CONTAINER
NaOH/HCl. Broth 5-10ml Culture tube
4. Microbiological culture media should not be Agar Slants 10ml Culture tube
turbid and should contain no sediment. Agar Stabs 7 ml Culture tube
Suspended matter can be removed by Agar Plates 200 ml (20- 500 ml flask
centrifugation and/or filtration. For filtration, 25 ml per (2/3 full)
cotton wool or one to two sheets of folded filter petri dish)
paper are usually sufficient.
5. Much time and work can be saved by using Storage of Culture Media
commercially produced media. These are The composition of the medium, the method of
available in powdered, granulated or liquid preparation, the pH, the conditions of
forms. sterilization, and the date of preparation must
be indicated on the label of the medium
Dispensing the medium container. It is a good practice to store media
The media are usually dispensed into: for several days at the temperature at which
a. test tubes of various sizes (Test tubes and they are eventually to be used. At the end of
flasks are usually closed with cotton plugs or the incubation, any microbial contamination
metal caps) may be identified.
b. screw-capped bottles.
Media should be stored in a dust-free,
If media are stored in cotton-plugged cold (about 4 °C), dark (or protected
vessels the plug should be covered with from direct sunlight) and not too humid
aluminum foil or cellophane room.
Contamination of the mouths of tubes Screw-capped bottles are suitable for
and flask must be avoided because it storing media. They have aluminum
leads to adhesion of the cotton plug to caps or plastic caps, both of which
the glass.
withstand autoclaving. Screw-capped 1. Prepare the glassware to be
bottles can be tightly closed. sterilized and place them in the dry
Media stored for periods longer than 6 air oven.
months should be checked before use. 2. Set the thermostat at 170 °C and
sterilize the materials for one hour.
TO PREPARE A CULTURE MEDIUM THERE IS 2. Sterilization by Moist Heat (autoclave) -
A COMPUTATION Culture media, solutions and cotton are usually
EXAMPLE: If the label of the bottle directs sterilized by this method. The shortest time for
you to add 28 grams of medium to 1000 mL effective sterilization is that required for
of distilled water, how many grams of destruction of the most heat-resistant
powdered medium should you weigh to microorganisms. At an approximately neutral
make 200 mL? pH, bacterial spores are the most resistant.
28 g Xg Steam under pressure at varying temperature-
SOLUTIONS: =
1000 g 200 g and-time intervals, depending on the materials
1000 x = 28 x 200 sterilized, is used. A temperature of 121 °C for
5600 15 lbs steam per square inch, for 15 minutes is
X=
1000 used for most media.
X= 5.6 grams
3. Fractional Sterilization in Flowing Steam
Tyndallization, or intermittent sterilization, is
PART 4: STERILIZATION used when steam pressure heating is too
rigorous. The material to be sterilized is
Sterility- absence of any kind of living organism. exposed to short periods of steaming at
intervals of 12 to 14 hours. Between steaming,
METHODS OF STERILIZATION the media is often placed at incubator
1. Sterilization by Dry Heat (conventional oven) temperatures.
- glassware, petri dish and pipette are sterilized
4. Filtration -Microbial cells can be removed
in the dry state in the hot air oven, exposed to
from fluids and gases by filtration. In industrial
160 to 165 C for 2hrs or 170 to 180 C for 1hr.
microbiological processes, heat-sensitive fluids
The period of heating commences from the
as well as the laboratory air are sterilized by
time the oven temperature reaches 160 °C.
filtration.
- Large volumes of air, such as are required in
Heating to redness. Wire needles and loops
clean rooms and laminar-flow cabinets, are
used for inoculation can be sterilized by heating
sterilized by filters. Dryness is a pre-requisite of
the wire to redness. The straight wire and the
the effectiveness of any air filter.
loop should be held obliquely in the upper part
of the Bunsen flame, both before and after use, 5. Radiation (UV) - the penetration capacity of
until they begin to glow. UV light is very low, this kind of radiation is
effective only for the sterilization of clean
Flaming. When glass vessels, test tubes or
rooms and laboratory surfaces. Radiation of
flasks are open, their mouths and metal caps
wavelength 253 to 265 nm is the most
should be flamed to prevent microbial
effective. However, care must be taken to
contamination of cultures. Scalpels, spatulas
protect the skin and eyes.
and glass rods should be immersed in alcohol
before flaming. 6. Cleaning and Disinfection
Cleaning. It is important to remove surface-
PROCEDURES IN DRY STERILIZATION
active substances by thorough cleaning and
rinsing before sterilization. For glassware,
soaking in detergent and washing with a brush bacterial suspension on the surface of an agar
is the most applicable method medium and streaking or spreading over the
surface. Spread plates are usually streaked with
- Cleaned glassware and instruments should a sterile bent glass rod. This process thins out
be rinsed with tap water followed by the bacteria on the agar surface so that
several rinses with distilled water, and individual bacteria are separated from each
after draining, dried in a drying cabinet. other.
