Professional Documents
Culture Documents
ALKYNE
ALKYNE
Uploaded by
Princess Mae EstabilloOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ALKYNE
ALKYNE
Uploaded by
Princess Mae EstabilloCopyright:
Available Formats
Page|1
Republic of the Philippines
ISABELA STATE UNIVERSITY
Jones, Isabela
COLLEGE OF CRIMINAL JUSTICE
EDUCATION
Second Semester, School Year 2021-2022
ORGANIC CHEMISTRY
I. TITLE OF THE MODULE
Chapter Four (4): ALKYNE
II. INTRODUCTION
Alkynes are hydrocarbons that contain carbon-carbon triple bonds. Many of the
reactions of alkynes are similar to the corresponding reactions of alkenes because both
involve πbonds between two carbon atoms. Like the π-bond of an alkene, the π-bonds of
an alkyne are also electron rich, and readily undergo addition reactions.
III. TABLE OF CONTENT
i. Unsaturated Hydrocarbons: Alkyne
ii. IUPAC Nomenclature of Alkynes
iii. Physical Properties of Alkynes
iv. Chemical Properties of Alkynes
v. Alkyne Synthesis
vi. Alkyne Preparation
vii. Additional Reactions of Alkynes
viii. Natural Occurring Alkynes
IV. LEARNING OUTCOME
At the end of this module, the students are expected to:
Name alkynes using IUPAC (systematic) and selected common name nomenclature;
Draw the structure of alkynes from IUPAC (systematic) and selected common names;
Distinguish the difference of saturated and unsaturated hydrocarbons; and
Distinguish reactions of alkenes and alkynes and their properties.
V. LEARNING CONTENT
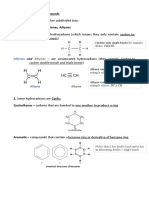
i. UNSATURATED HYDROCARBONS: ALKYNE
Alkynes also called acetylenes represent the second class of unsaturated
hydrocarbons. An alkyne is an acyclic unsaturated hydrocarbon that contains one or more
carbon–carbon triple bonds. The alkyne functional group is, thus, a C≡C group. As the
family name alkyne indicates, the characteristic “ending” associated with a triple bond is -
yne.
The general formula for an alkyne with one triple bond is CnH2n+2. Thus the
simplest member of this type of alkyne has the formula C2H2, and the next member, with
n = 3, has the formula C3H4.
The presence of a carbon–carbon triple bond in a molecule always results in a linear
arrangement for the two atoms attached to the carbons of the triple bond. Thus, ethyne is a
linear molecule.
The simplest alkyne, ethyne (C2H2), is the most important alkyne from an
industrial standpoint. A colorless gas, it goes by the common name acetylene and is used
in oxy- acetylene torches, high-temperature torches used for cutting and welding material
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
Page|2
ii. IUPAC NOMENCLATURE OF ALKYNES
The rules for naming alkynes are identical to those used to name alkenes, except the
ending -yne is used instead of -ene.
Find the longest carbon chain that includes both carbons of the triple bond.
Number the longest chain starting at the end closest to the triple bond.
After numbering the longest chain with the lowest number assigned to the
alkyne, label each of the substituents at its corresponding carbon. While writing
out the name of the molecule, arrange the substituents in alphabetical order.
4-chloro-6,6-diiodo-7-methyl-2-nonyne
If there are more than one of the same substituent use the prefixes di, tri, and
tetra for two, three, and four substituents respectively.
When there are two triple bonds in the molecule, find the longest carbon chain
including both the triple bonds. Number the longest chain starting at the end
closest to the triple bond that appears first. The suffix that would be used to
name this molecule would be –diyne. For example:
1,5-octadiyne
Substituents containing a triple bond are called alkynyl.
4-bromo-1-chloro-1-ethynylcyclohexane
A molecule that contains both double and triple bonds is called an alkenyne.
The chain can be numbered starting with the end closest to the functional
group that appears first. For example:
6-ethyl-3-methylnon-4-en-1-yne
iii. PHYSICAL PROPERTIES OF ALKYNES
The physical properties of alkynes pretty much follow the same pattern of those
of alkanes and alkenes.
Alkynes are unsaturated carbon that shares a triple bond at the carbon site
All alkynes are odorless and colorless with the exception of ethylene, which has
a slight distinctive odor.
