Professional Documents
Culture Documents
APTT (PTT) Kit With Normal Control Package Insert
APTT (PTT) Kit With Normal Control Package Insert
Uploaded by
bassam alharazi0 ratings0% found this document useful (0 votes)
56 views2 pagesThis document provides instructions for performing an activated partial thromboplastin time (PTT) assay using a liquid PTT reagent. Key details include:
- The PTT assay measures the intrinsic coagulation pathway and is used to monitor heparin therapy and detect coagulation factor deficiencies.
- The procedure involves mixing citrated plasma with PTT reagent and incubating, then adding calcium chloride to initiate clotting. The clotting time is the PTT.
- Storage conditions for the PTT reagent are 2-8°C for up to 30 days. Quality control testing with normal and abnormal controls is recommended daily.
Original Description:
Original Title
APTT (PTT) Kit with Normal Control Package Insert
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides instructions for performing an activated partial thromboplastin time (PTT) assay using a liquid PTT reagent. Key details include:
- The PTT assay measures the intrinsic coagulation pathway and is used to monitor heparin therapy and detect coagulation factor deficiencies.
- The procedure involves mixing citrated plasma with PTT reagent and incubating, then adding calcium chloride to initiate clotting. The clotting time is the PTT.
- Storage conditions for the PTT reagent are 2-8°C for up to 30 days. Quality control testing with normal and abnormal controls is recommended daily.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
56 views2 pagesAPTT (PTT) Kit With Normal Control Package Insert
APTT (PTT) Kit With Normal Control Package Insert
Uploaded by
bassam alharaziThis document provides instructions for performing an activated partial thromboplastin time (PTT) assay using a liquid PTT reagent. Key details include:
- The PTT assay measures the intrinsic coagulation pathway and is used to monitor heparin therapy and detect coagulation factor deficiencies.
- The procedure involves mixing citrated plasma with PTT reagent and incubating, then adding calcium chloride to initiate clotting. The clotting time is the PTT.
- Storage conditions for the PTT reagent are 2-8°C for up to 30 days. Quality control testing with normal and abnormal controls is recommended daily.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
an anticoagulant, nine parts of freshly collected whole PTT Reagent can be stored at 2-8°C for 30 days
blood should be immediately added to one part of after opening.
anticoagulant. Centrifuge the whole blood specimen at PTT Reagent should not be frozen and thawed.
ACTIVATED PARTIAL THROMBOPLASTIN TIME (PTT) 2500xg for 15 minutes. Separate the plasma using a Do not mix or use the components of this kit with
(LIQUID REAGENT) plastic pipette and place it in a plastic test tube. the components of any other kit with different lot
For In-Vitro diagnostic and professional use only Perform the activated partial thromboplastin time assay numbers.
within 4 hours. Throughout testing all test tubes, syringes and
Store at: 2-8°C 2. Add 1ml distilled water to vail mix well and allow it to pipettes should be plastic.
stand for 15 minutes at ambient temperature to ensure Throughout testing all test tubes, incubation time
APPLICATION it dissolved completely. should keep in constant and incubation
Activated Partial Thromboplastin Time (PTT) is commonly 3. Allow the PTT reagent to reach room temperature temperature should keep at 36.5-37.5°C.
used for pre-surgical screening for intrinsic factor deficiency, before use. Each laboratory should establish a Quality Control
monitoring heparin therapy, in the detection of Lupus A. Manual method program that includes both normal and abnormal
Anticoagulants, and quantitative determination of the 1. Bring all reagents, controls and sample to room control plasmas to evaluate instrument, reagent
Factor VIII, IX, XI, and XII relevant with the intrinsic temperature 15 minutes prior to testing. tested daily prior to performing tests on patient
coagulation system. 2. Pipette 50 µl of PTT reagent to each tube. plasmas. Monthly quality control charts are
3. Pipette 50 µl of sample, controls to the tubes prepared recommended to determine the mean and
PRINCIPLE in step 2. standard deviation of each of the daily control
In the test, citrated test plasma is mixed with PTT reagent 4. Incubate for 3 minutes at 37°C. plasma. All assays should include controls, and if
for a specified period of time. The time required for 5. Add 25µl of CaCl2 solution to each tube, start the stop any of the controls are outside the established
clotting formation is the activated partial thromboplastin watch, mix in a water bath (37°C) for 20 seconds, then reference ranges, then the assay should be
time (PTT). The degree of prolongation is proportional to record the time required for clot formation. considered invalid and no patient results should
the severity of single factor deficiency, or in a cumulative B. Automate Method be reported.
deficiency of all the factors involved. To perform this test, refer to the appropriate Turbid solution of CaCl2 may be indicative of
Instrument Operator’s Manual for detailed product deterioration.