Disinfectants- used to reduce the numbers of 2. Pour Plate Technique- involves the principle
microorganisms on surfaces. Ethanol is widely of dilution or thinning out the concentration of
used as a disinfectant for metal instruments, the bacterial suspension. Agar medium is
working surfaces, hands, rubber tubes and poured into the culture plate, allowed to solidify
rubber stoppers. It is most effective as an and incubate. Some organisms may be trapped
aqueous solution of about 70%. beneath the surface of the medium when it
- Other disinfectants are calcium gels. With this, the pour plate exhibits both
hypochlorite or sodium hypochlorite, surface and sub-surface colonies.
iodophores, formaldehyde and
quarternary ammonium compounds. A
CHARACTERISTICS OF COLONY
2% solution of hypochlorite contains 0.5%
active chlorine. Iodophores are aqueous
TRANSFORMATION
solutions of iodine and non-ionic surface-
active compounds. Formaldehyde is used
in 1% to 5% solutions. Quarternary
ammonium compounds are used in the
form of warm solutions. Agar plate cultures are obtained by the
PART 5: CULTURAL pour plate technique or the spread
CHARACTERISTCS OF plate technique.
MICROORGANISM Agar slant cultures are prepared by
making a streak inoculation up the
Culture- when bacteria or other microorganism middle of the sloped surface with a
grow in laboratory medium. Different species of transfer needle.
bacteria growing on the same kind of medium For the stab culture, the transfer needle
may appear different. carrying the inoculum is introduced in a
Microorganisms exist in nature as a straight line from the top to the bottom
mixed culture. However, to determine of the tube and removed along the
the characteristics of a particular same path.
species, it is important that the Tubes of broth can be inoculated with
organism be isolated and grown in the the transfer needle or loop.
laboratory as a pure culture (a culture Agar Plate Colonies
containing only one species of
organism). Size. Colonies range in size from extremely
small (pinpoint) measuring only a fraction of a
TECHNIQUES OF ISOLATING PURE CULTURE millimeter in diameter, to large colonies
measuring 5 to 10 mm in diameter.
1. Streak Plate or Spread Plate Technique. The Margin or Edge. The periphery of bacterial
streak plate or spread plate is obtained by colonies makes many different patterns,
transferring a portion (usually 0.1 mL) of a depending upon the species. It may be very
evenly circular, like the edge of a droplet, or it
may show such irregularities as rounded
projections, irregular notches, threadlike
projections or root-like projections.
Elevation. The colonies may be very thin (flat)
to thick (raised). Raised colonies exhibit varying
degrees of a convex structure.
Chromogenesis. Colonies may be colored
(pigmented) or not (nonpigmented). Various
shades of red, yellow, brown and violet
characterized certain species. Growth in Nutrient Broth
Optical features. May be opaque, translucent,
or opalescent. Amount of Growth. Scanty, moderate or
abundant.
Distribution of growth throughout the
broth. Uniformly distributed (evenly turbid);
growth confined to surface of broth as a scum
or film (pellicle); growth accumulated as
sediment, which may be granular or viscous.
Odor. May be putrid, fruity or aromatic, or
negligible.
Agar Slant Growth
Amount. Scanty, moderate or abundant.
Margin or Edge of Growth. Uniform or even or Growth in Agar Stabs
exhibiting various irregularities similar to the Amount of Growth. Scanty, moderate or
irregularities described or colonies. abundant.
Consistency of mass of growth. Butyrous or Margin or Edge of Growth. Uniform or even or
butter-like consistency; viscous or stringy; dry exhibiting various irregularities similar to the
and brittle. irregularities described or colonies.
Chromogenesis. Similar to that described for Chromogenesis. Similar to that described for
colonies. colonies
Aspergillus flavus
penicillium
Alternaria
Botrytis Cinerea
YEAST COLONIES - Young colonies are
ointment-like in consistency, while older ones Rhizopus Stolonifer
are denser, tending to dry out, and may form
pigments which give the organism a
characteristic color (yellow, orange, pink, brown
or black). The appearance of yeasts in liquid Geotricum candidum
culture may also be used to identify the
organism. Some species grow on the bottom of
the culture vessel forming a sediment, whereas
other species exhibit a uniform turbidity.
Another group grows only at the surface,
forming a thicker layer. PART 6: MORPHOLOGY OF
BACTERIA
Morphological Characteristics
Size of the cells - is determined by using
a microscope equipped with an ocular
micrometer (a disk with etched,
MOLD COLONIES-The presence of molds in food equidistant lines, the distance between
is characterized by the fuzzy, cottony them pre-determined by reference to a
appearance that tend to scatter on the surface stage micrometer.
as it grows. described based on the color of the
mycelium and the extent at which the organism - The unit for bacterial measurement
spread in the medium. is the micrometer, which is equivalent to
1/1000 mm or 1/25,400 inch.
