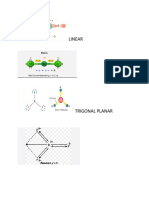
Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
7 viewsHTML
HTML
Uploaded by
brajanosmaniChemical formulae use symbols to represent the chemical elements and subscripts to indicate the number of atoms in a chemical compound or molecule. The simplest types are empirical formulae which show the numerical proportions of each type of atom, while molecular formulae indicate the actual numbers of each atom in a molecule without structural information. Examples of different types of chemical formulae are given, including ones showing isotopes using superscript prefixes to denote specific radioactive or stable isotopes.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- HTML For Beginers PDFDocument3 pagesHTML For Beginers PDFMd.Muzibur RahmanNo ratings yet
- FormulasDocument2 pagesFormulashaticesila204No ratings yet
- College Bca HTML Practical FileDocument13 pagesCollege Bca HTML Practical FileMalathi SankarNo ratings yet
- HTML CodesDocument3 pagesHTML CodesShaakir AbdirihmanNo ratings yet
- Practising HTML Tag1Document15 pagesPractising HTML Tag1Raj saranyaNo ratings yet
- Code 1Document10 pagesCode 1Vasu SharmaNo ratings yet
- Formulae Indicate The Simple Numbers of Each Type of Atom in A Molecule, With No Information OnDocument1 pageFormulae Indicate The Simple Numbers of Each Type of Atom in A Molecule, With No Information OnVinod NairNo ratings yet
- Module in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentDocument33 pagesModule in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentJohn Mark JuarezNo ratings yet
- Notes On HTML 51.docxclass 7Document7 pagesNotes On HTML 51.docxclass 7Madhu PratapNo ratings yet
- NewDocument1 pageNewchiraghimranNo ratings yet
- Web Authoring (HTML)Document64 pagesWeb Authoring (HTML)Rochana RamanayakaNo ratings yet
- HTML ExampleDocument33 pagesHTML ExampleCross SignalNo ratings yet
- HTML SDocument2 pagesHTML Slc2023asnNo ratings yet
- Smart Technologies: Ammber NosheenDocument71 pagesSmart Technologies: Ammber NosheenSyed ArslanNo ratings yet
- What Is HTMLDocument22 pagesWhat Is HTML98149No ratings yet
- Htmlprograms1 12Document26 pagesHtmlprograms1 12ananthakrishnanNo ratings yet
- Example: Deccansoft Software Services HTML TagsDocument7 pagesExample: Deccansoft Software Services HTML TagsJaswanth GoudNo ratings yet
- HTML Cheat SheetDocument7 pagesHTML Cheat Sheetsam debNo ratings yet
- HTML TagsDocument6 pagesHTML TagsEliza Joy GasesNo ratings yet
- Ravi WordpadDocument27 pagesRavi Wordpadravibca 2024No ratings yet
- HTML Elements: Unit-4Document13 pagesHTML Elements: Unit-4Awash Zilong SharmaNo ratings yet
- Java Script Form For New Student AdmissionDocument2 pagesJava Script Form For New Student Admissionrohitt69No ratings yet
- Can You Recall What Attributes Are?Document52 pagesCan You Recall What Attributes Are?Sheila Bliss J. Goc-ongNo ratings yet
- HTML CodesDocument4 pagesHTML Codesravina10008No ratings yet
- 10 Ca Lab WorkDocument28 pages10 Ca Lab Workluckykumarsingh726No ratings yet
- An Introduction To HTMLDocument118 pagesAn Introduction To HTMLarjitcseNo ratings yet
- HTMLtugasDocument29 pagesHTMLtugasRoy SihombingNo ratings yet
- Practical 3Document1 pagePractical 3Devil man StarNo ratings yet
- It ProgramsDocument40 pagesIt ProgramsIsha Katiyar100% (1)
- A Computer ProjectDocument31 pagesA Computer Projectsanjaijsu09No ratings yet
- Class 6 HTMLnotesDocument4 pagesClass 6 HTMLnotesALI ONENo ratings yet
- Wa0005Document7 pagesWa0005Queen AnnaNo ratings yet
- Font Size "6"Document7 pagesFont Size "6"Queen AnnaNo ratings yet
- HTML Basics PracticalsDocument18 pagesHTML Basics PracticalsNoman QadeerNo ratings yet
- HTMLDocument7 pagesHTMLanon_6694774No ratings yet
- HTML5 NotesDocument39 pagesHTML5 NotesTanusha handeNo ratings yet
- HTMLDocument44 pagesHTMLManzu PokharelNo ratings yet
- FormDocument6 pagesFormdeafsisirNo ratings yet
- Web Authoring (HTML)Document64 pagesWeb Authoring (HTML)Rochana RamanayakaNo ratings yet
- HTMLDocument26 pagesHTMLgdineshcareNo ratings yet
