Professional Documents
Culture Documents
Callister7e SM ch12 23
Callister7e SM ch12 23
Uploaded by
Wellington AparecidoCopyright:
Available Formats
You might also like
- Activity 3 Data SheetDocument9 pagesActivity 3 Data SheetEli GabuatNo ratings yet
- Callister Solutions of Ch08Document42 pagesCallister Solutions of Ch08Malika Navaratna100% (1)
- Chapter # 03Document68 pagesChapter # 03mc170202750 Alishba WazahatNo ratings yet
- Chapter # 03Document68 pagesChapter # 03mc170202750 Alishba WazahatNo ratings yet
- ch03 PDFDocument93 pagesch03 PDFSharuvindan NairNo ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet
- Material Science and Engineering 9th Solution-4Document93 pagesMaterial Science and Engineering 9th Solution-4호박No ratings yet
- ch03-Q&A HW3Document5 pagesch03-Q&A HW3Ingi Abdel Aziz Srag100% (1)
- Pages From Solution Mannual - Chapter 3 - 8th EditionDocument29 pagesPages From Solution Mannual - Chapter 3 - 8th EditionARUNKUMAR ANo ratings yet
- Estructura CristalinaDocument19 pagesEstructura CristalinaLucasNo ratings yet
- Homework 2 StructureDocument5 pagesHomework 2 StructureDouglas FelixNo ratings yet
- Chapter 3 in Class Problems SolutionsDocument7 pagesChapter 3 in Class Problems Solutionsarnob vsd100% (2)
- Chapter 3 The Structure of Crystalline Solids Problem SolutionsDocument139 pagesChapter 3 The Structure of Crystalline Solids Problem SolutionsRandom RandomNo ratings yet
- Graded Problems Indicated in BoldDocument7 pagesGraded Problems Indicated in BoldJigoku KuroakaNo ratings yet
- Callister7e SM Ch12 22aDocument1 pageCallister7e SM Ch12 22aWellington AparecidoNo ratings yet
- Lecture-27, Atomic Radius, Packing FactorDocument4 pagesLecture-27, Atomic Radius, Packing FactorKhawaz HossainNo ratings yet
- Unit - 1 The Solid StateDocument21 pagesUnit - 1 The Solid StateExodusNo ratings yet
- HW 1 Mie 201Document5 pagesHW 1 Mie 20100gp1200rNo ratings yet
- Homework 2Document3 pagesHomework 2summerspringduriNo ratings yet
- Wa0008.Document30 pagesWa0008.Pruthvi BNNo ratings yet
- Chapter Three (Nuclear Models)Document25 pagesChapter Three (Nuclear Models)White HeartNo ratings yet
- Solid State Questions: 12th StandardDocument7 pagesSolid State Questions: 12th StandardShriya RameshNo ratings yet
- Example 3-4 (Askeland Example 03-15) Packing Factor in Diamond CubicDocument4 pagesExample 3-4 (Askeland Example 03-15) Packing Factor in Diamond Cubicoza_kawaiiNo ratings yet
- HW1 (1) SolutionDocument6 pagesHW1 (1) SolutionRahmat WidodoNo ratings yet
- EP UNIT NewDocument20 pagesEP UNIT Newtejav2468No ratings yet
- Crystal StructureDocument52 pagesCrystal StructureTausif TausNo ratings yet
- Solid State PDF by Manish Sir ChemistryDocument6 pagesSolid State PDF by Manish Sir ChemistryANJANA SHARMANo ratings yet
- Basic Concepts of Crystal StructureDocument10 pagesBasic Concepts of Crystal StructureMlkhr lgndbNo ratings yet
- 3.solid StateExerciseDocument32 pages3.solid StateExerciseVignesh Raja.PNo ratings yet
- II Puc Chemistry Important Question and Answer For Midterm Exam 2021Document29 pagesII Puc Chemistry Important Question and Answer For Midterm Exam 2021om sri sai enterprisesNo ratings yet
- Sol Self Test ExDocument5 pagesSol Self Test Exthomaszoy zoyNo ratings yet
- Module 1 RevisedDocument26 pagesModule 1 RevisedManu ManoharNo ratings yet
- Crystallography SolutionsDocument2 pagesCrystallography SolutionsashleytanakakapangaNo ratings yet
- Crystal Structure PPT JntuDocument118 pagesCrystal Structure PPT JntuKapil Siddhant Devulapalli100% (3)
- Wi2006 ECE331 Homework #1 SolutionDocument4 pagesWi2006 ECE331 Homework #1 Solutionaradhana231No ratings yet
- Packing Fraction - Calculat.Document7 pagesPacking Fraction - Calculat.Mukesh BohraNo ratings yet
