Professional Documents
Culture Documents
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Uploaded by
ISABEL RODRIGUES ROSADOCopyright:
Available Formats
You might also like
- 100 Days of Sunlight by Abbie EmmonsDocument315 pages100 Days of Sunlight by Abbie EmmonsJessie Ann Aquino100% (11)
- Neurology Multiple Choice Questions With Explanations: Volume IFrom EverandNeurology Multiple Choice Questions With Explanations: Volume IRating: 4 out of 5 stars4/5 (7)
- The Neuroscience of Language. On Brain Circuits of Words and Serial Order by Friedemann PulvermüllerDocument332 pagesThe Neuroscience of Language. On Brain Circuits of Words and Serial Order by Friedemann PulvermüllerMadhu sudarshan ReddyNo ratings yet
- Brain StructureDocument11 pagesBrain Structureabhijeett31No ratings yet
- Meningoencephalitis of Unknown Origin in Dogs CR - 881Document6 pagesMeningoencephalitis of Unknown Origin in Dogs CR - 881Ezequiel Davi Dos SantosNo ratings yet
- The Veterinary Journal2008SmithFindings On Low-Field Cranial MR Images in Epileptic Dogs That Lack Interictal Neurological DeficitsDocument6 pagesThe Veterinary Journal2008SmithFindings On Low-Field Cranial MR Images in Epileptic Dogs That Lack Interictal Neurological DeficitsCabinet VeterinarNo ratings yet
- Subacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Document7 pagesSubacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Bhanu PratapNo ratings yet
- Kolicheski Et Al-2017-Journal of Veterinary Internal MedicineDocument9 pagesKolicheski Et Al-2017-Journal of Veterinary Internal MedicineDulce Lucia ChacónNo ratings yet
- Del Toro 2006Document5 pagesDel Toro 2006NATALIA MARTINEZ CORDOBANo ratings yet
- Encefalitis EquinaDocument8 pagesEncefalitis EquinaAndrésNo ratings yet
- SM Pediatric Balolike 10072 - 2022 - Article - 6396Document3 pagesSM Pediatric Balolike 10072 - 2022 - Article - 6396Tunde CsepanyNo ratings yet
- CVJ 10 1091Document3 pagesCVJ 10 1091Rayza LubisNo ratings yet
- Prion BrainDocument2 pagesPrion BrainD SNo ratings yet
- Germinoma With Involvement of Midline and Off-Midline Intracranial StructuresDocument6 pagesGerminoma With Involvement of Midline and Off-Midline Intracranial StructuresDiah LubisNo ratings yet
- Migraine Like Episodic Pain Behaviour in A DogDocument7 pagesMigraine Like Episodic Pain Behaviour in A DogWilliam ChandlerNo ratings yet
- Neurological Complications Associated With Spontaneously Occurring Feline Diabetes MellitusDocument13 pagesNeurological Complications Associated With Spontaneously Occurring Feline Diabetes MellitusA J U N ANo ratings yet
- Aicardi-Goutie'res Syndrome: Neuroradiologic Findings and Follow-UpDocument6 pagesAicardi-Goutie'res Syndrome: Neuroradiologic Findings and Follow-UpclaypotgoldNo ratings yet
- Evaluation of Treatment With A Combination of Azathioprine and Prednisone in Dogs With Meningoencephalomyelitis of Undetermined Etiology - 40 Cases (2000-2007)Document7 pagesEvaluation of Treatment With A Combination of Azathioprine and Prednisone in Dogs With Meningoencephalomyelitis of Undetermined Etiology - 40 Cases (2000-2007)Aline Carvalho CaradonnaNo ratings yet
- Leukodystrophy Paper August 27thDocument7 pagesLeukodystrophy Paper August 27thZorbey TurkalpNo ratings yet
- Spectrum of Neurological Manifestations in Scorpion StingDocument6 pagesSpectrum of Neurological Manifestations in Scorpion Stingiaset123No ratings yet
- Doenças Do Sistema Nervoso Central em CãesDocument14 pagesDoenças Do Sistema Nervoso Central em CãesMaria Luiza Appoloni ZambomNo ratings yet
- Necrotizing Cerebellitis Neospora JVIM 2010Document8 pagesNecrotizing Cerebellitis Neospora JVIM 2010Rafael Porto GoncalvesNo ratings yet
- Afectiuni Congenitale Ale FelinelorDocument34 pagesAfectiuni Congenitale Ale FelinelorVeaceslavNo ratings yet
- Neurite Aguda Do Nervo Trigêmeo em Pastor Alemão: Acute Trigeminal Nerve Neuritis in German ShepherdDocument5 pagesNeurite Aguda Do Nervo Trigêmeo em Pastor Alemão: Acute Trigeminal Nerve Neuritis in German ShepherdGuilherme de FreitasNo ratings yet
- Full Text 01Document8 pagesFull Text 01Zunaira AmberNo ratings yet
- Creutzfeldt JakobDocument15 pagesCreutzfeldt JakobNicolas RodriguezNo ratings yet
- Neurological Complications in Psittacosis: A Case Report and Literature ReviewDocument2 pagesNeurological Complications in Psittacosis: A Case Report and Literature ReviewMíguel Sastre LázaroNo ratings yet
- Apathy and Hypersomnia Are Common Features of Myotonic DystrophyDocument7 pagesApathy and Hypersomnia Are Common Features of Myotonic DystrophyAnnisa HusainNo ratings yet
- Caso de EncefalitisDocument4 pagesCaso de EncefalitisJonathan CuevaNo ratings yet
- Canine DistemperDocument16 pagesCanine DistemperFelipe GonzalezNo ratings yet
- Macrophage Activation Syndrome Secondary To Mixed Connective Tissue Disease: A Rare Case in A Tertiary Care Hospital of Eastern IndiaDocument5 pagesMacrophage Activation Syndrome Secondary To Mixed Connective Tissue Disease: A Rare Case in A Tertiary Care Hospital of Eastern IndiaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- References: Meningoencephalitis As A Presentation Form of Behc EtDocument2 pagesReferences: Meningoencephalitis As A Presentation Form of Behc EtAndys Mayest BuntanusNo ratings yet
- Https:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Document3 pagesHttps:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Lucas XavierNo ratings yet
- Proof of Progression Over Time Finally Fulminant Brain Muscle An 2009 SeiDocument3 pagesProof of Progression Over Time Finally Fulminant Brain Muscle An 2009 Seibilal hadiNo ratings yet
- Acute Disseminated Encephalomyelitis in Chicken Pox: Case ReportDocument2 pagesAcute Disseminated Encephalomyelitis in Chicken Pox: Case ReportCorneliu VladNo ratings yet
- Jvim 15681Document39 pagesJvim 15681daniruizcasNo ratings yet
- Encefalitis Autoinmune 2011 PDFDocument11 pagesEncefalitis Autoinmune 2011 PDFDiana Vanessa SuarezNo ratings yet
- Brain Lesions, Obesity, and Other Disturbances in Mice Treated With Monosodium Glutamate (Olney 1969)Document3 pagesBrain Lesions, Obesity, and Other Disturbances in Mice Treated With Monosodium Glutamate (Olney 1969)Aprilia OanimaNo ratings yet
- A Case of Sporadic Creutzfeldt-JakobDocument5 pagesA Case of Sporadic Creutzfeldt-JakobVlad PopescuNo ratings yet
- 2017 32 3 264 267 EngDocument4 pages2017 32 3 264 267 Engemmanuellagrace06No ratings yet