NAME IMAGE
the shape
Mold culture arrangement of the cells in groups
the differentiation of their fine
structures.
3 General forms of bacterial cells
1. Spherical or ellipsoidal- occur singly, in
pairs (diplococcus), in chains
(streptococcus), in irregular grapelike
clusters (staphylococcus), as group of cocci
in a quadratic arrangement (tetrads), or as
package-like cubes of eight cocci (sarcina).
Arrangement of the round-shaped
bacteria
Preparation of Smears and Simple
Staining
Aniline dyes- most stains used in microbiology
derived from benzene.
- usually salts, although a few are acids or
bases, composed of charged colored ions.
Chromophore – ion that is colored.
Methylene blue chloride Methylene
blue + Cl−
+
(chromophore)
2. Cylindrical or rod-shaped- [bacillus; bacilli If the chromophore is a positive ion, like
(pl)] do not arrange themselves in a variety of methylene blue, the stain is considered a basic
patters similar to the cocci. They often appear stain; if a negative ion, it is an acidic stain.
as single cells, but many cells occur in pairs Most bacteria are stained when a basic stain
(diplobacillus) or form chains (streptobacillus). permeates the cell wall and adheres by weak
ionic bonds to the negative charges of the
bacterial cell.
Simple Stains- procedure that use only one
stain. (Methylene blue, crystal violet, malachite
green, basic fuschsin carbolfuschin and
safranin)
- Simple stains can be used to determine cell
morphology, size and arrangement.
Direct Stain- a simple stain that
colors the bacteria
3. Spiral or helical- [spirillum; spirilla (pl)] occur
predominantly as individual cells. Negative Stain- colors the
background but leaves the bacteria
unstained. (eosine acid fuchsin, in the cell to form a crystal violet-iodine
rose Bengal and congo red) complex (CV – I).
Smear is made by spreading a bacterial 3. Apply decolorizing agent (ethyl alcohol
suspension on a clean slide and allowing it to or ethyl alcohol-acetone). The primary
air-dry. stain is washed out (decolorized) of
- The dry smear is passed through a Bunsen some bacteria, while others are
burner flame several times to heat fix the unaffected.
bacteria. 4. Apply secondary stain or counterstain
- Heat fixing denatures bacterial enzymes, (safranin). This basic dye stains the
preventing them from digesting cell parts, decolorized bacteria red.
which causes the cell o break, a process
known as autolysis.
Gram- negative- decolorize
- The alcohol dissolves the outer
lipopolysaccharide layer and the CV-I
washes out through the thin layer of
peptidoglycan
Gram- positive- those that retain the primary
stain
- cell walls consist of many layers of
peptidoglycan.
- The CV-I is larger than the crystal violet or
iodine molecules that initially entered the
cell and cannot pass through the thick
peptidoglycan. In gram-negative cells, the
alcohol dissolves the outer
lipopolysaccharide layer, and the CV-I
washes out through the thin layer of pept
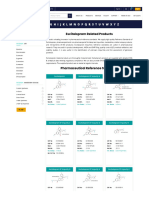
The Gram Stain
Gram stain - allows you to classify bacteria as
either gram-positive or gram-negative.
- useful stain for identifying and classifying
bacteria.
The staining technique consists of the
following steps:
1. Apply primary stain (crystal violet). All
bacteria are stained purple by this basic
dye.
2. Apply mordant (Gram’s iodine). The
iodine combines with the crystal violet RESULT OF GRAM STAINING
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Cultural Characteristics of MicroorganismsDocument5 pagesCultural Characteristics of MicroorganismsCharlene MartinezNo ratings yet
- Micro 12 - Lesson 4 - Food SpoilageDocument39 pagesMicro 12 - Lesson 4 - Food SpoilageCharlene MartinezNo ratings yet
- Lab Activity No. 4Document2 pagesLab Activity No. 4Charlene MartinezNo ratings yet
- Entrep Module 12 ReportDocument20 pagesEntrep Module 12 ReportCharlene MartinezNo ratings yet
- D ValueDocument2 pagesD ValueCharlene MartinezNo ratings yet
- Laundry Business PlanDocument29 pagesLaundry Business PlanCharlene MartinezNo ratings yet
- RotcDocument51 pagesRotcCharlene MartinezNo ratings yet
- DEPAC CleanerDocument9 pagesDEPAC CleanerArdiansyah Oktavianus WahyudiNo ratings yet
- Data Sheets Ball Valves Material Compatibility KTM en en 5197068 PDFDocument6 pagesData Sheets Ball Valves Material Compatibility KTM en en 5197068 PDFMAHESH CHANDNo ratings yet
- QB ChemistryDocument69 pagesQB ChemistryLeeladhar SharmaNo ratings yet
- CH 10 SlidesDocument10 pagesCH 10 SlidesKnightdale RauschenbergNo ratings yet
- Mole Concept: Relative Atomic, Molecular and Formula Masses Relative Atomic Mass (RAM)Document7 pagesMole Concept: Relative Atomic, Molecular and Formula Masses Relative Atomic Mass (RAM)Aria PersaudNo ratings yet
- Product: Poly Vinyl Chloride (PVC) Trade Name: PVC K67Document2 pagesProduct: Poly Vinyl Chloride (PVC) Trade Name: PVC K67RoohullahParvezNo ratings yet
- CHE3703-Exam OctNov 2023Document15 pagesCHE3703-Exam OctNov 2023leratobaloyi386No ratings yet
- Advanced Chemical Escape Hood: Oil & Gas - Petrochemical and Chemical Processing - Mining - DefenceDocument4 pagesAdvanced Chemical Escape Hood: Oil & Gas - Petrochemical and Chemical Processing - Mining - DefenceJan VenterNo ratings yet
- Basicity and AcidityDocument20 pagesBasicity and AcidityshruthiNo ratings yet
- Physical Chemistry - Redox ReactionDocument4 pagesPhysical Chemistry - Redox ReactionDivyanshuMittalNo ratings yet
- 2021 Sepulveda Microarqueology and Archaeology of Color QIDocument19 pages2021 Sepulveda Microarqueology and Archaeology of Color QIMarcela SepulvedaNo ratings yet
- Amorphous SiO2C Composite As Anode Material For Lithium-Ion BatteriesDocument7 pagesAmorphous SiO2C Composite As Anode Material For Lithium-Ion BatteriesSabrina BrittoNo ratings yet
- Premier Supplier of Escitalopram API & Reference Standards - SynZeal ResearchDocument5 pagesPremier Supplier of Escitalopram API & Reference Standards - SynZeal ResearchsynzealNo ratings yet
- DFF 3Document9 pagesDFF 3Umar SiddiqueNo ratings yet
- Product Temperature: Specific GravityDocument8 pagesProduct Temperature: Specific GravityRyan BacalaNo ratings yet
- 0620 m19 QP 42 PDFDocument16 pages0620 m19 QP 42 PDFAlmeeraNo ratings yet
- Lecture Structure Of-A TerpineolDocument12 pagesLecture Structure Of-A TerpineolRk ShituNo ratings yet
- Bacterial Transformation: 1-Calcium Ion TreatmentDocument3 pagesBacterial Transformation: 1-Calcium Ion TreatmentE MeerNo ratings yet
- Surfactants For Use As CodispersantsDocument10 pagesSurfactants For Use As Codispersantsramitkatyal21881No ratings yet
- The Effect of Regrind Mills On The Separation of Chalcopyrite From Pyrite in Cleaner Flotation 2015 Minerals EngineeringDocument11 pagesThe Effect of Regrind Mills On The Separation of Chalcopyrite From Pyrite in Cleaner Flotation 2015 Minerals EngineeringW ZuoNo ratings yet
- Energy Recovery - TspaceDocument26 pagesEnergy Recovery - TspaceAlfonso Blanco100% (1)
- ChE Basic QuestionsDocument114 pagesChE Basic QuestionsKrizzete HernandezNo ratings yet
- Be Summer 2023Document2 pagesBe Summer 2023Abc 194748No ratings yet
- Preinsem PaperDocument1 pagePreinsem PaperDev PratapNo ratings yet
- Membrane Transport (Module 3)Document20 pagesMembrane Transport (Module 3)Aldine MabulacNo ratings yet
- Articles: Optimization of Chitin Extraction From Shrimp ShellsDocument7 pagesArticles: Optimization of Chitin Extraction From Shrimp ShellsJuan Pablo EspinosaNo ratings yet
- ASTM D5402-93 R99 Assessing The Solvent Resistance of Organic CoatingsDocument3 pagesASTM D5402-93 R99 Assessing The Solvent Resistance of Organic CoatingsJuan Carlos Contreras CherresNo ratings yet
- FDC FlexDuctConn SubmittalDocument2 pagesFDC FlexDuctConn SubmittalRaj SekharNo ratings yet
- Extraction of Active Principles From Natural SourcesDocument9 pagesExtraction of Active Principles From Natural SourcesSiddarth PalletiNo ratings yet
- Shrimp FinaleDocument19 pagesShrimp FinaleGrace Saagundo DubdubanNo ratings yet