MATERIALS instructions.
MATERIALS PROVIDED: NORMAL REFERENCE/CONTROL HUMAN PLASMA
PTT Liquid Reagent. REFERENCE VALUES: The PT, PTT, TT , and FIB assay procedure are routinely
Plasma Normal Control. Normal control sample(s): 26-36 seconds; used to identify and quantitate deficiencies in clotting
MATERIALS REQUIRED BUT NOT PROVIDED: For best results, each laboratory should determine a mechanism as well as to monitor anticoagulant therapy.
CaCl2 (25mmol) reference range for its particular population and instrument All human blood donations used in the preparation of our
reagent system. products have been tested individually and found to be
Specimen collection Storage and Stability: negative for HbsAg, HCV and HIV antibody. However, we
1. Plasma obtained from whole blood samples that had recommend that all material of human origin should be
been collected in a tube with 0.109M sodium citrate as STORAGE AND STABILITY considered as potentially hazardous and be handled with
appropriate care. Stability after reconstitution:
INTENDED USE At 2oC-8oC4 hours
NCP use in factor II, V, VII, VIII, IX, and it is used as a control ATLAS MEDICAL
of precision and accuracy for PT, PTT, TT , FIB. REFERANCE RANGE William James House, Cowley Road,
Product Reference Value Cambridge, CB4 0WX
REAGENTS PT liquid 11-14 sec Tel: ++44 (0) 1223 858 910
PTT liquid 20-40 sec
NCP (Lyophilized product) Fax: ++44 (0) 1223 858 524
Fib liquid 3.03 g/L
TT liquid 10-14 sec PPI699A01
PREPARATION AND TEST PROCEDURE PT lyophilized 11-14 sec Rev D (26.10.2015)
1. Gently add 1ml distilled water to each vail of Fib lyophilized 3.03g/L
TT lyophilized 10-14 sec
thrombin, mix well and allow it to stand for 15 Catalogue
Store at
NOTE Number
minutes at ambient temperature to ensure it For In-Vitro
Each laboratory should determine a reference range for its Caution
influence completeness. Diagnostic use
particular population and instrument reagent system. Number of tests in Read product insert
2. According to the different examination the pack before use
experiment, other steps same with the samples Lot (batch)
REFERENCES: Manufacturer
number
which to testing.
1. Basu, D; Gallus.; Hirsh, j. N. Eng. J. Med. 287: Fragile, handle
Expiry date
with care
324,1972
ATTENTION Manufacturer fax Do not use if
2. Young, D.; Pestaner, L.; Gibberman, V. Clin. Chen. number package is damaged
1. For in vitro diagnostic use only, avoid taking in.
21: 355 D, 1975 Manufacturer
when operation should defer to the patient
3. Quick A. J., The PARTIAL THROMBOPLASTIN TIME telephone number
plasma treatment, keep one's eyes peeled.
in Hemophilia and in Obstructive
2. Each experiment best determines two NCP every
Jaundice.J.Biol.Chem,:109,73-74:1935
day, and establishment anticipated QC range for
4. Biggs R.ed, Human Blood Coagulation Hemostasis
own instrument-reagent system.
and Thrombosis Second Ed.Blackwell Scientific
3. The plasma root in Human's matter, crossed the
Publications, London 1976.
HTLV-III/HIV, HBsAg and the HCV testing, only
5. Peterson C.E., K waan H.C., Current Conce PT s of
donations with negative findings are used for
Warfarin Therapy, Arch Intern. Med.
manufacture. Nevertheless, since absence of
146:581-584,1986
infections agents cannot be proven, all materials
obtained from human blood should always be
handled with due care, observing the precautions
recommended for biohazardous material.
STORAGE AND STABILITY
Storage at 2oC-8oC, NCP can be used labeled expiry date.