- Case Report A Chinese Patient With Spinocerebellar Ataxia Finally Confirmed As Gerstmann-Sträussler-Scheinker Syndrome With P102L MutationDocument16 pagesCase Report A Chinese Patient With Spinocerebellar Ataxia Finally Confirmed As Gerstmann-Sträussler-Scheinker Syndrome With P102L MutationLídia YoshiharaNo ratings yet
- Case Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiaDocument4 pagesCase Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiahypnonautNo ratings yet
- Frontotemporal Dementia NeuropathologyDocument14 pagesFrontotemporal Dementia Neuropathologyppico1963No ratings yet
- BH 010060 ENGDocument2 pagesBH 010060 ENGCarlosNo ratings yet
- Seizure: European Journal of EpilepsyDocument10 pagesSeizure: European Journal of EpilepsyDanilo AssisNo ratings yet
- Encefalitis LimbicaDocument11 pagesEncefalitis LimbicaRandy UlloaNo ratings yet
- Anti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaDocument6 pagesAnti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaAbhishek KayalNo ratings yet
- Cerebral PyogranulomaDocument4 pagesCerebral PyogranulomamaiaraNo ratings yet
- Trabalho para A Semana de 03 de MarcoDocument5 pagesTrabalho para A Semana de 03 de MarcoTiago FernandesNo ratings yet
- GeneticsDocument10 pagesGeneticsapi-3835615No ratings yet
- Clinically Inapparent Gallbladder Cancer Presenting With Paraneoplastic Cranial Neuropathies Aunali S Khaku, MD Lisa Aenlle MD, Irene A Malaty, MD University of Florida Department of NeurologyDocument1 pageClinically Inapparent Gallbladder Cancer Presenting With Paraneoplastic Cranial Neuropathies Aunali S Khaku, MD Lisa Aenlle MD, Irene A Malaty, MD University of Florida Department of Neurologyask1288No ratings yet
- High Sensitivity of Asymmetric F THK5351 PET Abnormality in Patients With Corticobasal SyndromeDocument9 pagesHigh Sensitivity of Asymmetric F THK5351 PET Abnormality in Patients With Corticobasal SyndromeBrent AllieNo ratings yet
- Pisa Syndrome Source Clin Neuropharmacol SO 2015 Jul Aug 38 4 135 40 (PMIDT26166239)Document6 pagesPisa Syndrome Source Clin Neuropharmacol SO 2015 Jul Aug 38 4 135 40 (PMIDT26166239)dai shujuanNo ratings yet
- April 2019 1554202000 8152018Document2 pagesApril 2019 1554202000 8152018KantiNareshNo ratings yet
- Rewiew-Clinical Features and Prognosis of CanineDocument5 pagesRewiew-Clinical Features and Prognosis of CanineJuliana Ramos PereiraNo ratings yet
- Rett Syndrome: A Case ReportDocument6 pagesRett Syndrome: A Case ReportIJAR JOURNALNo ratings yet
- Algoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Document7 pagesAlgoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Farid Santiago Abedrabbo LombeydaNo ratings yet
- Guillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportDocument5 pagesGuillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportAsep MetrikaNo ratings yet
- ArticolDocument3 pagesArticolDiana DîrzuNo ratings yet
- 07 11 2017 - 14 10vet2450pvb 5339.R1LD PDFDocument10 pages07 11 2017 - 14 10vet2450pvb 5339.R1LD PDFfarah rachmahNo ratings yet
- A Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientDocument3 pagesA Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientInternational Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- Epileptic Encephalopathies SYNGAP1Document7 pagesEpileptic Encephalopathies SYNGAP1Saya OtonashiNo ratings yet
- Neuro 1Document5 pagesNeuro 1Ivan RisnaNo ratings yet
- Chapter 5 SummaryDocument10 pagesChapter 5 SummarykuzmanovicstanaNo ratings yet
- Networks in Temporal Lobe Epilepsy: Karina A. González Otárula,, Stephan SchueleDocument9 pagesNetworks in Temporal Lobe Epilepsy: Karina A. González Otárula,, Stephan SchueleDonald CabreraNo ratings yet
- Shaw Et Al. 2008Document9 pagesShaw Et Al. 2008Rodolfo Esteban Muñoz GómezNo ratings yet
- Chapter 3 Biological Foundations of BehaviourDocument20 pagesChapter 3 Biological Foundations of BehaviourAlex LiNo ratings yet
- CT Brain Part II-AnatomyDocument65 pagesCT Brain Part II-AnatomyXhenni XhenniNo ratings yet
- Gelastic and Cursive Seizures - Neurology - 1973Document11 pagesGelastic and Cursive Seizures - Neurology - 1973---No ratings yet
- Soal MCQ Blok 16Document14 pagesSoal MCQ Blok 16Mira Pandora100% (1)
- Mareschal Et Alii, 2007, Chapter 5 Sirois Et Alii, 2008, P. 325, and Figure 1 Below, Section IIIDocument15 pagesMareschal Et Alii, 2007, Chapter 5 Sirois Et Alii, 2008, P. 325, and Figure 1 Below, Section IIIHenrique Augusto Torres SimplícioNo ratings yet
- Obesity Related Cognitive Impairment The Role of Endo - 2019 - Neurobiology ofDocument18 pagesObesity Related Cognitive Impairment The Role of Endo - 2019 - Neurobiology ofSabrina YenNo ratings yet
- Sts Cheat Sheet of The BrainDocument30 pagesSts Cheat Sheet of The BrainRahula RakeshNo ratings yet
- The Cognitive Neuroscience of AutismDocument7 pagesThe Cognitive Neuroscience of AutismPatricia Roxana NeamțuNo ratings yet
- A System That Controls All of The Activities of The BodyDocument49 pagesA System That Controls All of The Activities of The BodynelsonNo ratings yet
- Online - OpenAccess - 978 3 7376 5093 9.OpenAccessDocument493 pagesOnline - OpenAccess - 978 3 7376 5093 9.OpenAccessPedro RodriguesNo ratings yet
- Week4-1 Brain DevelopmentDocument43 pagesWeek4-1 Brain DevelopmentJayden TseNo ratings yet
- Fundamental of EEG MeasurementDocument12 pagesFundamental of EEG MeasurementRyna Aulia Falamy100% (1)
- Fardo, Et Al., A Mechanism For Spatial Perception On Human Skin (Cognition 178), 2018Document8 pagesFardo, Et Al., A Mechanism For Spatial Perception On Human Skin (Cognition 178), 2018Franco ZárateNo ratings yet
- (ANA) 5.06 Organization of The Nervous SystemDocument19 pages(ANA) 5.06 Organization of The Nervous SystemAdrian MaterumNo ratings yet
- Outline of Functional Organization of Nervous SystemDocument6 pagesOutline of Functional Organization of Nervous SystemKarmina SantosNo ratings yet
- Jneurosci 5316-03 2004Document7 pagesJneurosci 5316-03 2004labsoneducationNo ratings yet
- Nervous System-Anatomy & PhysiologyDocument64 pagesNervous System-Anatomy & PhysiologyDeshi SportsNo ratings yet
- The Structure and Function of The BrainDocument25 pagesThe Structure and Function of The BrainNo NameNo ratings yet
- BIOL 4380 Emotion BIOL 4380 Emotion: Systems of Neuroscience (York University) Systems of Neuroscience (York University)Document10 pagesBIOL 4380 Emotion BIOL 4380 Emotion: Systems of Neuroscience (York University) Systems of Neuroscience (York University)Jiaying ChenNo ratings yet
- ขขอสอบ CU-TEP Reading Comprehension อสอบ CU-TEP Reading ComprehensionDocument18 pagesขขอสอบ CU-TEP Reading Comprehension อสอบ CU-TEP Reading Comprehensiontest testNo ratings yet
- Prelim Module in General PsychologyDocument9 pagesPrelim Module in General PsychologyAllona Zyra CambroneroNo ratings yet
- The Sleep-Deprived Human BrainDocument15 pagesThe Sleep-Deprived Human BrainGustavo CamiloNo ratings yet
- Anatomy and PhysiologyDocument10 pagesAnatomy and PhysiologyChris CHris ChRis100% (1)
- Histo Module 3Document67 pagesHisto Module 3Kathleen RodasNo ratings yet
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Uploaded by
ISABEL RODRIGUES ROSADOOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Clinical and Pathological Findings of Necrotizing Meningoencephalitis in A Maltese Dog.
Uploaded by
ISABEL RODRIGUES ROSADOCopyright:
Available Formats
Couto et al; Clinical and Pathological Findings of Necrotizing 31
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
Case Report
Clinical and Pathological Findings of Necrotizing
Meningoencephalitis in a Maltese Dog
Rodrigo M. Couto1, Silvia A. França1, Marina A. Rios1,
Isabel R. Rosado1, Paula M. Costa1, Roselene Ecco1*
1
Departamento de Clínica e Cirurgia Veterinária, Escola de Veterinária, Universidade Federal de Minas Gerais.
* Corresponding Author: Avenida Antônio Carlos, 6627. São Francisco. Belo Horizonte,
Minas Gerais, Brazil. CEP30123-970 E-mail ecco@vet.ufmg.br
Submitted February 21st 2013, Accepted March 25th 2013
Abstract
A 2-year-old, intact female Maltese dog was presented to the veterinarian with a history of acute neurological
signs. On neurological examination the dog showed deficit of mental status (apathy and depression), seizures, constant
howling, head turn and compulsive circling to the right side and falls to the left side. The treatment protocol using
prednisolone (for seizures remission) and cyclosporine (initiated in the chronic stage) did not stop the progression of the
disease and euthanasia was elected 65 days later. Necropsy revealed mild cerebral asymmetry, and in the frontal (more
affected) parietal and occipital lobes of the right hemisphere there were friable, depressed and yellowish areas
characterizing malacia. The left contralateral frontal lobe was edematous and slightly yellowish. At histopathology, the
lesions were characterized by marked, multifocal to coalescing necrotizing meningoencephalitis, characterized by focally
extensive areas of malacia, especially in the cortex of the frontal and right parietal lobes. Extension of lesions to white
matter was observed only in the caudal region of the right frontal lobe. Plasma cells and lymphocytes infiltration was
observed around vessels, leptomeninges and in the neuroparenchyma. In addition, the non-cavitation areas were also
characterized by neuropil vacuolization, neuronal necrosis, neuronophagia, astroglyosis with various gemistocytes,
endothelial hyperplasia and hypertrophy. The immunohistochemical analysis showed predominance of CD3 positive T
lymphocytes in proportion to CD79 positive cells. Clinical signs, character and distribution of neurological lesions were
compatible with necrotizing meningoencephalitis (NME). This condition, initially reported only in Pugs, currently affects
other breeds and attention should be given to the differential diagnosis with other neuropathies in dogs.
Key Words: brain, clinical signs, seizures, malacia, histopathology, immunohistochemistry, dogs.
Introduction dogs (14, 21). However, similar antibody levels occur in
Necrotizing meningoencephalitis (NME) is a the CSF of dogs with GME, brain tumors and even in
central nervous system (CNS) nonsuppurative some clinically normal dogs (20, 21). A genomic study in
inflammatory disorder of dogs, with poorly understood Pugs with NME showed a single strong association with
etiopathogenesis. Necrotizing leukoencephalitis (NLE) dog leukocyte antigen (DLA) class II, and supports the
and granulomatous meningoencephalomyelitis (GME) are role of the immune system in the disorder (6). Genetic
also CNS idiopathic inflammatory conditions. predisposition has been also confirmed in Pug dogs with
Nevertheless, each disease has unique histopathological NME but it is believed that there are additional influences
features (18). An autoimmune pathogenesis has been contributing to the phenotypic expression of the disease (3,
suggested for NME based on the presence of anti- 6). A recent study showed that viral pathogens are not
astrocytic and anti-glial fibrillary acid protein (GFAP) common in the brain of dogs with NME. However,
autoantibodies in the cerebrospinal fluid (CSF) of affected Mycoplasma canis was found in some cases of NME and
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
Couto et al; Clinical and Pathological Findings of Necrotizing 32
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
further investigation is warranted to determine the menace response. Because clinical signs improved
importance of M. canis in this condition (2). gradually, the initial prescription was maintained. Fifty
NME has been reported in various toy breeds three days after the first presentation the dog was checked
including Pug dogs, Yorkshire terrier, Maltese, Chihuahua again. The owner reported that the initial dosage of
(5, 18), Shih Tzu (5), West highland white terrier, Boston prednisolone was maintained but, arbitrarily the client did
terrier, Spitz Japanese and Pinscher (18), Pekingese (8) not maintained the medicine after three weeks, and the
and French bulldog (13). NME seems to be more common seizures episodes started again after discontinuing the
in females (5, 10) and, has been diagnosed in dogs with six corticoid therapy. The veterinarian detected that all
months to seven years of age (18). However, the more clinical signs mentioned above worsened. A new treatment
common age range from two (18) to four years (5). protocol adding cyclosporine (6 mg/kg bid) to
The clinical signs associated with NME are prednisolone was prescribed. However, ten days later, the
rapidly progressive and associate with the clinical signs worsened dramatically. The dog presented
neuroanatomical localization (18). The most common severe changes of the mental status (disorientation,
signs include seizures, depression, circling, (5, 6, 22), aggression and apathy), increased postural deficit, and
visual deficit (5, 6), postural reaction deficits (6) and bilateral deficit to menace response and cluster seizures.
vestibule-cerebellar signs (5, 16). Due to the poor prognosis, the owner elected euthanasia.