- In Order To Format An HTML DocumentDocument16 pagesIn Order To Format An HTML DocumentSanthosh KumarNo ratings yet
- HTML BasicDocument8 pagesHTML Basicanon_116484471No ratings yet
- Subscript HTMLDocument5 pagesSubscript HTMLshlok patelNo ratings yet
- Det 2Document1 pageDet 2klaudia takuNo ratings yet
- Common Lab Manual PrintDocument136 pagesCommon Lab Manual PrintSanthosh SiyanshNo ratings yet
- Html&java Script ProgramsDocument19 pagesHtml&java Script Programssreeroyal986No ratings yet
- Lab 1 Chapter 2&3: Sử dụng Front-Page để thực hiện bài thực hành nàyDocument6 pagesLab 1 Chapter 2&3: Sử dụng Front-Page để thực hiện bài thực hành nàylehaphuong03No ratings yet
- HTML and Some ScriptingDocument42 pagesHTML and Some ScriptingrichtomNo ratings yet
- HTML File by ShivamDocument24 pagesHTML File by ShivamJatin GulsatyaNo ratings yet
- HTML TagsDocument10 pagesHTML TagsMark PanganibanNo ratings yet
- Praktikum 01. Pengenalan HTMLDocument7 pagesPraktikum 01. Pengenalan HTMLHeng GiNo ratings yet
- That PecticleDocument30 pagesThat Pecticlesanjaijsu09No ratings yet
- HTML Practical File - Chapter 8 Short Answer Type QuestionsDocument16 pagesHTML Practical File - Chapter 8 Short Answer Type QuestionsVikas ShuklaNo ratings yet
- 20BEC111 WT Practical4Document9 pages20BEC111 WT Practical4Dhruv PrajapatiNo ratings yet
- Learning HTMLDocument38 pagesLearning HTMLIX-B-MAYANK YADAV (5466)100% (1)
- Hyper Text Markup LanguageDocument34 pagesHyper Text Markup LanguageRishabh JainNo ratings yet
- M2 Part2Document19 pagesM2 Part2AmanNo ratings yet
- MeerktsDocument1 pageMeerktsbrajanosmaniNo ratings yet
- Academic DiscussionsDocument1 pageAcademic DiscussionsbrajanosmaniNo ratings yet
- Types of NumbersDocument11 pagesTypes of NumbersbrajanosmaniNo ratings yet
- I Agree With The Second Statement Saying That Some People Think Access To So Much Information Creates ProblemsDocument2 pagesI Agree With The Second Statement Saying That Some People Think Access To So Much Information Creates ProblemsbrajanosmaniNo ratings yet
- Classification of Living OrganismsDocument23 pagesClassification of Living OrganismsbrajanosmaniNo ratings yet
- Where Are Alkynes Found in The WorldDocument2 pagesWhere Are Alkynes Found in The WorldbrajanosmaniNo ratings yet
- Diseases Caused by PathogensDocument2 pagesDiseases Caused by PathogensbrajanosmaniNo ratings yet
- Now Days Parents Are Being More OpenDocument1 pageNow Days Parents Are Being More OpenbrajanosmaniNo ratings yet
- Thermoelectric TorchDocument1 pageThermoelectric TorchbrajanosmaniNo ratings yet
- CèilidhDocument1 pageCèilidhbrajanosmaniNo ratings yet
- Scottish Wedding TraditionsDocument6 pagesScottish Wedding TraditionsbrajanosmaniNo ratings yet
- Projekt BiologjiDocument3 pagesProjekt BiologjibrajanosmaniNo ratings yet
- EnglishDocument4 pagesEnglishbrajanosmaniNo ratings yet
- The Professor Made Several Points Regarding The Notion That RDocument1 pageThe Professor Made Several Points Regarding The Notion That RbrajanosmaniNo ratings yet
- While The Passage States That The Pollution Theory Is Seemed As The More Likely Cause of Sea Otters DeclineDocument1 pageWhile The Passage States That The Pollution Theory Is Seemed As The More Likely Cause of Sea Otters DeclinebrajanosmaniNo ratings yet
- Shape of Molecules PolariyyDocument4 pagesShape of Molecules PolariyybrajanosmaniNo ratings yet
HTML
HTML
Uploaded by
brajanosmani0 ratings0% found this document useful (0 votes)
7 views2 pagesChemical formulae use symbols to represent the chemical elements and subscripts to indicate the number of atoms in a chemical compound or molecule. The simplest types are empirical formulae which show the numerical proportions of each type of atom, while molecular formulae indicate the actual numbers of each atom in a molecule without structural information. Examples of different types of chemical formulae are given, including ones showing isotopes using superscript prefixes to denote specific radioactive or stable isotopes.