You might also like
- Insert - Elecsys T3.ms - 11731360122.v26.enDocument4 pagesInsert - Elecsys T3.ms - 11731360122.v26.enGuneyden Guneyden100% (3)
- Panthromtin SLDocument6 pagesPanthromtin SLVanina LuzNo ratings yet
- The Digital SelfDocument24 pagesThe Digital SelfKyla Torrado86% (7)
- 04 FS Package InsertDocument6 pages04 FS Package InsertImas NurhayatiNo ratings yet
- Counselling Skills For NursesDocument129 pagesCounselling Skills For Nursesadyna26100% (1)
- Guru Vandana or गुरु मन्त्रDocument6 pagesGuru Vandana or गुरु मन्त्रveeram100% (3)
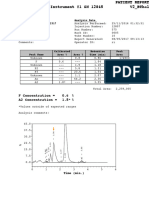
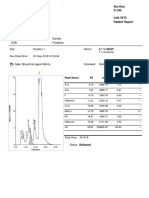
- ApttDocument2 pagesApttNonameNo ratings yet
- PT Calcium Package InsertDocument2 pagesPT Calcium Package InsertManikanta Sai KumarNo ratings yet
- Thrombin TimeDocument2 pagesThrombin Timesofia anneNo ratings yet
- Lquiplastine - PTDocument2 pagesLquiplastine - PTm sdNo ratings yet
- 01 Control N-1Document1 page01 Control N-1Imas NurhayatiNo ratings yet
- Tecopts Rev8Document1 pageTecopts Rev8satujuli23No ratings yet
- Thrombin Time ReagentDocument14 pagesThrombin Time Reagentyousra zeidanNo ratings yet
- (Prothrombin Time) : Intended UseDocument2 pages(Prothrombin Time) : Intended UseDharmesh PatelNo ratings yet
- L Low Abnormal Control (Cat. No. 0020003205) : 10 Vials PrecisionDocument3 pagesL Low Abnormal Control (Cat. No. 0020003205) : 10 Vials PrecisionAlfonso KSNo ratings yet
- Biolabo PTDocument2 pagesBiolabo PTSendy Fadilla OktoraNo ratings yet
- Bbcois02 2012 PTDocument2 pagesBbcois02 2012 PTlucia acasieteNo ratings yet
- Tpla2: Sample Volumes Sample Sample DilutionDocument3 pagesTpla2: Sample Volumes Sample Sample DilutionhairiNo ratings yet
- ProthrombinDocument3 pagesProthrombinD. latumNo ratings yet
- HEMOCLOT Thrombin Time (T.T.) : Kit For The Thrombin Time Determination On PlasmaDocument2 pagesHEMOCLOT Thrombin Time (T.T.) : Kit For The Thrombin Time Determination On PlasmaTanveerNo ratings yet
- Anti-Thyroid Peroxidase Antibody (Anti-TPO) CLIA: 2 X 50 Test 52025070Document2 pagesAnti-Thyroid Peroxidase Antibody (Anti-TPO) CLIA: 2 X 50 Test 52025070p11.sethiaNo ratings yet
- NOTE: When Testing Thrombin Time (TT) Assay (5 ML 3.0Document3 pagesNOTE: When Testing Thrombin Time (TT) Assay (5 ML 3.0Alfonso KSNo ratings yet
- PI e TLN 8Document1 pagePI e TLN 8TRITEST TRITESTNo ratings yet
- PI e TLN 9Document1 pagePI e TLN 9Hadi BitarNo ratings yet
- ft4 III 2020-04 v3Document4 pagesft4 III 2020-04 v3Ismael CulquiNo ratings yet
- Hemostat Thromboplastin: Determination of Prothrombin Time (PT)Document2 pagesHemostat Thromboplastin: Determination of Prothrombin Time (PT)luisoft88100% (1)
- A02a00002aen Yumizen G PT LiqDocument2 pagesA02a00002aen Yumizen G PT LiqpapyNo ratings yet
- A TpoDocument4 pagesA TpoJimboreanu György PaulaNo ratings yet
- Co PtsiDocument1 pageCo Ptsifarouktaher592No ratings yet
- Ft4 Ii: Free ThyroxineDocument4 pagesFt4 Ii: Free ThyroxinehairiNo ratings yet
- Tugas Pak AzharDocument9 pagesTugas Pak Azhardedi gunawanNo ratings yet
- Quality Control in Coagulation TestingDocument5 pagesQuality Control in Coagulation TestingAmine CHAHIDNo ratings yet
- Elecsys FT4 II: 06437281 190 Cobas e 411 Cobas e 601 Cobas e 602 English System InformationDocument4 pagesElecsys FT4 II: 06437281 190 Cobas e 411 Cobas e 601 Cobas e 602 English System InformationAli KING FREDDYNo ratings yet
- Hemostat Thromboplastin-SI: Determination of Prothrombin Time (PT)Document2 pagesHemostat Thromboplastin-SI: Determination of Prothrombin Time (PT)Lemi MaluluNo ratings yet
- Prothrombin Time SOPDocument3 pagesProthrombin Time SOPGamal Eldien FathiNo ratings yet
- Ctni Rapid Quantitative Test: Limitations of ProcedureDocument2 pagesCtni Rapid Quantitative Test: Limitations of ProcedureTrần Thanh Viện100% (1)
- Normal Control 1 - 0020013900: ENGLISH - Insert Revision 04/2018Document3 pagesNormal Control 1 - 0020013900: ENGLISH - Insert Revision 04/2018Brady AndersonNo ratings yet
- A02a00004aen Yumizen G APTT PDFDocument2 pagesA02a00004aen Yumizen G APTT PDFNAKANWAGI JOSLYLINENo ratings yet
- Routine Laboratory Evaluation of CoagulationDocument32 pagesRoutine Laboratory Evaluation of CoagulationArshie08No ratings yet
- LKSGDTT11-I TPHA Rev.7Document1 pageLKSGDTT11-I TPHA Rev.7Dau TranvanNo ratings yet
- mp0050 MKDocument6 pagesmp0050 MKRayane Teles de FreitasNo ratings yet
- Ifu - 771100 771101 en - 2017 12 19Document1 pageIfu - 771100 771101 en - 2017 12 19Khaled AlkhawaldehNo ratings yet
- APTT MispaClogDocument1 pageAPTT MispaClogDharmesh PatelNo ratings yet
- anti-TPO (aTPO) : Assay For The Detection of Autoantibodies Against Thyroid PeroxidaseDocument10 pagesanti-TPO (aTPO) : Assay For The Detection of Autoantibodies Against Thyroid PeroxidaseGuneyden GuneydenNo ratings yet
- Insert PREA 0108252645190c503 V4 enDocument3 pagesInsert PREA 0108252645190c503 V4 enVegha NedyaNo ratings yet
- Hema 2 Lab: Coagulation Tests: PT and ApttDocument5 pagesHema 2 Lab: Coagulation Tests: PT and ApttGerly MaglangitNo ratings yet
- Elecsys BRAHMS PCT: ProcalcitoninDocument5 pagesElecsys BRAHMS PCT: ProcalcitoninDóra BenczeNo ratings yet
- IFU Dia TT EN 20170915Document2 pagesIFU Dia TT EN 20170915P managerNo ratings yet
- Used For The Quantitative Determination: PT TestDocument2 pagesUsed For The Quantitative Determination: PT TestApril Lady Faith P. PaundogNo ratings yet
- ACTHDocument6 pagesACTHşaban günerNo ratings yet
- Fibrinogen 506 EngDocument6 pagesFibrinogen 506 Engالواثقة باللهNo ratings yet
- INS CR EN CRP Rev.22 - 181109Document3 pagesINS CR EN CRP Rev.22 - 181109Ahmed Ben NjahNo ratings yet
- AAT2 enDocument3 pagesAAT2 enSyahdie FahledieNo ratings yet
- Lksgdtt11-I Tpha 01-2008Document1 pageLksgdtt11-I Tpha 01-2008Maguiliwè BELEINo ratings yet
- All - Bc-Ret Control - en - V2Document1 pageAll - Bc-Ret Control - en - V2Huy Trung GiápNo ratings yet
- Prothrombin Time (aPTT) Mr. Khaled AlzatariDocument21 pagesProthrombin Time (aPTT) Mr. Khaled AlzatariKhaled ZatariNo ratings yet
- (Provided in Separate Box) : (Toll Free)Document2 pages(Provided in Separate Box) : (Toll Free)Dave LuceroNo ratings yet
- At 13704Document2 pagesAt 13704dr_joe23No ratings yet
- Allplex 2019-Ncov Assay: Intended UseDocument3 pagesAllplex 2019-Ncov Assay: Intended UseAsiyahNo ratings yet
- Activated Partial Thromboplastin Time (aPTT) : Activity #10Document14 pagesActivated Partial Thromboplastin Time (aPTT) : Activity #10Kei Ef SiNo ratings yet
- Liquicellin E: For APTT DeterminationDocument2 pagesLiquicellin E: For APTT Determinationm sdNo ratings yet
- 5 Top Soft Skills of Exceptional Laboratory LeadersDocument3 pages5 Top Soft Skills of Exceptional Laboratory Leadersbassam alharaziNo ratings yet
- Pienn01 Needles r00Document6 pagesPienn01 Needles r00bassam alharaziNo ratings yet
- Poster 19 Steps of Venous Blood Collection A1 en Rev00 1120Document1 pagePoster 19 Steps of Venous Blood Collection A1 en Rev00 1120bassam alharaziNo ratings yet
- Developing The Next Generation of Laboratory LeadersDocument4 pagesDeveloping The Next Generation of Laboratory Leadersbassam alharaziNo ratings yet
- A AN SOP 040302 214 Rev01Document4 pagesA AN SOP 040302 214 Rev01bassam alharaziNo ratings yet
- Collection of Swabs For CultureDocument4 pagesCollection of Swabs For Culturebassam alharaziNo ratings yet
- Lab Safety Rules and GuidelinesDocument11 pagesLab Safety Rules and Guidelinesbassam alharaziNo ratings yet
- Therapeutic DrugsDocument2 pagesTherapeutic Drugsbassam alharaziNo ratings yet
- Sartorius Infographic Finalized-1Document1 pageSartorius Infographic Finalized-1bassam alharaziNo ratings yet
- Tibc Arc ChemDocument8 pagesTibc Arc Chembassam alharaziNo ratings yet
- Three Keys To Making Quick DecisionsDocument2 pagesThree Keys To Making Quick Decisionsbassam alharaziNo ratings yet
- 7 Steps To Succession Planning in Clinical LabsDocument4 pages7 Steps To Succession Planning in Clinical Labsbassam alharaziNo ratings yet
- Employee Engagement - The Role of The Laboratory LeaderDocument6 pagesEmployee Engagement - The Role of The Laboratory Leaderbassam alharaziNo ratings yet
- 10 Types of Cognitive Biases That Can Impact Decision-Making in The LabDocument5 pages10 Types of Cognitive Biases That Can Impact Decision-Making in The Labbassam alharaziNo ratings yet
- Iron Arc ChemDocument8 pagesIron Arc Chembassam alharaziNo ratings yet
- Igg Arc ChemDocument8 pagesIgg Arc Chembassam alharaziNo ratings yet
- How To Transition From Technical Lead To Senior Laboratory LeaderDocument3 pagesHow To Transition From Technical Lead To Senior Laboratory Leaderbassam alharaziNo ratings yet
- Test Information FIBRODocument8 pagesTest Information FIBRObassam alharaziNo ratings yet
- Igm Arc ChemDocument8 pagesIgm Arc Chembassam alharaziNo ratings yet
- Vitamin B12 Deficiency EvaluationDocument1 pageVitamin B12 Deficiency Evaluationbassam alharaziNo ratings yet
- Haptoglobin ARC CHEMDocument8 pagesHaptoglobin ARC CHEMbassam alharaziNo ratings yet
- BQ Ps 37hba1c-Dir en WebDocument2 pagesBQ Ps 37hba1c-Dir en Webbassam alharaziNo ratings yet
- Activated Protein C Resistance V Factor V LiedinDocument59 pagesActivated Protein C Resistance V Factor V Liedinbassam alharaziNo ratings yet
- Laboratory Records VARIANT II Beta Thal Short Program Le Lamentin 1 54Document3 pagesLaboratory Records VARIANT II Beta Thal Short Program Le Lamentin 1 54bassam alharaziNo ratings yet
- Educational References VARIANT II Beta Thal Short Program J Iran 1 16Document4 pagesEducational References VARIANT II Beta Thal Short Program J Iran 1 16bassam alharaziNo ratings yet
- SPEG DFT Handbook v1.0Document25 pagesSPEG DFT Handbook v1.0bassam alharaziNo ratings yet
- Diabetes Disease Modified DrugsDocument103 pagesDiabetes Disease Modified Drugsbassam alharaziNo ratings yet
- Library of Variants New D 100 HbA1c Sinai Baltimore 0 24Document3 pagesLibrary of Variants New D 100 HbA1c Sinai Baltimore 0 24bassam alharaziNo ratings yet
- Inflammatory Markersv1Document1 pageInflammatory Markersv1bassam alharaziNo ratings yet
- American J Hematol - 2019 - BainDocument1 pageAmerican J Hematol - 2019 - Bainbassam alharaziNo ratings yet
- Hoki 2019 PDFDocument95 pagesHoki 2019 PDFPutriNo ratings yet
- The Effect of Digitalization On Business Performance An Applied StudyDocument8 pagesThe Effect of Digitalization On Business Performance An Applied StudyFrank MendozaNo ratings yet
- MIND Guide To YogaDocument9 pagesMIND Guide To YogarajendraNo ratings yet
- Differences Between British and American EnglishDocument6 pagesDifferences Between British and American EnglishDana Barwal100% (1)
- Social Innovation Project: Science, Technology and SocietyDocument3 pagesSocial Innovation Project: Science, Technology and SocietyRobert GarlandNo ratings yet
- Flyer ESIM Concept 171017Document8 pagesFlyer ESIM Concept 171017Anonymous dqOOagF8LNo ratings yet
- A Study On The Challenges HR Managers Face TodayDocument10 pagesA Study On The Challenges HR Managers Face TodayJAY MARK TANONo ratings yet
- Digest Manila Insurance v. Sps AmuraoDocument3 pagesDigest Manila Insurance v. Sps AmuraoYsa UbanaNo ratings yet
- Seminar On Contemparary Issues in ManagementDocument4 pagesSeminar On Contemparary Issues in ManagementNaimish BodarNo ratings yet
- Revision For The First Semester Exam Unit 4-5-6 (Lop 12)Document35 pagesRevision For The First Semester Exam Unit 4-5-6 (Lop 12)Bao TranNo ratings yet
- Labor Law Review Case Digest Duka - Book I To Book IIDocument24 pagesLabor Law Review Case Digest Duka - Book I To Book IINiñoMaurinNo ratings yet
- In The Name of DemocracyDocument3 pagesIn The Name of DemocracycitizenpakistanNo ratings yet
- BLEACH Charac Part 1.Document53 pagesBLEACH Charac Part 1.Al LanNo ratings yet
- JFL Volume7 Issue28 Pages121-134Document15 pagesJFL Volume7 Issue28 Pages121-134Karina WedhantiNo ratings yet
- Harris1976 PDFDocument7 pagesHarris1976 PDFRoger RamosNo ratings yet
- Airconditioning DayaDocument2 pagesAirconditioning DayaMike AdvinculaNo ratings yet
- Rules Relating To Service of SummonDocument5 pagesRules Relating To Service of Summonamlan DeyNo ratings yet
- Test Your Memory, Test Your Listening: Friends EveryoneDocument1 pageTest Your Memory, Test Your Listening: Friends EveryoneYahya Al-AmeriNo ratings yet
- Early Adolescence (Ages 10 To 13) : Females MalesDocument5 pagesEarly Adolescence (Ages 10 To 13) : Females MalesBea Valerie GrislerNo ratings yet
- Malcolm X Papers SchomburgDocument49 pagesMalcolm X Papers Schomburgdrferg50% (2)
- Teaching The Writing Process As A First and SecondDocument7 pagesTeaching The Writing Process As A First and Secondchitra selviNo ratings yet
- WC Level 11 Review Cevaz: 1. Complete The Sentences With The Correct Form of The Verb To Make Second Conditional ClausesDocument5 pagesWC Level 11 Review Cevaz: 1. Complete The Sentences With The Correct Form of The Verb To Make Second Conditional ClausesJose RoncayoloNo ratings yet
- Presentation Title: Debugging Simulation ModelsDocument74 pagesPresentation Title: Debugging Simulation ModelsflyingdreamsNo ratings yet
- Movie Review: - Efforts By-Riya Maan - Rishabh Saxena - 12 CDocument11 pagesMovie Review: - Efforts By-Riya Maan - Rishabh Saxena - 12 CRishabh SaxenaNo ratings yet
- Doctor and Other Healthcare Professional 1Document34 pagesDoctor and Other Healthcare Professional 1Pavan chowdaryNo ratings yet
- Ebook DevOps ToolchainDocument11 pagesEbook DevOps ToolchainKalanidhiNo ratings yet
- Foreign Court and JudgementDocument9 pagesForeign Court and JudgementLavanya NairNo ratings yet