Definitive antemortem diagnosis is challenging At necropsy, there was mild asymmetry
because histopathology is mandatory. For most cases, the between the cerebral hemispheres. In the right hemisphere
clinical diagnosis is presumptive, associating clinical signs there were multifocal to coalescing depressed, markedly
and neuroanatomic localization, CSF analysis and friable and yellowish approximately 0.5 to 1.0 cm-
advanced imaging tests to exclude other causes (5, 16, 18). diameter areas. In the frontal lobe there was a locally
The prognosis of NME cases is poor due to the extensive 2.0 cm-diameter malacic area. The left
progressive course of the disease, with lower survival rate contralateral frontal lobe was edematous, slightly
in animals with seizures (5). Clinical signs in correlation yellowish and friable (Figure 1). On the corresponding cut
with the localization of CNS lesions and surface of the right frontal lobe, there was partial loss of
immunohistochemical findings of a NME case in a cortical parenchyma and demarcation between grey and
Maltese dog are described. white matter was not evident. The frontal lobes of both
hemispheres were markedly affected followed by parietal
Case report and occipital lobes. No gross lesions were observed in the
A 2-year-old, intact female Maltese dog was temporal lobe, hippocampus, cerebellum and brain stem.
presented to the veterinarian with a history of acute Extraneural gross lesions included moderate diffuse
neurological signs characterized by seizures, constant pulmonary congestion and edema. In the liver there was
howling, and circling to the right side. The owner moderate and diffuse glycogen degeneration.
informed that the immunization schedule had been
followed. On neurological examination, the dog presented
deficit of mental status (apathy and depression), head turn,
compulsive circling to the right side and falls to the left
side. During ambulation the animal turned away from
obstacles, indicating no visual deficit. Abnormal
movements and proprioception deficit to the left side were
detected. There was also left deficit to menace response
and cervical sensibility. A multifocal intracranial lesion
involving the telencephalon was suspected. The complete
blood count and serum chemistry profiling were
unremarkable. Magnetic resonance imaging (MRI) and
CSF analysis could not be performed because of the
client’s refusal. According to breed, age, history and
neuroanatomic localization of the lesions, an inflammatory
neurological disease of unknown cause was suspected. On
the basis of the suspicion, phenobarbital (6mg/kg bid),
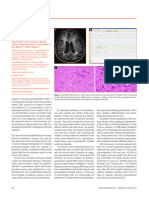
Figure 1. Dog, encephalon. In the frontal, parietal and occiptal lobes of
doxycycline (10mg/kg bid), prednisolone (2mg/kg bid) the right cerebral hemisphere, depressed and yellowish areas can be
and A and B vitamins were prescribed. A re-check five observed characterizing multifocal to coalescing malacia.
days after the onset of therapy was performed and no
changes were detected when thorough clinical The whole brain and samples of multiple organs
examination was performed. On neurological examination, were fixed in 10% buffered formalin and routinely
reduction of circling and seizures was observed but there processed for histopathology. Histologically, there was
was no improvement in posture, proprioception and marked multifocal to coalescing, necrotizing
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
Couto et al; Clinical and Pathological Findings of Necrotizing 33
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
nonsuppurative meningoencephalitis. The cerebral cortex lymphocytes perivascular cuffs associated with mild
showed areas with markedly increased cellularity vacuolization and axonal degeneration. No lesions were
interspersed with multifocal cavitations, partially filled found in the hippocampus, diencephalon (thalamus and
with numerous gitter cells, characterizing malacia (Figure hypothalamus), mesencephalon, cerebellum and medulla
2). Moderate to marked infiltration of cells, such as plasma oblongata. Paraffin embedded cerebral sections were
cells and lymphocytes were observed around vessels and selected and immunohistochemistry for CD3 (lymphocytes
diffuse in the leptomeninges. In addition, the non- T marker) and CD 79a (lymphocytes B marker) was
cavitation areas were characterized by neuropil performed as formerly described (1). The
vacuolization, neuronal necrosis, neuronophagia, immunohistochemical analysis showed that positive CD3
astroglyosis with various gemistocytes, endothelial cells were predominant in the perivascular cuffs,
hyperplasia and hypertrophy (Figure 3 and 4). leptomeninges and also in the neuroparenchyma (Figure 5
and 6). Less numbers of positive CD79a cells were
observed in the perivascular cuffs and leptomeninges but
they were rarely observed in the neuroparenchyma.
Figure 2. Dog, cortical cerebrum. Extensive malacia located within the
grey matter. Nonsuppurative inflammatory infiltration can be observed in Figure 4. Dog, cortical cerebrum. Vacuolization of the neuropil with
the leptomeninges, especially around blood vessels. Hematoxylin and proliferation of glial cells including gemistocytes (arrows) and
Eosin, 10x. perivascular lymphocytes. Hematoxylin and Eosin, 400x.
Gross and clinical findings were consistent with a
multifocal to coalescing malacia involving the cerebral
cortex. The histopathology allowed the definitive
diagnosis of NME characterized by multifocal to
coalescing necrotizing nonsuppurative
meningoencephalitis, affecting the grey matter of frontal,
parietal and occipital lobes, predominating in the right
cerebral hemisphere.
NME is an inflammatory and necrotizing
disease with great tropism for cerebral hemispheres. The
lesions occur preferentially in the telencephalon,
especially in the grey matter (occasionally can be observed
in the subcortical white matter) and leptomeninges with
multifocal distribution (5, 13, 16, 22).