Original Description:
Original Title
html
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentChemical formulae use symbols to represent the chemical elements and subscripts to indicate the number of atoms in a chemical compound or molecule. The simplest types are empirical formulae which show the numerical proportions of each type of atom, while molecular formulae indicate the actual numbers of each atom in a molecule without structural information. Examples of different types of chemical formulae are given, including ones showing isotopes using superscript prefixes to denote specific radioactive or stable isotopes.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
7 views2 pagesHTML
HTML
Uploaded by
brajanosmaniChemical formulae use symbols to represent the chemical elements and subscripts to indicate the number of atoms in a chemical compound or molecule. The simplest types are empirical formulae which show the numerical proportions of each type of atom, while molecular formulae indicate the actual numbers of each atom in a molecule without structural information. Examples of different types of chemical formulae are given, including ones showing isotopes using superscript prefixes to denote specific radioactive or stable isotopes.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
<html>
<title> Exercise </title>
<body>
<p style="color:green; font-size:30px"> Chemical formulae </p>
<p> A <u> chemical formulae</u> is a way of presenting <i> information </i> about the <mark> chemical properties </mark>
of atoms that constitute a particular <b> chemical compound </b> or molecule, <i> using chemical element symbols</i>,
numbers, and sometimes also other <b>symbols</b>, such as
<br>
parantheses, <mark> dashes </mark>, brackets, commas and <del>plus </del> (+) and <del> minus </del> (-) signs. </p>
<br>
<p style="color:green"> The simplest types of chemical formulae are called empirical formulae, which use letters and
numbers indicating the numerical proportions of atoms of each type. Molecular formulae indicate the simplest numbers of each
type of atom in a molecule,
<br>
with no information on structure, For example, the empirical formulae for glucose is CH<sub>2</sub>O (twice as many
hydrogens atoms as carbon and oxygen), while in molecular formula is C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>(12
hydrogen atoms, six carbon and oxygen atoms). </p>
<br>
<br>
<p style="color:orange; font-size:30px"> Examples </p>
<br>
<br>
<p> CH<sub>3 </sub>CH<sub>2 </sub>CH<sub>2 </sub>CH<sub>2</sub>CH<sub>2 </sub>CH<sub>3 </sub> </p>
<br>
<br>
<p> H <sub>3</sub>O<sup>+</sup> or SO<sub>4</sub> <sup> 2- </sup> </p>
<br>
<br>
<p style="color:orange; font-size:30px"> Isotopes </p>
<br>
<br>
<p> Although <mark> isotopes </mark> are more relevant to <b> nuclear chemistry</mark> or <u>stable isotopes
chemistry </u> than to conventional chemistry, different isotopes may be indicated with a prefixed superscript in a chemical
formulae. For example, the phosphate ion
<br>
containing radioactive phosphorus -32 is [<sup>32</sup>PO<sub>4</sub>4]<sup>3-</sup>. Also a study involving
stable isotope ratios might include the molecule <sup>18</sup>O<sup>16</sup> <sup>O. </p>
</body>
</html>
You might also like
- HTML For Beginers PDFDocument3 pagesHTML For Beginers PDFMd.Muzibur RahmanNo ratings yet