- Part - I Class - XII Chemistry Chapter - 01 The Solid State: Material Downloaded From andDocument5 pagesPart - I Class - XII Chemistry Chapter - 01 The Solid State: Material Downloaded From andJyotsanaNo ratings yet
- Lec9 Crystal Structure01Document59 pagesLec9 Crystal Structure01rukeerusselNo ratings yet
- Derivations 2nd Puc NewDocument5 pagesDerivations 2nd Puc NewVinayak BhatNo ratings yet
- Crystal Structure (21!10!2011)Document71 pagesCrystal Structure (21!10!2011)Swetha PrasadNo ratings yet
- Chemistry Question BankDocument86 pagesChemistry Question BankNagesh SharmaNo ratings yet
- Structures of Metals and CeramicsDocument21 pagesStructures of Metals and CeramicsZacchariah ZerefNo ratings yet
- 634599660691024384Document44 pages634599660691024384Vijay Kumar100% (1)
- Midterm Passing PackageDocument23 pagesMidterm Passing PackageChinmayee NNo ratings yet
- Midterm Passing PackageDocument23 pagesMidterm Passing PackageRohit Reddy100% (2)
- 3 - Imperfection of SolidsDocument20 pages3 - Imperfection of SolidsANGELICA MAE BELOSONo ratings yet
- CH18Document79 pagesCH18deepakkr22781100% (1)
- Finding The Bond Angle in Tetrahedral-Shaped Molecule: Christopher J. KawaDocument2 pagesFinding The Bond Angle in Tetrahedral-Shaped Molecule: Christopher J. KawaRudolf KiraljNo ratings yet
- Rob Kusner and John M. Sullivan - Comparaing The Weaire-Phelan Equal-Volume Foam To Kelvin's FoamDocument10 pagesRob Kusner and John M. Sullivan - Comparaing The Weaire-Phelan Equal-Volume Foam To Kelvin's FoamFlaoeramNo ratings yet
- Engg MaterialsEMP - L3Document39 pagesEngg MaterialsEMP - L3Engr ZainNo ratings yet
- Cation Vs AnionDocument16 pagesCation Vs AnionnobleskyNo ratings yet
- CH 211 TutorialDocument3 pagesCH 211 TutorialSupun KahawaththaNo ratings yet
- PDFDocument6 pagesPDFAna Lorraine DalilisNo ratings yet
- IpomoPdfDownloadDirect PDFDocument31 pagesIpomoPdfDownloadDirect PDFPRUTHVI YBNo ratings yet
- Crystal Structure Notes: 1. Explain The Following Terms BrieflyDocument12 pagesCrystal Structure Notes: 1. Explain The Following Terms BrieflyNitesh ShahNo ratings yet
- 4 XRDDocument59 pages4 XRDMaaz ZafarNo ratings yet
- Covering Octahedral VoidsDocument2 pagesCovering Octahedral VoidsJitendra KumarNo ratings yet
- Self-Assembling Systems: Theory and SimulationFrom EverandSelf-Assembling Systems: Theory and SimulationLi-Tang YanNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2No ratings yet
- Callister7e SM Ch12 18Document1 pageCallister7e SM Ch12 18Wellington AparecidoNo ratings yet
- Callister7e SM ch12 16Document1 pageCallister7e SM ch12 16Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 25aDocument1 pageCallister7e SM Ch12 25aWellington AparecidoNo ratings yet
- Callister7e SM Ch12 25bDocument1 pageCallister7e SM Ch12 25bWellington AparecidoNo ratings yet
- Callister7e SM Ch12 22aDocument1 pageCallister7e SM Ch12 22aWellington AparecidoNo ratings yet
- Callister7e SM ch12 19Document1 pageCallister7e SM ch12 19Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet
Callister7e SM ch12 23
Callister7e SM ch12 23
Uploaded by
Wellington AparecidoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Callister7e SM ch12 23
Callister7e SM ch12 23
Uploaded by
Wellington AparecidoCopyright:
Available Formats
12-27
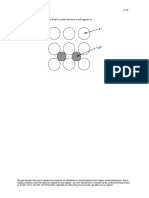
12.23 We are asked in this problem to compute the atomic packing factor for the diamond cubic crystal
structure, given that the angle between adjacent bonds is 109.5°. The first thing that we must do is to determine the
unit cell volume VC in terms of the atomic radius r. From Problem 12.15 the following relationship was developed
a = 4 y sin θ
in which y = 2r and θ = 35.25°. Furthermore, since the unit cell is cubic, VC = a3; therefore