The dog of the present study showed clinical
Figure 3. Dog, cortical cerebrum. Extensive malacia characterized by signs rapidly progressive and associated with the
loss of parenchyma and replacement by numerous gitter cells (arrows) neuroanatomic localization of the lesions as observed in
and few lymphocytes. Hematoxylin and Eosin, 400x.
other studies (18). The lesions and clinical signs of most
cases of NME are found exclusively in the cerebrum,
Single or binucleate gemistocytes were more
distinctive characteristic for this condition (8, 13).
commonly seen adjacent to necrotic areas. These areas
Detailed neurological examination allows to determining
were more intense in the frontal and parietal lobes of the
the sites of the lesions, which are extremely important for
right hemisphere. In the white matter subjacent to the
presumptive diagnosis and treatment. In addition to
necrotic right frontal cortex there were plasma cells and
neurological examination for supporting the clinical
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
Couto et al; Clinical and Pathological Findings of Necrotizing 34
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
suspicion, are fundamental the epidemiological data, CSF deficit of the left thoracic and pelvic limbs) suggested that
analysis, cross-sectional imaging via computed the lesion was worse in the right cerebral hemisphere.
tomography (CT) scan or magnetic resonance imaging Unilateral or asymmetric lesions that affect the frontal
(MRI) of the CNS and infectious diseases testing. Ct- lobe of the cerebrum can determine walking in circles,
guided brain biopsy and histopathological evaluation of generally to the same side as the lesion and head turn (11).
brain tissue may be considered in cases of suspected NME Most clinical signs noted in this dog could be related to the
(5, 18). gray cerebral matter, especially of the frontal lobe. Lesions
were not found in the cerebellum and brain stem; the eyes,
inner ear, and the vestibular nerve were not examined. The
vestibular system is constituted of neural cells in the inner
ear and their nuclei are in the brain stem (11). Lesions in
this system can cause nistagmus, ataxia and proprioceptive
deficits (12). Thus, in this dog, proprioception deficit
could not be definitely related to the vestibular system.
Most cases of NME in Pug dogs showed lesions in the
cerebrum only. Nevertheless, a high proportion of affected
dogs showed lesions also in the brain stem and in the
cerebellum (10).
The treatment protocol initiated after
presumptive diagnosis of meningoencephalitis improved
the neurological clinical picture (remission of seizures),
however, some signs persisted and discontinuous
prednisolone protocol allowed the return of seizures and
Figure 5. Dog, cortical cerebrum. CD3-positive T lymphocytes are all other clinical signs worsened. The resumption of
observed around blood vessels and within the cortical neuroparenchyma. therapy (prednisolone and cyclosporine) did not avoid the
Chromogen DAB. Harris hematoxylin counterstain, 200x.
clinical deterioration. Clinical signs remission and long-
term survival after prednisolone and cyclosporine therapy
was reported in a Pekingese dog (8). In the present report,
the discontinued prednisolone therapy by the client and the
later administration of cyclosporine, an
immunosuppressive agent directly suppressing T
lymphocyte activation and proliferation (4), possibly
determined the failure of the treatment.
The differential diagnosis of NME using
histopathology must include other CNS disorders: NLE,
GME, canine distemper, as well as tumors (13). NLE is
characterized by inflammatory and necrotic lesions similar
to NME, however, the lesions are predominately observed
in the white matter. Inflammatory cells infiltration is
prominent in the ependyma and mild in the choroid
plexus. GME is another idiopathic canine disorder
affecting mainly the cerebellum and brainstem. The
Figure 6. Dog, cortical cerebrum. CD3-positive T lymphocytes are disease is characterized by nodular granulomatous lesions
observed in the leptomeninges and in the cortical neuroparenchyma. containing macrophages and epithelioid cells, mainly in
Chromogen DAB. Harris hematoxylin counterstain, 200x.
subcortical regions. In addition, there are perivascular
cuffs constituted of lymphocytes, plasma cells,
Signs of depression, disorientation, aggression,
macrophages, and some neutrophils (13, 18). Neurological
circling, menace response and frequent seizures were the
cases of canine distemper are characterized by
main neurological signs in the dog of this report. The
demyelination of axons in the white matter of the
clinical signs observed are related to lesions in the cerebral
cerebellum and brainstem; most cases are associated with
cortex and/or thalamus (12). The mental depression could
nonsuppurative encephalitis, malacia and in some cases,
be also associated with lesions in the brain stem,
inclusion bodies in glial cells. Occasional cases are
particularly in the mesencephalon (11, 12). Seizures and
characterized by laminar cortical necrosis of the
circling could be also related to lesions in the
telencephalon (15). Co-infection of canine distemper and
hypothalamus (12). Nevertheless, no lesions were seen in
toxoplasmosis may occur, determining malacia associated
the mesencephalon and hypothalamus of this dog, and no
with parasitic cysts (9,15). The dog of this report did not
explanations for those signs are found at this moment
present any changes for diseases included in the
Initially, the clinical signs in this dog (proprioceptive
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
Couto et al; Clinical and Pathological Findings of Necrotizing 35
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
differential diagnosis and also had been appropriately meningoencephalitis in pug dogs. J. Hered., 2011,
vaccinated. 102, 540-546.
The immunohistochemical study showed 4. BENNET WM., NORMAN DJ. Action and toxicity
predominance of T lymphocytes in the leptomeninges, of cyclosporine. Ann. Rev. Med., 1986, 37, 215-224.
around vessels and in the neuroparenchyma similar to the 5. GRANGER N., SMITH PM., JEFFERY ND.
observed in other studies in dogs with NME (7, 13). For Clinical findings and treatment of non-infectious
viral encephalites like canine distemper, T lymphocytes meningoencephalomyelitis in dogs: A systematic
are found diffusely around blood vessels and review of 457 published cases from 1962 to 2008.
predominately in the white matter (19). For protozoans Vet. J., 2010, 184, 290-297.
and bacterial diseases, B lymphocytes are found 6. GREER KA., SCHATZBERG SJ., PORTER BF.,
predominately around blood vessels (17). A study using JONES KA., FAMULA, TR, MURPHY, KE.
double-labeling immunofluorescence antibody Heritability and transmission analysis of necrotizing
demonstrated predominance of CD3+ T lymphocytes in meningoencephalitis in the Pug. Res. Vet. Sci., 2009,
close proximity or attached to astrocytes, and the 86, 438–442.
cytoplasm and astrocytic processes were positive for IgG 7. HIGGINS RJ., DICKINSON PJ., KUBE SA.,
in the NME and NLE lesions (13). The involvement of the MOORE PF., COUTO SS., VERNAU KM.,
autoantibody to astrocytes in the NME cases supports the STURGES BK., LECOUTEUR RA. Necrotizing
immune-mediated pathogenesis hypothesis however, does meningoencephalitis in five chihuahua dogs. Vet.
not confirm if anti-astrocytic and anti-GFAP antibodies Pathol., 2008, 45, 336–346.
are a primary cause or a secondary consequence of NME 8. JUNG DI., KIM JW., PARK HM. Long-term
(13, 21). immunosuppressive therapy with cyclosporine plus
In conclusion, this study shows that prednisolone for necrotizing meningoencephalitis in
neurological examination related to neuroanatomical a Pekingese dog. J. Vet. Med. Sci., 2012, 74, 765-
localization can be useful for clinical presumptive 769.
diagnosis of NME. This condition, initially reported as a 9. KOESTNER A., COLE CR. Neuropathology of
Pug disease, currently affects other breeds, like the canine toxoplasmosis. Am. J. Vet. Res., 1960, 21,
Maltese from this report and, attention should be given to 831-844.
the differential diagnosis with other neuropathies in dogs. 10. LEVINE JM., FOSGATE GT., PORTER B.,
SCHATZBERG SJ., GREER K. Epidemiology of
ACKNOWLEDGMENTS Necrotizing Meningoencephalitis in Pug Dogs. J.