- FormulasDocument2 pagesFormulashaticesila204No ratings yet
- College Bca HTML Practical FileDocument13 pagesCollege Bca HTML Practical FileMalathi SankarNo ratings yet
- HTML CodesDocument3 pagesHTML CodesShaakir AbdirihmanNo ratings yet
- Practising HTML Tag1Document15 pagesPractising HTML Tag1Raj saranyaNo ratings yet
- Code 1Document10 pagesCode 1Vasu SharmaNo ratings yet
- Formulae Indicate The Simple Numbers of Each Type of Atom in A Molecule, With No Information OnDocument1 pageFormulae Indicate The Simple Numbers of Each Type of Atom in A Molecule, With No Information OnVinod NairNo ratings yet
- Module in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentDocument33 pagesModule in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentJohn Mark JuarezNo ratings yet
- Notes On HTML 51.docxclass 7Document7 pagesNotes On HTML 51.docxclass 7Madhu PratapNo ratings yet
- NewDocument1 pageNewchiraghimranNo ratings yet
- Web Authoring (HTML)Document64 pagesWeb Authoring (HTML)Rochana RamanayakaNo ratings yet
- HTML ExampleDocument33 pagesHTML ExampleCross SignalNo ratings yet
- HTML SDocument2 pagesHTML Slc2023asnNo ratings yet
- Smart Technologies: Ammber NosheenDocument71 pagesSmart Technologies: Ammber NosheenSyed ArslanNo ratings yet
- What Is HTMLDocument22 pagesWhat Is HTML98149No ratings yet
- Htmlprograms1 12Document26 pagesHtmlprograms1 12ananthakrishnanNo ratings yet
- Example: Deccansoft Software Services HTML TagsDocument7 pagesExample: Deccansoft Software Services HTML TagsJaswanth GoudNo ratings yet
- HTML Cheat SheetDocument7 pagesHTML Cheat Sheetsam debNo ratings yet
- HTML TagsDocument6 pagesHTML TagsEliza Joy GasesNo ratings yet
- Ravi WordpadDocument27 pagesRavi Wordpadravibca 2024No ratings yet
- HTML Elements: Unit-4Document13 pagesHTML Elements: Unit-4Awash Zilong SharmaNo ratings yet
- Java Script Form For New Student AdmissionDocument2 pagesJava Script Form For New Student Admissionrohitt69No ratings yet
- Can You Recall What Attributes Are?Document52 pagesCan You Recall What Attributes Are?Sheila Bliss J. Goc-ongNo ratings yet
- HTML CodesDocument4 pagesHTML Codesravina10008No ratings yet
- 10 Ca Lab WorkDocument28 pages10 Ca Lab Workluckykumarsingh726No ratings yet
- An Introduction To HTMLDocument118 pagesAn Introduction To HTMLarjitcseNo ratings yet
- HTMLtugasDocument29 pagesHTMLtugasRoy SihombingNo ratings yet
- Practical 3Document1 pagePractical 3Devil man StarNo ratings yet
- It ProgramsDocument40 pagesIt ProgramsIsha Katiyar100% (1)
- A Computer ProjectDocument31 pagesA Computer Projectsanjaijsu09No ratings yet
- Class 6 HTMLnotesDocument4 pagesClass 6 HTMLnotesALI ONENo ratings yet
- Wa0005Document7 pagesWa0005Queen AnnaNo ratings yet
- Font Size "6"Document7 pagesFont Size "6"Queen AnnaNo ratings yet
- HTML Basics PracticalsDocument18 pagesHTML Basics PracticalsNoman QadeerNo ratings yet
- HTMLDocument7 pagesHTMLanon_6694774No ratings yet
- HTML5 NotesDocument39 pagesHTML5 NotesTanusha handeNo ratings yet
- HTMLDocument44 pagesHTMLManzu PokharelNo ratings yet
- FormDocument6 pagesFormdeafsisirNo ratings yet
- Web Authoring (HTML)Document64 pagesWeb Authoring (HTML)Rochana RamanayakaNo ratings yet
- HTMLDocument26 pagesHTMLgdineshcareNo ratings yet