3
VC = (4 y sin θ) 3 = [(4)(2 r )(sin 35.25°)] = 98.43 r 3
Now, it is necessary to determine the sphere volume in the unit cell, VS, in terms of r. For this unit cell (Figure
12.15) there are 4 interior atoms, 6 face atoms, and 8 corner atoms. The entirety of the interior atoms, one-half of
each face atom, and one-eighth of each corner atom belong to the unit cell. Therefore, there are 8 equivalent atoms
per unit cell; hence
⎛4 ⎞
VS = (8)⎜ π r 3 ⎟ = 33.51 r 3
⎝3 ⎠
Finally, the atomic packing factor is just
VS 33.51 r 3
APF = = = 0.340
VC 98.43 r 3
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to
students enrolled in courses for which the textbook has been adopted. Any other reproduction or translation of this work beyond that permitted
by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
You might also like
- Activity 3 Data SheetDocument9 pagesActivity 3 Data SheetEli GabuatNo ratings yet
- Callister Solutions of Ch08Document42 pagesCallister Solutions of Ch08Malika Navaratna100% (1)
- Chapter # 03Document68 pagesChapter # 03mc170202750 Alishba WazahatNo ratings yet
- Chapter # 03Document68 pagesChapter # 03mc170202750 Alishba WazahatNo ratings yet
- ch03 PDFDocument93 pagesch03 PDFSharuvindan NairNo ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet
- Material Science and Engineering 9th Solution-4Document93 pagesMaterial Science and Engineering 9th Solution-4호박No ratings yet
- ch03-Q&A HW3Document5 pagesch03-Q&A HW3Ingi Abdel Aziz Srag100% (1)
- Pages From Solution Mannual - Chapter 3 - 8th EditionDocument29 pagesPages From Solution Mannual - Chapter 3 - 8th EditionARUNKUMAR ANo ratings yet
- Estructura CristalinaDocument19 pagesEstructura CristalinaLucasNo ratings yet
- Homework 2 StructureDocument5 pagesHomework 2 StructureDouglas FelixNo ratings yet
- Chapter 3 in Class Problems SolutionsDocument7 pagesChapter 3 in Class Problems Solutionsarnob vsd100% (2)
- Chapter 3 The Structure of Crystalline Solids Problem SolutionsDocument139 pagesChapter 3 The Structure of Crystalline Solids Problem SolutionsRandom RandomNo ratings yet
- Graded Problems Indicated in BoldDocument7 pagesGraded Problems Indicated in BoldJigoku KuroakaNo ratings yet
- Callister7e SM Ch12 22aDocument1 pageCallister7e SM Ch12 22aWellington AparecidoNo ratings yet
- Lecture-27, Atomic Radius, Packing FactorDocument4 pagesLecture-27, Atomic Radius, Packing FactorKhawaz HossainNo ratings yet
- Unit - 1 The Solid StateDocument21 pagesUnit - 1 The Solid StateExodusNo ratings yet
- HW 1 Mie 201Document5 pagesHW 1 Mie 20100gp1200rNo ratings yet
- Homework 2Document3 pagesHomework 2summerspringduriNo ratings yet
- Wa0008.Document30 pagesWa0008.Pruthvi BNNo ratings yet
- Chapter Three (Nuclear Models)Document25 pagesChapter Three (Nuclear Models)White HeartNo ratings yet
- Solid State Questions: 12th StandardDocument7 pagesSolid State Questions: 12th StandardShriya RameshNo ratings yet
- Example 3-4 (Askeland Example 03-15) Packing Factor in Diamond CubicDocument4 pagesExample 3-4 (Askeland Example 03-15) Packing Factor in Diamond Cubicoza_kawaiiNo ratings yet
- HW1 (1) SolutionDocument6 pagesHW1 (1) SolutionRahmat WidodoNo ratings yet
- EP UNIT NewDocument20 pagesEP UNIT Newtejav2468No ratings yet
- Crystal StructureDocument52 pagesCrystal StructureTausif TausNo ratings yet
- Solid State PDF by Manish Sir ChemistryDocument6 pagesSolid State PDF by Manish Sir ChemistryANJANA SHARMANo ratings yet
- Basic Concepts of Crystal StructureDocument10 pagesBasic Concepts of Crystal StructureMlkhr lgndbNo ratings yet
- 3.solid StateExerciseDocument32 pages3.solid StateExerciseVignesh Raja.PNo ratings yet