The first three authors have a research fellowship Vet. Intern. Med., 2008, 22, 961–968.
from CAPES (Coordenação de Aperfeiçoamento de 11. OLIVER JE., LORENZ MD., KORNEGAY JN.
Pessoal de Nível Superior), FAPEMIG (Fundação de Handbook of Veterinary Neurology. 3 ed.
Amparo à Pesquisa do Estado de Minas Gerais) and CNPq Philadelphia: W.B. Saunders Company, 1997.
(Conselho Nacional de Desenvolvimento Científico e 12. O’NEILL EJ., MERRETT D., JONES B.
Tecnológico), respectivelly. Granulomatous meningoencephalomyelitis in dogs:
A review. Ir. Vet. J., 2005, 58 (2), 86-92.
References 13. PARK ES, UCHIDA K, NAKAYAMA H.
Comprehensive immunohistochemical studies on
1. ARAÚJO MR., PREIS IS., LAVALLE GE., canine necrotizing meningoencephalitis (NME),
CASSALI GD., ECCO R. Histomorphological and necrotizing leukoencephalitis (NLE), and
immunohistochemical characterization of 172 granulomatous meningoencephalomyelitis (GME).
cutaneous round cell tumours in dogs. Pesq. Vet. Vet Pathol. 2012, 49 (4):682-92.
Bras., 2012, 32, 772-780. 14. SHIBUYA MN., FUJIWARA K., IMAJOH-OHMI
2. BARBER RM., PORTER BF., LI Q., MAY M., S., FUKUDA H., PHAM NT., TAMAHARA S.,
CLAIBORNE MK., ALLISON AB., HOWERTH ONO K. Autoantibodies against glial fibrillary acidic
EW., BUTLER A., WEI S., LEVINE JM., LEVINE protein (GFAP) in cerebrospinal fluids from Pug
GJ., BROWN DR., SCHATZBERG SJ. Broadly dogs with necrotizing meningoencephalitis. J. Vet.
reactive polymerase chain reaction for pathogen Med. Sci., 2007, 69(3), 241-245.
detection in canine granulomatous 15. SILVA MC., FIGHERA AR., BRUM SJ., GRAÇA
meningoencephalomyelitis and necrotizing LD., KOMMERS DG., IRIGOYEN L.F.; BARROS
meningoencephalitis. J. Vet. Intern. Med., 2012, 26, SLC. Aspectos clinicopatológicos de 620 casos
962-968. neurológicos de cinomose em cães. Pesq. Vet. Bras.,
3. BARBER RM., SCHATZBERG SJ., 2007, 27 (5), 215-220.
CORNEVEAUX JJ., ALLEN AN, PORTER BF, 16. SPITZBARTH I., SCHENK HC., TIPOLD A,
PRUZIN JJ, PLATT SR, KENT M, HUENTELMAN BEINEKE A. Immunohistochemical
MJ. Identification of risk loci for necrotizing Characterization of Inflammatory and Glial
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
Couto et al; Clinical and Pathological Findings of Necrotizing 36
Meningoencephalitis in a Maltese Dog. Braz J Vet Pathol, 2013, 6 (1), 31 - 36
Responses in a Case of Necrotizing canine distemper virus infection. Acta Neuropathol.,
Leucoencephalitis in a French Bulldog. J. Comp. 1999, 97, 45–56.
Path., 2010, 142, 235-241. 20. TODA Y., MATSUKI N., SHIBUYA M., FUJIOKA
17. SUMMERS BA. Inflammatory diseases of the I., TAMAHARA S., ONO K. Glial fibrillary acidic
central nervous system. In: Veterinary protein (GFAP) and anti-GFAP autoantibody in
Neuropathology, ed. SUMMERS BA., canine necrotising meningoencephalitis. Vet Rec.
CUMMINGS JF., LAHUNTA A., St. Louis: Mosby, 2007, 161, 261-264.
1995, 111–114. 21. UCHIDA K., HASEGAWA T., IKEDA M.,
18. TALARICO LR., SCHATZBERG SJ. Idiopathic YAMAGUCHI R., TATEYAMA S. Detection of an
granulomatous and necrotizing inflammatory autoantibody from Pug dogs with necrotizing
disorders of the canine central nervous system: a encephalitis (Pug dog encephalitis). Vet Pathol.,
review and future perspectives. J. Sm. Anim. Pract., 1999, 36, 301-307.
2010, 51, 138-149. 22. VIOLIN KB., QUEIROZ NGT., HOSOMI FYM.,
19. TIPOLD A., MOORE PF., ZURBRIGGEN A., RAMOSI AT., AMARAL HA., KOGIKA MM.,
BURGENER I., BARBEN G., VANDEVELDE M. GISELE FABRINO MACHADO GF., MAIORKA
Early T cell response in the central nervous system in PC. Meningoencefalite necrotizante de cão Maltês.
Ciência Rural, 2008, 38, 836-838.
Brazilian Journal of Veterinary Pathology. www.bjvp.org.br . All rights reserved 2007.
You might also like
- 100 Days of Sunlight by Abbie EmmonsDocument315 pages100 Days of Sunlight by Abbie EmmonsJessie Ann Aquino100% (11)
- Neurology Multiple Choice Questions With Explanations: Volume IFrom EverandNeurology Multiple Choice Questions With Explanations: Volume IRating: 4 out of 5 stars4/5 (7)
- The Neuroscience of Language. On Brain Circuits of Words and Serial Order by Friedemann PulvermüllerDocument332 pagesThe Neuroscience of Language. On Brain Circuits of Words and Serial Order by Friedemann PulvermüllerMadhu sudarshan ReddyNo ratings yet
- Brain StructureDocument11 pagesBrain Structureabhijeett31No ratings yet
- Meningoencephalitis of Unknown Origin in Dogs CR - 881Document6 pagesMeningoencephalitis of Unknown Origin in Dogs CR - 881Ezequiel Davi Dos SantosNo ratings yet
- The Veterinary Journal2008SmithFindings On Low-Field Cranial MR Images in Epileptic Dogs That Lack Interictal Neurological DeficitsDocument6 pagesThe Veterinary Journal2008SmithFindings On Low-Field Cranial MR Images in Epileptic Dogs That Lack Interictal Neurological DeficitsCabinet VeterinarNo ratings yet
- Subacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Document7 pagesSubacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Bhanu PratapNo ratings yet
- Kolicheski Et Al-2017-Journal of Veterinary Internal MedicineDocument9 pagesKolicheski Et Al-2017-Journal of Veterinary Internal MedicineDulce Lucia ChacónNo ratings yet
- Del Toro 2006Document5 pagesDel Toro 2006NATALIA MARTINEZ CORDOBANo ratings yet
- Encefalitis EquinaDocument8 pagesEncefalitis EquinaAndrésNo ratings yet
- SM Pediatric Balolike 10072 - 2022 - Article - 6396Document3 pagesSM Pediatric Balolike 10072 - 2022 - Article - 6396Tunde CsepanyNo ratings yet
- CVJ 10 1091Document3 pagesCVJ 10 1091Rayza LubisNo ratings yet
- Prion BrainDocument2 pagesPrion BrainD SNo ratings yet
- Germinoma With Involvement of Midline and Off-Midline Intracranial StructuresDocument6 pagesGerminoma With Involvement of Midline and Off-Midline Intracranial StructuresDiah LubisNo ratings yet
- Migraine Like Episodic Pain Behaviour in A DogDocument7 pagesMigraine Like Episodic Pain Behaviour in A DogWilliam ChandlerNo ratings yet
- Neurological Complications Associated With Spontaneously Occurring Feline Diabetes MellitusDocument13 pagesNeurological Complications Associated With Spontaneously Occurring Feline Diabetes MellitusA J U N ANo ratings yet
- Aicardi-Goutie'res Syndrome: Neuroradiologic Findings and Follow-UpDocument6 pagesAicardi-Goutie'res Syndrome: Neuroradiologic Findings and Follow-UpclaypotgoldNo ratings yet
- Evaluation of Treatment With A Combination of Azathioprine and Prednisone in Dogs With Meningoencephalomyelitis of Undetermined Etiology - 40 Cases (2000-2007)Document7 pagesEvaluation of Treatment With A Combination of Azathioprine and Prednisone in Dogs With Meningoencephalomyelitis of Undetermined Etiology - 40 Cases (2000-2007)Aline Carvalho CaradonnaNo ratings yet
- Leukodystrophy Paper August 27thDocument7 pagesLeukodystrophy Paper August 27thZorbey TurkalpNo ratings yet
- Spectrum of Neurological Manifestations in Scorpion StingDocument6 pagesSpectrum of Neurological Manifestations in Scorpion Stingiaset123No ratings yet
- Doenças Do Sistema Nervoso Central em CãesDocument14 pagesDoenças Do Sistema Nervoso Central em CãesMaria Luiza Appoloni ZambomNo ratings yet
- Necrotizing Cerebellitis Neospora JVIM 2010Document8 pagesNecrotizing Cerebellitis Neospora JVIM 2010Rafael Porto GoncalvesNo ratings yet
- Afectiuni Congenitale Ale FelinelorDocument34 pagesAfectiuni Congenitale Ale FelinelorVeaceslavNo ratings yet
- Neurite Aguda Do Nervo Trigêmeo em Pastor Alemão: Acute Trigeminal Nerve Neuritis in German ShepherdDocument5 pagesNeurite Aguda Do Nervo Trigêmeo em Pastor Alemão: Acute Trigeminal Nerve Neuritis in German ShepherdGuilherme de FreitasNo ratings yet
- Full Text 01Document8 pagesFull Text 01Zunaira AmberNo ratings yet
- Creutzfeldt JakobDocument15 pagesCreutzfeldt JakobNicolas RodriguezNo ratings yet
- Neurological Complications in Psittacosis: A Case Report and Literature ReviewDocument2 pagesNeurological Complications in Psittacosis: A Case Report and Literature ReviewMíguel Sastre LázaroNo ratings yet
- Apathy and Hypersomnia Are Common Features of Myotonic DystrophyDocument7 pagesApathy and Hypersomnia Are Common Features of Myotonic DystrophyAnnisa HusainNo ratings yet
- Caso de EncefalitisDocument4 pagesCaso de EncefalitisJonathan CuevaNo ratings yet
- Canine DistemperDocument16 pagesCanine DistemperFelipe GonzalezNo ratings yet
- Macrophage Activation Syndrome Secondary To Mixed Connective Tissue Disease: A Rare Case in A Tertiary Care Hospital of Eastern IndiaDocument5 pagesMacrophage Activation Syndrome Secondary To Mixed Connective Tissue Disease: A Rare Case in A Tertiary Care Hospital of Eastern IndiaInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- References: Meningoencephalitis As A Presentation Form of Behc EtDocument2 pagesReferences: Meningoencephalitis As A Presentation Form of Behc EtAndys Mayest BuntanusNo ratings yet
- Https:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Document3 pagesHttps:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Lucas XavierNo ratings yet
- Proof of Progression Over Time Finally Fulminant Brain Muscle An 2009 SeiDocument3 pagesProof of Progression Over Time Finally Fulminant Brain Muscle An 2009 Seibilal hadiNo ratings yet
- Acute Disseminated Encephalomyelitis in Chicken Pox: Case ReportDocument2 pagesAcute Disseminated Encephalomyelitis in Chicken Pox: Case ReportCorneliu VladNo ratings yet
- Jvim 15681Document39 pagesJvim 15681daniruizcasNo ratings yet
- Encefalitis Autoinmune 2011 PDFDocument11 pagesEncefalitis Autoinmune 2011 PDFDiana Vanessa SuarezNo ratings yet
- Brain Lesions, Obesity, and Other Disturbances in Mice Treated With Monosodium Glutamate (Olney 1969)Document3 pagesBrain Lesions, Obesity, and Other Disturbances in Mice Treated With Monosodium Glutamate (Olney 1969)Aprilia OanimaNo ratings yet
- A Case of Sporadic Creutzfeldt-JakobDocument5 pagesA Case of Sporadic Creutzfeldt-JakobVlad PopescuNo ratings yet
- 2017 32 3 264 267 EngDocument4 pages2017 32 3 264 267 Engemmanuellagrace06No ratings yet
- Case Report A Chinese Patient With Spinocerebellar Ataxia Finally Confirmed As Gerstmann-Sträussler-Scheinker Syndrome With P102L MutationDocument16 pagesCase Report A Chinese Patient With Spinocerebellar Ataxia Finally Confirmed As Gerstmann-Sträussler-Scheinker Syndrome With P102L MutationLídia YoshiharaNo ratings yet
- Case Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiaDocument4 pagesCase Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiahypnonautNo ratings yet
- Frontotemporal Dementia NeuropathologyDocument14 pagesFrontotemporal Dementia Neuropathologyppico1963No ratings yet
- BH 010060 ENGDocument2 pagesBH 010060 ENGCarlosNo ratings yet
- Seizure: European Journal of EpilepsyDocument10 pagesSeizure: European Journal of EpilepsyDanilo AssisNo ratings yet
- Encefalitis LimbicaDocument11 pagesEncefalitis LimbicaRandy UlloaNo ratings yet
- Anti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaDocument6 pagesAnti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaAbhishek KayalNo ratings yet
- Cerebral PyogranulomaDocument4 pagesCerebral PyogranulomamaiaraNo ratings yet
- Trabalho para A Semana de 03 de MarcoDocument5 pagesTrabalho para A Semana de 03 de MarcoTiago FernandesNo ratings yet
- GeneticsDocument10 pagesGeneticsapi-3835615No ratings yet
- Clinically Inapparent Gallbladder Cancer Presenting With Paraneoplastic Cranial Neuropathies Aunali S Khaku, MD Lisa Aenlle MD, Irene A Malaty, MD University of Florida Department of NeurologyDocument1 pageClinically Inapparent Gallbladder Cancer Presenting With Paraneoplastic Cranial Neuropathies Aunali S Khaku, MD Lisa Aenlle MD, Irene A Malaty, MD University of Florida Department of Neurologyask1288No ratings yet
- High Sensitivity of Asymmetric F THK5351 PET Abnormality in Patients With Corticobasal SyndromeDocument9 pagesHigh Sensitivity of Asymmetric F THK5351 PET Abnormality in Patients With Corticobasal SyndromeBrent AllieNo ratings yet
- Pisa Syndrome Source Clin Neuropharmacol SO 2015 Jul Aug 38 4 135 40 (PMIDT26166239)Document6 pagesPisa Syndrome Source Clin Neuropharmacol SO 2015 Jul Aug 38 4 135 40 (PMIDT26166239)dai shujuanNo ratings yet
- April 2019 1554202000 8152018Document2 pagesApril 2019 1554202000 8152018KantiNareshNo ratings yet
- Rewiew-Clinical Features and Prognosis of CanineDocument5 pagesRewiew-Clinical Features and Prognosis of CanineJuliana Ramos PereiraNo ratings yet
- Rett Syndrome: A Case ReportDocument6 pagesRett Syndrome: A Case ReportIJAR JOURNALNo ratings yet
- Algoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Document7 pagesAlgoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Farid Santiago Abedrabbo LombeydaNo ratings yet
- Guillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportDocument5 pagesGuillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportAsep MetrikaNo ratings yet
- ArticolDocument3 pagesArticolDiana DîrzuNo ratings yet
- 07 11 2017 - 14 10vet2450pvb 5339.R1LD PDFDocument10 pages07 11 2017 - 14 10vet2450pvb 5339.R1LD PDFfarah rachmahNo ratings yet
- A Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientDocument3 pagesA Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientInternational Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- Epileptic Encephalopathies SYNGAP1Document7 pagesEpileptic Encephalopathies SYNGAP1Saya OtonashiNo ratings yet
- Neuro 1Document5 pagesNeuro 1Ivan RisnaNo ratings yet
- Chapter 5 SummaryDocument10 pagesChapter 5 SummarykuzmanovicstanaNo ratings yet
- Networks in Temporal Lobe Epilepsy: Karina A. González Otárula,, Stephan SchueleDocument9 pagesNetworks in Temporal Lobe Epilepsy: Karina A. González Otárula,, Stephan SchueleDonald CabreraNo ratings yet
- Shaw Et Al. 2008Document9 pagesShaw Et Al. 2008Rodolfo Esteban Muñoz GómezNo ratings yet
- Chapter 3 Biological Foundations of BehaviourDocument20 pagesChapter 3 Biological Foundations of BehaviourAlex LiNo ratings yet
- CT Brain Part II-AnatomyDocument65 pagesCT Brain Part II-AnatomyXhenni XhenniNo ratings yet
- Gelastic and Cursive Seizures - Neurology - 1973Document11 pagesGelastic and Cursive Seizures - Neurology - 1973---No ratings yet
- Soal MCQ Blok 16Document14 pagesSoal MCQ Blok 16Mira Pandora100% (1)
- Mareschal Et Alii, 2007, Chapter 5 Sirois Et Alii, 2008, P. 325, and Figure 1 Below, Section IIIDocument15 pagesMareschal Et Alii, 2007, Chapter 5 Sirois Et Alii, 2008, P. 325, and Figure 1 Below, Section IIIHenrique Augusto Torres SimplícioNo ratings yet
- Obesity Related Cognitive Impairment The Role of Endo - 2019 - Neurobiology ofDocument18 pagesObesity Related Cognitive Impairment The Role of Endo - 2019 - Neurobiology ofSabrina YenNo ratings yet
- Sts Cheat Sheet of The BrainDocument30 pagesSts Cheat Sheet of The BrainRahula RakeshNo ratings yet
- The Cognitive Neuroscience of AutismDocument7 pagesThe Cognitive Neuroscience of AutismPatricia Roxana NeamțuNo ratings yet
- A System That Controls All of The Activities of The BodyDocument49 pagesA System That Controls All of The Activities of The BodynelsonNo ratings yet
- Online - OpenAccess - 978 3 7376 5093 9.OpenAccessDocument493 pagesOnline - OpenAccess - 978 3 7376 5093 9.OpenAccessPedro RodriguesNo ratings yet
- Week4-1 Brain DevelopmentDocument43 pagesWeek4-1 Brain DevelopmentJayden TseNo ratings yet
- Fundamental of EEG MeasurementDocument12 pagesFundamental of EEG MeasurementRyna Aulia Falamy100% (1)
- Fardo, Et Al., A Mechanism For Spatial Perception On Human Skin (Cognition 178), 2018Document8 pagesFardo, Et Al., A Mechanism For Spatial Perception On Human Skin (Cognition 178), 2018Franco ZárateNo ratings yet
- (ANA) 5.06 Organization of The Nervous SystemDocument19 pages(ANA) 5.06 Organization of The Nervous SystemAdrian MaterumNo ratings yet
- Outline of Functional Organization of Nervous SystemDocument6 pagesOutline of Functional Organization of Nervous SystemKarmina SantosNo ratings yet
- Jneurosci 5316-03 2004Document7 pagesJneurosci 5316-03 2004labsoneducationNo ratings yet
- Nervous System-Anatomy & PhysiologyDocument64 pagesNervous System-Anatomy & PhysiologyDeshi SportsNo ratings yet
- The Structure and Function of The BrainDocument25 pagesThe Structure and Function of The BrainNo NameNo ratings yet
- BIOL 4380 Emotion BIOL 4380 Emotion: Systems of Neuroscience (York University) Systems of Neuroscience (York University)Document10 pagesBIOL 4380 Emotion BIOL 4380 Emotion: Systems of Neuroscience (York University) Systems of Neuroscience (York University)Jiaying ChenNo ratings yet
- ขขอสอบ CU-TEP Reading Comprehension อสอบ CU-TEP Reading ComprehensionDocument18 pagesขขอสอบ CU-TEP Reading Comprehension อสอบ CU-TEP Reading Comprehensiontest testNo ratings yet
- Prelim Module in General PsychologyDocument9 pagesPrelim Module in General PsychologyAllona Zyra CambroneroNo ratings yet
- The Sleep-Deprived Human BrainDocument15 pagesThe Sleep-Deprived Human BrainGustavo CamiloNo ratings yet
- Anatomy and PhysiologyDocument10 pagesAnatomy and PhysiologyChris CHris ChRis100% (1)
- Histo Module 3Document67 pagesHisto Module 3Kathleen RodasNo ratings yet