- In Order To Format An HTML DocumentDocument16 pagesIn Order To Format An HTML DocumentSanthosh KumarNo ratings yet
- HTML BasicDocument8 pagesHTML Basicanon_116484471No ratings yet
- Subscript HTMLDocument5 pagesSubscript HTMLshlok patelNo ratings yet
- Det 2Document1 pageDet 2klaudia takuNo ratings yet
- Common Lab Manual PrintDocument136 pagesCommon Lab Manual PrintSanthosh SiyanshNo ratings yet
- Html&java Script ProgramsDocument19 pagesHtml&java Script Programssreeroyal986No ratings yet
- Lab 1 Chapter 2&3: Sử dụng Front-Page để thực hiện bài thực hành nàyDocument6 pagesLab 1 Chapter 2&3: Sử dụng Front-Page để thực hiện bài thực hành nàylehaphuong03No ratings yet
- HTML and Some ScriptingDocument42 pagesHTML and Some ScriptingrichtomNo ratings yet
- HTML File by ShivamDocument24 pagesHTML File by ShivamJatin GulsatyaNo ratings yet
- HTML TagsDocument10 pagesHTML TagsMark PanganibanNo ratings yet
- Praktikum 01. Pengenalan HTMLDocument7 pagesPraktikum 01. Pengenalan HTMLHeng GiNo ratings yet
- That PecticleDocument30 pagesThat Pecticlesanjaijsu09No ratings yet
- HTML Practical File - Chapter 8 Short Answer Type QuestionsDocument16 pagesHTML Practical File - Chapter 8 Short Answer Type QuestionsVikas ShuklaNo ratings yet
- 20BEC111 WT Practical4Document9 pages20BEC111 WT Practical4Dhruv PrajapatiNo ratings yet
- Learning HTMLDocument38 pagesLearning HTMLIX-B-MAYANK YADAV (5466)100% (1)
- Hyper Text Markup LanguageDocument34 pagesHyper Text Markup LanguageRishabh JainNo ratings yet
- M2 Part2Document19 pagesM2 Part2AmanNo ratings yet
- MeerktsDocument1 pageMeerktsbrajanosmaniNo ratings yet
- Academic DiscussionsDocument1 pageAcademic DiscussionsbrajanosmaniNo ratings yet
- Types of NumbersDocument11 pagesTypes of NumbersbrajanosmaniNo ratings yet
- I Agree With The Second Statement Saying That Some People Think Access To So Much Information Creates ProblemsDocument2 pagesI Agree With The Second Statement Saying That Some People Think Access To So Much Information Creates ProblemsbrajanosmaniNo ratings yet
- Classification of Living OrganismsDocument23 pagesClassification of Living OrganismsbrajanosmaniNo ratings yet
- Where Are Alkynes Found in The WorldDocument2 pagesWhere Are Alkynes Found in The WorldbrajanosmaniNo ratings yet
- Diseases Caused by PathogensDocument2 pagesDiseases Caused by PathogensbrajanosmaniNo ratings yet
- Now Days Parents Are Being More OpenDocument1 pageNow Days Parents Are Being More OpenbrajanosmaniNo ratings yet
- Thermoelectric TorchDocument1 pageThermoelectric TorchbrajanosmaniNo ratings yet
- CèilidhDocument1 pageCèilidhbrajanosmaniNo ratings yet
- Scottish Wedding TraditionsDocument6 pagesScottish Wedding TraditionsbrajanosmaniNo ratings yet
- Projekt BiologjiDocument3 pagesProjekt BiologjibrajanosmaniNo ratings yet
- EnglishDocument4 pagesEnglishbrajanosmaniNo ratings yet
- The Professor Made Several Points Regarding The Notion That RDocument1 pageThe Professor Made Several Points Regarding The Notion That RbrajanosmaniNo ratings yet
- While The Passage States That The Pollution Theory Is Seemed As The More Likely Cause of Sea Otters DeclineDocument1 pageWhile The Passage States That The Pollution Theory Is Seemed As The More Likely Cause of Sea Otters DeclinebrajanosmaniNo ratings yet
- Shape of Molecules PolariyyDocument4 pagesShape of Molecules PolariyybrajanosmaniNo ratings yet