- II Puc Chemistry Important Question and Answer For Midterm Exam 2021Document29 pagesII Puc Chemistry Important Question and Answer For Midterm Exam 2021om sri sai enterprisesNo ratings yet
- Sol Self Test ExDocument5 pagesSol Self Test Exthomaszoy zoyNo ratings yet
- Module 1 RevisedDocument26 pagesModule 1 RevisedManu ManoharNo ratings yet
- Crystallography SolutionsDocument2 pagesCrystallography SolutionsashleytanakakapangaNo ratings yet
- Crystal Structure PPT JntuDocument118 pagesCrystal Structure PPT JntuKapil Siddhant Devulapalli100% (3)
- Wi2006 ECE331 Homework #1 SolutionDocument4 pagesWi2006 ECE331 Homework #1 Solutionaradhana231No ratings yet
- Packing Fraction - Calculat.Document7 pagesPacking Fraction - Calculat.Mukesh BohraNo ratings yet
- Part - I Class - XII Chemistry Chapter - 01 The Solid State: Material Downloaded From andDocument5 pagesPart - I Class - XII Chemistry Chapter - 01 The Solid State: Material Downloaded From andJyotsanaNo ratings yet
- Lec9 Crystal Structure01Document59 pagesLec9 Crystal Structure01rukeerusselNo ratings yet
- Derivations 2nd Puc NewDocument5 pagesDerivations 2nd Puc NewVinayak BhatNo ratings yet
- Crystal Structure (21!10!2011)Document71 pagesCrystal Structure (21!10!2011)Swetha PrasadNo ratings yet
- Chemistry Question BankDocument86 pagesChemistry Question BankNagesh SharmaNo ratings yet
- Structures of Metals and CeramicsDocument21 pagesStructures of Metals and CeramicsZacchariah ZerefNo ratings yet
- 634599660691024384Document44 pages634599660691024384Vijay Kumar100% (1)
- Midterm Passing PackageDocument23 pagesMidterm Passing PackageChinmayee NNo ratings yet
- Midterm Passing PackageDocument23 pagesMidterm Passing PackageRohit Reddy100% (2)
- 3 - Imperfection of SolidsDocument20 pages3 - Imperfection of SolidsANGELICA MAE BELOSONo ratings yet
- CH18Document79 pagesCH18deepakkr22781100% (1)
- Finding The Bond Angle in Tetrahedral-Shaped Molecule: Christopher J. KawaDocument2 pagesFinding The Bond Angle in Tetrahedral-Shaped Molecule: Christopher J. KawaRudolf KiraljNo ratings yet
- Rob Kusner and John M. Sullivan - Comparaing The Weaire-Phelan Equal-Volume Foam To Kelvin's FoamDocument10 pagesRob Kusner and John M. Sullivan - Comparaing The Weaire-Phelan Equal-Volume Foam To Kelvin's FoamFlaoeramNo ratings yet
- Engg MaterialsEMP - L3Document39 pagesEngg MaterialsEMP - L3Engr ZainNo ratings yet
- Cation Vs AnionDocument16 pagesCation Vs AnionnobleskyNo ratings yet
- CH 211 TutorialDocument3 pagesCH 211 TutorialSupun KahawaththaNo ratings yet
- PDFDocument6 pagesPDFAna Lorraine DalilisNo ratings yet
- IpomoPdfDownloadDirect PDFDocument31 pagesIpomoPdfDownloadDirect PDFPRUTHVI YBNo ratings yet
- Crystal Structure Notes: 1. Explain The Following Terms BrieflyDocument12 pagesCrystal Structure Notes: 1. Explain The Following Terms BrieflyNitesh ShahNo ratings yet
- 4 XRDDocument59 pages4 XRDMaaz ZafarNo ratings yet
- Covering Octahedral VoidsDocument2 pagesCovering Octahedral VoidsJitendra KumarNo ratings yet
- Self-Assembling Systems: Theory and SimulationFrom EverandSelf-Assembling Systems: Theory and SimulationLi-Tang YanNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2No ratings yet
- Callister7e SM Ch12 18Document1 pageCallister7e SM Ch12 18Wellington AparecidoNo ratings yet
- Callister7e SM ch12 16Document1 pageCallister7e SM ch12 16Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 25aDocument1 pageCallister7e SM Ch12 25aWellington AparecidoNo ratings yet
- Callister7e SM Ch12 25bDocument1 pageCallister7e SM Ch12 25bWellington AparecidoNo ratings yet
- Callister7e SM Ch12 22aDocument1 pageCallister7e SM Ch12 22aWellington AparecidoNo ratings yet
- Callister7e SM ch12 19Document1 pageCallister7e SM ch12 19Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet