Professional Documents
Culture Documents
Amines: Physical Properties Preperation Physical Properties
Amines: Physical Properties Preperation Physical Properties
Uploaded by
Chandra Vamsi AdhikariOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Amines: Physical Properties Preperation Physical Properties
Amines: Physical Properties Preperation Physical Properties
Uploaded by
Chandra Vamsi AdhikariCopyright:
Available Formats
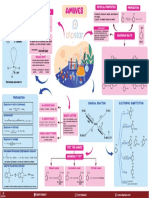
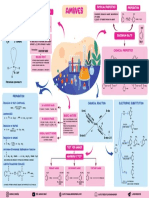
PHYSICAL PROPERTIES PREPERATION
STRUCTURE
PHYSICAL PROPERTIES AMINES Colourless, soluble in water, decompose in
dry state
NH2
-
N2+X
NH3 Ammonia PHYSICAL STATE C6H5N2+Cl- is readily soluble in water
NaNO + 2HCl
2
273− 278K
→ + NaCl + 2H2O
Lower aliphatic amines are gases,
:
intermediate members are liquid
R-NH2 1° AMine (fishy odour), while higher members
N2+X
-
:
IMPORTANCE
are solid.
In preperation of substituded
R2NH 2° Amine SOLUBILITY
aromatic compounds which
cannot be prepared by direct DIAZONIUM SALTS
substituion in benzene or
Lower aliphatic amines are soluble substistude benzene.
R3N 3° Amine in water due to H-bonding, while higher RN2+X
-
amines (> C6) are insoluble in water.
1
So lub ility ∝
CHEMICAL PROPERTIES
:
Molecular weight
BOILING POINT X X
G
Primary and secondary amines at
te
rm
Cu
on
an + HX cti
forms intermolecular H-Bonding Re
HX
Cu
+
Re
a
ac er
while tertiary does not. tio ey
n N2+ X − dm
N F Sa
n
CN
R R Primary > Secondary > Tertiary
HBF
∆
4 CuC
N/KC
N
θ > 109° Amine Amine Amine
H 3PO 2 +
+ H2
O
KI
Cu
I
R OH
- C
6H
O H, H 2O ∆ 5N
H
H5 + 2, H
Pyramidal geometry C 6 Cu +
N=N OH N=N NH2
OH
PREPARATION
• Reduction of Nitro Compounds. CHEMICAL REACTION ELECTROPHIC SUBSTITUTION
IN GASESOUS PHASE
Sn/HCl or Fe/HCl NH2
RNO2 → RNH2 3° Amine > 2° Amine > 1° Amine > NH3
OrH2/pd
R-NC R-OH 3 Br2
B2 Br
C
Ca H3 Cl
H2O
• Ammonolysis BASIC NATURE O 2
rb +
yla KO HN Br
IN AQUEOUS PHASE
te min H
NH3 Due to the presence of lone
R-NH2 + H2O + Na+ X-
- NaoH
R-X R-NH3X NH2
pair on nitrogen amines are st
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
(C2H5)2NH > (C2H5)3N > (C2H5)NH2 > NH3
basic.
e
R-NH2 O (i) CH3COCl
Factors affecting basicity R'-
• Reduction of Nitriles (i) Inductive effect C-
Cl
(ii) Br2, CH3COOH
(Major)
Ac
R'
H2 /Ni
+
R− C ≡ N → R − CH2 − NH2 (ii) solvation effect yla
Na(Hg)/C 2H 5oH
OVERALL BASICITY ORDER
(iii) Steric hinderance tio
n
O Br
R-NH-R' NH2 NH2 NH2
Aliphatic Amine > Ammonia R-NH-C-R'
• Reduction of Amides > Aromatic Amine
HNO3
NO2
O
(i) LiAlH4 + +
=
R − C − NH2 R − CH2 − NH2 H2SO4, 288 K NO2
(i) H2O NH2 (51%) (47%) (2%)
TEST FOR AMINES NO2
• Hoffmann Bromamide Degradation reaction NH2
O
R − C − NH2 + Br2 + 4NaOH
→ R − NH2 + Na2 CO3 + 2NaBr +2H 2O HINSBERG'S TEST (i) CH3COCl
(ii) Br2, CH3COOH
one carbon less amine is formed as compared to amides
NO2
• Gabriel Phthalimide synthesis NH2
O O PRIMARY AMINE SECONDARY AMINE TERTIARY AMINE
H2SO4
C C SO2Cl SO2-NHR SO2Cl SO2-NR2 SO2Cl
KOH NaOH
N - H
RX
→ N - R → RNH2
C C (1° Amine) + RNH2 + R2NH + R 3N No Reaction SO3H
O O ppt. soluble in ppt. insoluble in
CH3/AlCl3 No reaction
or CH3OCl/AlCl3
alkali alkali (due to salt formation)
Aromatic primary amines cannot be prepared by this method.
You might also like
- Determination of Ka of Weak Acids Lab ReportDocument3 pagesDetermination of Ka of Weak Acids Lab ReportNick Schweitzer78% (9)
- Colours of Elements and Compounds: Rasheed Ahmad A / O Level Chemistry 0333-4277385Document3 pagesColours of Elements and Compounds: Rasheed Ahmad A / O Level Chemistry 0333-4277385Malaika AkramNo ratings yet
- Ballad: Moderato. RomanticoDocument3 pagesBallad: Moderato. RomanticoVasilescuLucianNo ratings yet
- 715G6550 P01 001 002HDocument1 page715G6550 P01 001 002HNicolas Rueda100% (1)
- Qualitative Analysis of Elements in Organic CompoundsDocument3 pagesQualitative Analysis of Elements in Organic CompoundsJeremy Dawn100% (1)
- C13 AminesDocument1 pageC13 AminesDeivaNo ratings yet
- C13 AminesDocument1 pageC13 AminesPARAMBATH ANUP KUMARNo ratings yet
- Amines: Physical Properties Preperation Physical PropertiesDocument1 pageAmines: Physical Properties Preperation Physical PropertiesGargi PathakNo ratings yet
- The P-Block Elements - Mind Maps - Lakshya JEE 2024Document1 pageThe P-Block Elements - Mind Maps - Lakshya JEE 2024librarybooks.tashiNo ratings yet
- On The Track of Elements : LanthanidesDocument1 pageOn The Track of Elements : LanthanidesAmanda BarrosoNo ratings yet
- Periodic TableDocument1 pagePeriodic Tablepropheticman605No ratings yet
- Ggs Atex: Zone 2, Iic, T1: L204 - Cold InsulatedDocument1 pageGgs Atex: Zone 2, Iic, T1: L204 - Cold InsulatedShahzad AhmedNo ratings yet
- Neet Ug 2023 Chemistry PaperDocument11 pagesNeet Ug 2023 Chemistry Paperchakochi2007No ratings yet
- Shivaki STV-24LED1 Chassis MSD306Document11 pagesShivaki STV-24LED1 Chassis MSD306pikomobNo ratings yet
- Metabolic Pathways Poster PDFDocument1 pageMetabolic Pathways Poster PDFAlex DatsiukNo ratings yet
- Yare PDF/Yare Tenor SaxofonDocument4 pagesYare PDF/Yare Tenor Saxofonjorge luiNo ratings yet
- Cybernet Ptbm121d4x Part1Document1 pageCybernet Ptbm121d4x Part1evonaj25No ratings yet
- Poster de Las Vias Metabolicas.Document2 pagesPoster de Las Vias Metabolicas.Moises Rosales100% (1)
- 2'-8" WD. X 8" DP. STRIP Footing Detail Pad Footing Detail (F1)Document1 page2'-8" WD. X 8" DP. STRIP Footing Detail Pad Footing Detail (F1)Nizam RamkissoonNo ratings yet
- PCU Bridges 30 L.M 4lanes-General Notes1Document1 pagePCU Bridges 30 L.M 4lanes-General Notes1Anthony TangNo ratings yet
- Group 2 Electrical Circuit: 25L/30L/33L (LC) - 7MDocument7 pagesGroup 2 Electrical Circuit: 25L/30L/33L (LC) - 7MCamilo TorresNo ratings yet
- Table of Common Polyatomic Ions: HeliumDocument2 pagesTable of Common Polyatomic Ions: Helium4123245No ratings yet
- Hot Cold: PowerDocument3 pagesHot Cold: Powersergio villalbaNo ratings yet
- 9Document9 pages9deniden2013No ratings yet
- Area Inf IndirectaDocument1 pageArea Inf IndirectaEdgar VasquezNo ratings yet
- Intro Nhỏ ơiDocument1 pageIntro Nhỏ ơiHai DangNo ratings yet
- SMW 3 0Document1 pageSMW 3 0Zoran Poštin0% (1)
- Nucleotide Synthesis Purine Pyrimidine Synthesis Illustration AtfDocument1 pageNucleotide Synthesis Purine Pyrimidine Synthesis Illustration AtfJoax Wayne SanchezNo ratings yet
- Mukli Football StadiumDocument32 pagesMukli Football StadiumRoshan KejariwalNo ratings yet
- 715G18131 Circuit DiagramDocument2 pages715G18131 Circuit DiagramCarlos AlbertoNo ratings yet
- Group 2 Electrical Circuit: Frame/Engine Part Dashboard PartDocument7 pagesGroup 2 Electrical Circuit: Frame/Engine Part Dashboard PartAndré TarginoNo ratings yet
- Cpu Powercell 5000W: 05/02/2016 10:51:15 F 0.54 U:/Projetos/New Pcw/Pcw5K/Cpu5Kw2.Sch (Sheet: 1/1)Document1 pageCpu Powercell 5000W: 05/02/2016 10:51:15 F 0.54 U:/Projetos/New Pcw/Pcw5K/Cpu5Kw2.Sch (Sheet: 1/1)Claudinei FigueiraNo ratings yet
- K2 9742 121211 TDR TD: Can OpenDocument11 pagesK2 9742 121211 TDR TD: Can OpenMauro PerezNo ratings yet
- Mapa MetabólicoDocument1 pageMapa MetabólicoSuelenVernekMarquesNo ratings yet
- Sigma Metabolic Pathways-1Document1 pageSigma Metabolic Pathways-1Marianna Hipólito RochaNo ratings yet
- Premium Connections Thread CompoundsDocument1 pagePremium Connections Thread Compoundsjuan camiloNo ratings yet
- Specac Useful Spectroscopy PosterDocument1 pageSpecac Useful Spectroscopy PosterBechir ChammemNo ratings yet
- Classification of Elements (Javed)Document37 pagesClassification of Elements (Javed)Asim AliNo ratings yet
- FlowchartsDocument1 pageFlowchartsManvith KumarNo ratings yet
- Philips plhl-t827c 3pagc10005br SCHDocument1 pagePhilips plhl-t827c 3pagc10005br SCHMakhou SoyoNo ratings yet
- So Do Nguyen Ly 7TBDocument1 pageSo Do Nguyen Ly 7TBKhoatgTranNo ratings yet
- Boss RV 5 Digital Reverb SchematicDocument1 pageBoss RV 5 Digital Reverb Schematictofu157No ratings yet
- Fuente Ple90p PsuDocument1 pageFuente Ple90p PsuJibon DasNo ratings yet
- Fuente PLE90P PSUDocument1 pageFuente PLE90P PSUJibon DasNo ratings yet
- Detailed Command Area PlanDocument1 pageDetailed Command Area PlanVijaya Kumar GNo ratings yet
- Plano Reservorio ActualDocument1 pagePlano Reservorio ActualNatsu DragneelNo ratings yet
- 18 135100 0000100595 Acm STR DRG 102502 - ADocument1 page18 135100 0000100595 Acm STR DRG 102502 - Asaloman.vasuprada28No ratings yet
- DIGIT - Schematic - AMP Channel SectionDocument1 pageDIGIT - Schematic - AMP Channel Sectionvs055707No ratings yet
- Dynamic 12000 H 2 OhmDocument8 pagesDynamic 12000 H 2 OhmEspedito Alves SilvaNo ratings yet
- Dynamic 12000 H 2 OhmDocument8 pagesDynamic 12000 H 2 OhmMarcos SilvaNo ratings yet
- PV Single Line DiagramDocument1 pagePV Single Line DiagramMohammad ShayanNo ratings yet
- IP1725 IP1725: ResetDocument6 pagesIP1725 IP1725: ResetizzudinNo ratings yet
- fsp488-4f01 Power Supply SCHDocument1 pagefsp488-4f01 Power Supply SCHCarlos Junior PereiraNo ratings yet
- Pci Fonte Av3n + AmplificadorDocument1 pagePci Fonte Av3n + AmplificadorJeferson MazieroNo ratings yet
- Philips 715g6863-p01 PsuDocument3 pagesPhilips 715g6863-p01 Psuies837100% (1)
- Ground Floor PlanDocument1 pageGround Floor PlanSadaf HzNo ratings yet
- Manual de Instalare Controler de Acces Facial IP Hikvision DS-KD9203-E6Document1 pageManual de Instalare Controler de Acces Facial IP Hikvision DS-KD9203-E6FertuGabrielNo ratings yet
- Carvin R600 R1000 Rev-IDocument1 pageCarvin R600 R1000 Rev-Isoundman7157No ratings yet
- A10 低速驱动板40 013TDocument1 pageA10 低速驱动板40 013T易行胜No ratings yet
- Esquema Hidráulico Sany SY215C-AND-SY235Document1 pageEsquema Hidráulico Sany SY215C-AND-SY235juanchis650100% (1)
- Instant Assessments for Data Tracking, Grade 2: MathFrom EverandInstant Assessments for Data Tracking, Grade 2: MathNo ratings yet
- Amrita Btech Fee Structure Scholarship Updated 2023 2024Document3 pagesAmrita Btech Fee Structure Scholarship Updated 2023 2024Chandra Vamsi AdhikariNo ratings yet
- Coimbatore Induction Program Schedule 2023 1Document4 pagesCoimbatore Induction Program Schedule 2023 1Chandra Vamsi AdhikariNo ratings yet
- Btechpref2021 31061Document1 pageBtechpref2021 31061Chandra Vamsi AdhikariNo ratings yet
- Chemical Kinetics: Aa BB CC DD + +Document1 pageChemical Kinetics: Aa BB CC DD + +Chandra Vamsi AdhikariNo ratings yet
- Welcome To The Amrita Family, Adhikari Chandra Vamsi!!Document2 pagesWelcome To The Amrita Family, Adhikari Chandra Vamsi!!Chandra Vamsi AdhikariNo ratings yet
- 1Document1 page1Chandra Vamsi AdhikariNo ratings yet
- Quadratic EquationsDocument30 pagesQuadratic EquationsChandra Vamsi AdhikariNo ratings yet
- 3Document2 pages3Chandra Vamsi AdhikariNo ratings yet
- Maths - Coordinate Geometry (Prerequisites) (Notes)Document9 pagesMaths - Coordinate Geometry (Prerequisites) (Notes)Chandra Vamsi AdhikariNo ratings yet
- 7Document1 page7Chandra Vamsi AdhikariNo ratings yet
- Coordinate SystemDocument12 pagesCoordinate SystemChandra Vamsi AdhikariNo ratings yet
- Logarithms: MathsDocument13 pagesLogarithms: MathsChandra Vamsi Adhikari100% (1)
- Basic Maths. Lagoriths (Synopsis)Document3 pagesBasic Maths. Lagoriths (Synopsis)Chandra Vamsi AdhikariNo ratings yet
- Quadratic Expressions Assignment 1Document6 pagesQuadratic Expressions Assignment 1Chandra Vamsi AdhikariNo ratings yet
- Non MetalsDocument34 pagesNon MetalsDigvijay Patil100% (2)
- Redox TitrationDocument4 pagesRedox Titrationjeena josephNo ratings yet
- Bayer Process PDFDocument2 pagesBayer Process PDFFrandi CahyaNo ratings yet
- Important Concepts in Chemistry-2Document71 pagesImportant Concepts in Chemistry-2Saonah ZabaliNo ratings yet
- PPTDocument28 pagesPPTRama Arul SakthiNo ratings yet
- Chem.: 2) Nabf,/H, ODocument20 pagesChem.: 2) Nabf,/H, OMasumeh HashemiNo ratings yet
- Lecture 2Document9 pagesLecture 2Rahmeh EL saaiedehNo ratings yet
- Polymers: A Comprehensive Review On Corn Starch-Based Nanomaterials: Properties, Simulations, and ApplicationsDocument27 pagesPolymers: A Comprehensive Review On Corn Starch-Based Nanomaterials: Properties, Simulations, and ApplicationsIska AnggrainiNo ratings yet
- Fermentation: Metabolic BiodiversityDocument10 pagesFermentation: Metabolic BiodiversityEduar Moreno LondoñoNo ratings yet
- Floclog 702b Water Clarifier MsdsDocument2 pagesFloclog 702b Water Clarifier MsdsSouth Santee AquacultureNo ratings yet
- Activated Carbon: For Catalyst SupportDocument4 pagesActivated Carbon: For Catalyst SupportScott McLeanNo ratings yet
- 1st Midterm For Polymer Chemistry 11/20/2013 (Total:120 Points) Department of Materials Science and Engineering, NTUST Chapter 1,2,11Document2 pages1st Midterm For Polymer Chemistry 11/20/2013 (Total:120 Points) Department of Materials Science and Engineering, NTUST Chapter 1,2,11吳睿哲No ratings yet
- Plant Mineral NutritionDocument56 pagesPlant Mineral NutritionNam GonzalesNo ratings yet
- Tugas TLC MHSDocument2 pagesTugas TLC MHSCipoxz ParicukNo ratings yet
- 12th Class Guess Papers 2024 Chemistry ShortDocument7 pages12th Class Guess Papers 2024 Chemistry Shorttahajalil1074No ratings yet
- De TopicDocument10 pagesDe TopicHet ThankiNo ratings yet
- Physical Science: Quarter 1 - Module 3: Polarity of MoleculesDocument27 pagesPhysical Science: Quarter 1 - Module 3: Polarity of MoleculesArthur Laurel100% (1)
- Surface FinishingDocument7 pagesSurface Finishingcanveraza3122No ratings yet
- PolarityDocument27 pagesPolarityGiffNo ratings yet
- TEPZZ 894 - ZA - T: European Patent ApplicationDocument8 pagesTEPZZ 894 - ZA - T: European Patent Application76 NISHANT RANANo ratings yet
- Consumables Catalog: ShimadzuDocument208 pagesConsumables Catalog: ShimadzuHarveenkaur Malhan100% (1)
- Sample Questions - Chapter 27Document9 pagesSample Questions - Chapter 27Rasel IslamNo ratings yet
- Answered Paper of September 2020 Paper 1 ScienceDocument16 pagesAnswered Paper of September 2020 Paper 1 Sciencelakakal375No ratings yet
- Niehaus 1999Document19 pagesNiehaus 1999sudhu sudsNo ratings yet
- A Mechanism Study On Preparation of Rayon Based Carbon FibersDocument8 pagesA Mechanism Study On Preparation of Rayon Based Carbon FibersrkergunNo ratings yet
- MEPOXE MA. Methyl Ethyl Ketone Peroxide Type MA. Phthalate Plastilizer - 50 5% Active Oxygen - 9.5 0.5% Esters, Alcohols, Ketones, EthersDocument5 pagesMEPOXE MA. Methyl Ethyl Ketone Peroxide Type MA. Phthalate Plastilizer - 50 5% Active Oxygen - 9.5 0.5% Esters, Alcohols, Ketones, Etherseromax1No ratings yet
- Organic QuestionsDocument51 pagesOrganic Questionshemab30851No ratings yet