The first three alkynes are gases, and the next eight are liquids. All alkynes
higher than these eleven are solids
Alkynes are slightly polar in nature
The boiling point and melting point of alkynes increases as their molecular
structure grows bigger. The boiling point increases with increase in their
molecular mass. Also, the boiling points of alkynes are slightly higher than those
of their corresponding alkenes, due to the one extra bond at the carbon site.
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
Page|3
iv. CHEMICAL PROPERTIES OF ALKYNES
The triple-bond functional group of alkynes behaves chemically quite
similarly to the double-bond functional group of alkenes. Thus there are many
parallels between alkene chemistry and alkyne chemistry. The same substances that
add to double bonds (H2, HCl, Cl2, and so on) also add to triple bonds. However,
two molecules of a specific reactant can add to a triple bond, as contrasted to the
addition of one molecule of reactant to a double bond. In triple-bond addition, the
first molecule converts the triple bond into a double bond, and the second molecule
then converts the double bond into a single bond. For example, propyne reacts with
H2 to form propene first and then to form propane.
Alkynes, like alkenes and alkanes, are flammable, that is, they readily
undergo combustion reactions.
v. ALKYNE SYNTHESIS
CRACKING
- It is the process of breaking down heavier hydrocarbon molecule into a
lighter molecule through the introduction of heat and sometimes
catalysts.
vi. ALKYNE PREPARATION
The preparations of alkynes are very similar to those of the alkenes. The
main preparative reactions involve the elimination of groups or ions from
molecules, resulting in the formation of π bonds.
Dehydrohalogenation. The loss of a hydrogen atom and a halogen atom
from adjacent alkane carbon atoms leads to the formation of an alkene. The loss of
additional hydrogen and halogen atoms from the double‐bonded carbon atoms leads
to alkyne formation. The halogen atoms may be located on the same carbon
(a geminal dihalide) or on adjacent carbons (a vicinal dihalide).
Substitution. Larger alkynes can be generated by reacting an alkyl halide
with an acetylide ion, which is generated from a shorter alkyne.
Because acetylide ions are bases, elimination reactions can occur, leading
to the formation of an alkene from the alkyl halide. Because substitution and
elimination reactions proceed through the formation of a common intermediate,
these two types of reactions always occur simultaneously.
Ethyne (acetylene) preparation. Ethyne, which is commonly called
acetylene, is the simplest alkyne. Historically, it was prepared by reacting
calcium carbide with water.
Today, ethyne is normally prepared by the pyrolysis of methane. In this
procedure, a stream of methane gas is briefly heated to 1500°C in an airless
chamber. Air must be excluded from the reaction or oxidation (combustion) will
occur.
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
Page|4
vii. ADDITIONAL REACTIONS
The principal reaction of the alkynes is addition across the triple bond to form
alkanes. These addition reactions are analogous to those of the alkenes.
Hydrogenation. Alkynes undergo catalytic hydrogenation with the same catalysts
used in alkene hydrogenation: platinum, palladium, nickel, and rhodium.
Hydrogenation proceeds in a stepwise fashion, forming an alkene first, which
undergoes further hydrogenation to an alkane.
This reaction proceeds so smoothly that it is difficult, if not impossible, to stop the
reaction at the alkene stage, although by using palladium or nickel for the catalyst, the
reaction can be used to isolate some alkenes. Greater yields of alkenes are possible with
the use of poisoned catalysts. One such catalyst, the Lindlar catalyst, is composed of
finely divided palladium coated with quinoline and absorbed on calcium carbonate.
This treatment makes the palladium less receptive to hydrogen, so fewer hydrogen
atoms are available to react. When a catalyst is deactivated in such a manner, it is
referred to as being poisoned.
The mechanism of alkyne hydrogenation is identical to that of the alkenes. Because
the hydrogen is absorbed on the catalyst surface, it is supplied to the triple bond in a
syn manner.
Alkynes can also be hydrogenated with sodium in liquid ammonia at low
temperatures. This reaction is a chemical reduction rather than a catalytic reaction, so
the hydrogen atoms are not attached to a surface, and they may approach an alkene
from different directions, leading to the formation of trans alkenes.
Halogenation. The addition of halogens to an alkyne proceeds in the same manner
as halogen addition to alkenes. The halogen atoms add to an alkyne molecule in a
stepwise fashion, leading to the formation of the corresponding alkene, which
undergoes further reaction to a tetrahaloalkane.
Unlike most hydrogenation reactions, it is possible to stop this reaction at the alkene
stage by running it at temperatures slightly below 0°C.
Hydrohalogenation. Hydrogen halides react with alkynes in the same manner as
they do with alkenes.
Both steps in the above addition follow the Markovnikov rule. Thus, the addition
of hydrogen bromide to 1‐butyne gives 2‐bromo‐1‐butene as the major product of thefirst
step.
The reaction of 2‐bromo‐1‐butene in the second step gives 2,2‐dibromobutane asthe
major product.
Hydration. The addition of the elements of water across the triple bond of an
alkyne leads to the formation of aldehydes and ketones. Water addition to terminal
alkynes leads to the generation of aldehydes, while nonterminal alkynes and water
generate ketones.
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
Page|5
These products are produced by rearrangement of an unstable enol (vinyl alcohol)
intermediate. The term “enol” comes from the en in “alkene” and ol in “alcohol,”
reflecting that one of the carbon atoms in vinyl alcohol has both a double bond (alkene)
and an OH group (alcohol) attached to it. A vinyl group is
and a vinyl alcohol is
Water adds across the triple bond of an alkyne via a carbocation mechanism. Dilute
mineral acid and mercury(II) ions are needed for the reaction to occur.
Oxidation. Alkynes are oxidized by the same reagents that oxidize alkenes.
Disubstituted alkynes react with potassium permanganate to yield vicinal diketones or,
under more vigorous conditions, carboxylic acids.
Polymerization. Alkynes can be polymerized by both cationic and free‐radical
methods. The reactions and mechanisms are identical with those of the alkenes.
viii. NATURAL OCCURRING ALKYNES
Alkynes are organic or carbon-based compounds that contain a triple bond
between two carbon atoms. This triple bond ensures these compounds have different
structural and chemical properties from compounds with double-bonded carbons,
which are called alkenes, or alkanes that have single-bonded carbons only. Although
alkynes are uncommon in nature, one of them is important in industry and some
naturally occurring compounds do contain triple-bonded carbons.
Ethyne aka Acetylene
Ethyne is the simplest of the alkynes; its molecular formula is C2H2, and it consists
of two carbon atoms triple-bonded to each other with a hydrogen atom bonded to each
of the carbons. At room temperature it's a colorless gas. The short, electron-rich triple
bond has a high energy content and thus burning ethyne releases a lot of energy,
which makes it a popular fuel in welding torches and other similar applications. Most
welders refer to it by its common name, acetylene. In the early 20th century it was
also an important starting material for synthesis of industrial chemicals, although its
popularity waned as oil-based starting materials took its place.
Ethinyl Estradiol
Ethinyl Estradiol is a synthetic compound similar in structure to the naturally
occurring compound estradiol, which is an important estrogen hormone in the female
body. Like estradiol, it acts as an estrogen but it's extraordinarily potent and long
lasting -- and that's why it's administered as an active ingredient in oral
contraceptives. Introducing this compound into the body changes the body's hormone
balance so that the ovaries do not ovulate and the lining of the womb becomes thinner.
Poisonous Alkynes
Some organisms produce poisonous alkyne compounds to help them deal with
predators or prey. One example is ichthyothereol, a highly toxic alkyne found in the
leaves of a small herb named Ichthyothere terminalis in Brazil. Indians living in the
region once used this poison to kill fish. Another example is histrioconicotoxin, an
alkyne compound found in the skin of the poison arrow frog from the same region.
As the name suggests, its historical use was as a coating for arrowheads.
Medical Alkynes
A few compounds of medical interest aside from ethinyl estradiol also contain alkyne
units. One example is the compounds calicheamicin and esperamicin. Both are found
in nature and have similar structures. They are extremely toxic to cells and work by
breaking double-stranded DNA. Unfortunately, they are so toxic it's not possible to
use them untethered as cancer drugs because they're too good at killing healthy cells.
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
Page|6
Some researchers, however, have sought to attach calicheamicin to antibodies that
specifically target cancer cells so the poison will be delivered to the cancer cells only.
One such drug called gemtuzumab ozogamicin was approved by FDA for treatment
of acute myelogenous leukemia in 2000, but was withdrawn from the U.S. market in
2010.
VI. ASSESSMENTS
1. What is the general molecular formula for an alkyne in which two carbon–carbon triple
bonds are present?
2. What is the general molecular formula for a cycloalkyne in which one carbon–carbon
triple bond is present?
3. Assign an IUPAC name to each of the following unsaturated hydrocarbons.
4. Contrast alkynes and alkenes in terms of general physical properties.
5. Contrast alkynes and alkenes in terms of general chemical properties.
6. Draw skeletal structural formulas and give the IUPAC names for the three possible
alkyne isomers with the molecular formula C5H8.
VII. RESOURCES
Sachkheim, George I. and Lehman Denis D. Chemistry for the Health Sciences. 8th
Edition. Prentice-Hall, Inc.
Stoker, Stephen H. General, Organic, and Biological Chemistry. 5th Edition.
Brooks//Cole, Cenage Learning. 2010
Holm, Johanna Forgotten Chemistry. Barron’s Educational Series, Inc. 2006
Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th
Edition. New York: W. H. Freeman & Company, 2007.
https://sciencing.com/methanol-isopropyl-alcohol-same-thing-5652093.html
General Chemistry (Organic Chemistry) - Crim AdGE
Isabela State University – Jones Campus
You might also like
- A Requiem To Mother EarthDocument5 pagesA Requiem To Mother EarthSandra SabuNo ratings yet
- ALKENEDocument11 pagesALKENEPrincess Mae EstabilloNo ratings yet
- Bonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanDocument33 pagesBonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanIce BearNo ratings yet
- Chapter 1 Classes of Org. CpdsDocument100 pagesChapter 1 Classes of Org. CpdsezgmsayleakeNo ratings yet
- Alkenes AlkynesDocument40 pagesAlkenes AlkynesHanindya NugrahaNo ratings yet
- AlkynesDocument3 pagesAlkynesabdullahNo ratings yet
- AlkynesDocument5 pagesAlkynesJotillnaimNo ratings yet
- Gen Chem Organic Chemistry NotesDocument5 pagesGen Chem Organic Chemistry NotesVianneie Dominique BernadasNo ratings yet
- ChemistryDocument25 pagesChemistryMa. Angelica Claire LuayonNo ratings yet
- Organic Chemistry Ii (Och221T) : Chemical Engineering Class 2017BDocument31 pagesOrganic Chemistry Ii (Och221T) : Chemical Engineering Class 2017BSiphelele SimelaneNo ratings yet
- Organic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDocument43 pagesOrganic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDarius Gan100% (1)
- Chapter 3 Alkenes and Alkynes PowerpointDocument61 pagesChapter 3 Alkenes and Alkynes PowerpointFreya An YbanezNo ratings yet
- MODULE 4 Alkenes and AlkynesDocument19 pagesMODULE 4 Alkenes and AlkynesSittie Fahieda AloyodanNo ratings yet
- 3.3. Organic Chemistry I-1Document32 pages3.3. Organic Chemistry I-1DenisNo ratings yet
- Alkenes: Al-Farahidi University College of PharmacyDocument11 pagesAlkenes: Al-Farahidi University College of Pharmacyمؤمل كامل عبد العالي هريسNo ratings yet
- Al KynesDocument22 pagesAl KynesFikadu HabtuNo ratings yet
- AlkenesDocument5 pagesAlkenesSafa MarwanNo ratings yet
- AlkeneDocument26 pagesAlkeneOmar ElbhariyNo ratings yet
- Alkenes and AlkynesDocument24 pagesAlkenes and AlkynesRamil Jay IganaNo ratings yet
- Organic ChemistryDocument55 pagesOrganic ChemistryKim Taeha BTSNo ratings yet
- ORGANIC CHEMISTRY O LEVEL Hydrocarbons, Alcohols & Carboxylic AcidsDocument32 pagesORGANIC CHEMISTRY O LEVEL Hydrocarbons, Alcohols & Carboxylic AcidsCosmas DubeNo ratings yet
- Chemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsDocument2 pagesChemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsBenj Jamieson DuagNo ratings yet
- ALKENEDocument7 pagesALKENEKrystel Ann Demaosa CarballoNo ratings yet
- Activity 2 Nomenclature and StrucureDocument2 pagesActivity 2 Nomenclature and StrucureMANUEL, BUSTY P.No ratings yet
- Families of Organic CompoundsDocument8 pagesFamilies of Organic CompoundsJessa Mae LangcuyanNo ratings yet
- TdayandaDocument9 pagesTdayandach.konsam31No ratings yet
- AlkanesDocument8 pagesAlkanesBenjamen FolarinNo ratings yet
- Alkenes and AlkynesDocument27 pagesAlkenes and AlkynesS:M:ENo ratings yet
- CHEM1a EXPLORE NOTES WEEK 4Document5 pagesCHEM1a EXPLORE NOTES WEEK 4sjjsjsNo ratings yet
- Organic Chem U-2 Functional GroupDocument34 pagesOrganic Chem U-2 Functional Groupsinte beyuNo ratings yet
- Alkanes and AlkenesDocument30 pagesAlkanes and Alkenesrheanna.bartonNo ratings yet
- Alkanes, Alkenes, and AlkynesDocument2 pagesAlkanes, Alkenes, and AlkynesBacadon JerryNo ratings yet
- Organic ChemDocument18 pagesOrganic ChemKevinNo ratings yet
- Unsaturated Hydrocarbons: Suzette B DoctoleroDocument40 pagesUnsaturated Hydrocarbons: Suzette B DoctoleroJulius FrondaNo ratings yet
- Alkanes: Hybridisation of OrbitalsDocument11 pagesAlkanes: Hybridisation of OrbitalsIsaa gabNo ratings yet
- Alkenes and CycloalkenesDocument27 pagesAlkenes and CycloalkenesNicole ElnasNo ratings yet
- AlcoholDocument12 pagesAlcoholGNo ratings yet
- Alkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class ADocument14 pagesAlkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class AasaNo ratings yet
- AlkyneDocument30 pagesAlkyneKiela ArizobalNo ratings yet
- Exp.5-Reaction of Alkanes, Alkenes, and CycloalkanesDocument27 pagesExp.5-Reaction of Alkanes, Alkenes, and CycloalkaneszazoNo ratings yet
- Alkane Chemistry AssignmentDocument6 pagesAlkane Chemistry AssignmentNafees ImtiazNo ratings yet
- MIDTERMS CHEM - MazonDocument10 pagesMIDTERMS CHEM - MazonMazon, Dinah Melisse P.No ratings yet
- Alkanes 1-1Document22 pagesAlkanes 1-1Benjamen FolarinNo ratings yet
- Organic Chemistry - Organic Compounds Written ReportDocument16 pagesOrganic Chemistry - Organic Compounds Written ReportBiribiri Chikuchiku100% (1)
- Coek - Info AlkynesDocument12 pagesCoek - Info AlkynesDũng NgôNo ratings yet
- Organic Chemistry (Alkane, Alkene and Alkyne)Document7 pagesOrganic Chemistry (Alkane, Alkene and Alkyne)Dazell Varron100% (1)
- Alkane, Alkene, Alkyne PDFDocument17 pagesAlkane, Alkene, Alkyne PDFEra MelaniaNo ratings yet
- Al KynesDocument16 pagesAl KynesShivam GuptaNo ratings yet
- Chapter 6Document95 pagesChapter 6api-705775034No ratings yet
- Alkenes and Alkynes: Structure and Physical PropertiesDocument16 pagesAlkenes and Alkynes: Structure and Physical PropertiesSaloni JainNo ratings yet
- AlkenesDocument14 pagesAlkenesJotillnaimNo ratings yet
- Module 7 Aliphatic HydrocarbonsDocument16 pagesModule 7 Aliphatic HydrocarbonsDana MikaelaNo ratings yet
- Alcanos, Alquenos y AlquinosDocument52 pagesAlcanos, Alquenos y AlquinosagmirandaNo ratings yet
- CHEMISTRY G-10 UNIT 6Document20 pagesCHEMISTRY G-10 UNIT 6Yordanos SolomonNo ratings yet
- Alkenes IntroducingDocument20 pagesAlkenes Introducingmariaf.rojas26No ratings yet
- Alkenes and AlkynesDocument28 pagesAlkenes and AlkynesDorama AikaNo ratings yet
- Alkenes and AlkynesDocument39 pagesAlkenes and AlkynesMuhammed Emin KoçoğluNo ratings yet
- Alkanes (Paraffins)Document17 pagesAlkanes (Paraffins)Ahmed AmirNo ratings yet
- Chapter 11 - AlkynesDocument48 pagesChapter 11 - AlkynesTempuraNo ratings yet
- Practice Makes Perfect in Chemistry: Chemical Bonding with AnswersFrom EverandPractice Makes Perfect in Chemistry: Chemical Bonding with AnswersRating: 5 out of 5 stars5/5 (1)
- Alkyl HalideDocument8 pagesAlkyl HalidePrincess Mae EstabilloNo ratings yet
- ALKENEDocument11 pagesALKENEPrincess Mae EstabilloNo ratings yet
- Aromatic HydrocarbonsDocument7 pagesAromatic HydrocarbonsPrincess Mae EstabilloNo ratings yet
- Forensic ChemistryDocument6 pagesForensic ChemistryPrincess Mae EstabilloNo ratings yet
- Ethics PrelimDocument36 pagesEthics PrelimPrincess Mae EstabilloNo ratings yet
- Forensic Chemistry and ToxicologyDocument31 pagesForensic Chemistry and ToxicologyPrincess Mae EstabilloNo ratings yet
- 11 Chemistry Module 2Document19 pages11 Chemistry Module 2SpongeBob SquarePants Fidget ToysNo ratings yet
- HwyconstDocument23 pagesHwyconstAmulie JarjuseyNo ratings yet
- Flotrac Algorithm White PaperDocument4 pagesFlotrac Algorithm White PaperAnestesia 2017 UDECNo ratings yet
- Structural Engineering Professor Step III: Ucsd Academic Biography/Bibliography FormDocument30 pagesStructural Engineering Professor Step III: Ucsd Academic Biography/Bibliography FormCesar Paul Purihuaman MoraNo ratings yet
- Credit Card Fraud Detection Using Improved Deep Learning ModelsDocument22 pagesCredit Card Fraud Detection Using Improved Deep Learning ModelsrauhNo ratings yet
- Gear Trains: 8.1. Angular Velocity RatioDocument16 pagesGear Trains: 8.1. Angular Velocity RatioaddisudagneNo ratings yet
- Ag4q-212s KBDocument2 pagesAg4q-212s KBhtek.thunderainNo ratings yet
- Material Balance PDFDocument31 pagesMaterial Balance PDFApril Joy HaroNo ratings yet
- Scavenger Hunt 2019Document2 pagesScavenger Hunt 2019Rahul AdhikariNo ratings yet
- Problem: Determine The Total Volume of Earth To Be Excavated Up To Elevation 0Document17 pagesProblem: Determine The Total Volume of Earth To Be Excavated Up To Elevation 0gtech00100% (1)
- Visual Storytelling The Digital Video Documentary - Original PDFDocument44 pagesVisual Storytelling The Digital Video Documentary - Original PDFjparanoti100% (1)
- Masterglenium Ace: Solutions For The Pre-Cast IndustryDocument7 pagesMasterglenium Ace: Solutions For The Pre-Cast IndustryAlanNo ratings yet
- Mid Rex 7Document22 pagesMid Rex 7Sudeshna RoyNo ratings yet
- Chapter 20 Practice TestDocument19 pagesChapter 20 Practice TestCorei'Ana Conrad0% (1)
- Edible Oil - Case StudyDocument8 pagesEdible Oil - Case StudyVansh Raj GautamNo ratings yet
- Sewing Skills Checklist PDFDocument44 pagesSewing Skills Checklist PDFyemkem100% (1)
- TPS54160 1.5-A, 60-V, Step-Down DC/DC Converter With Eco-Mode™Document57 pagesTPS54160 1.5-A, 60-V, Step-Down DC/DC Converter With Eco-Mode™sbrhomeNo ratings yet
- Control of Spinal Anesthesia-Induced Hypotension in Adults - PMCDocument14 pagesControl of Spinal Anesthesia-Induced Hypotension in Adults - PMCAkash SharmaNo ratings yet
- Al55 66 Technical Manual v11 EngDocument60 pagesAl55 66 Technical Manual v11 EngProblem VelikiNo ratings yet
- FlapDocument100 pagesFlapRicha Agrawal100% (2)
- South Africa Hard Copy Lit 2Document20 pagesSouth Africa Hard Copy Lit 2Jennifer Española BernalNo ratings yet
- Final Firefly Reading & Writing AnswerDocument70 pagesFinal Firefly Reading & Writing AnswerVamshidhar ReddyNo ratings yet
- Kohima Nagaland LBDocument1 pageKohima Nagaland LBIndia TreadingNo ratings yet
- Gas Pressure Reducing: Gas Pressure Reducing & Shut-Off Valve & Shut-Off Valve Series 71P11A Series 71P11ADocument4 pagesGas Pressure Reducing: Gas Pressure Reducing & Shut-Off Valve & Shut-Off Valve Series 71P11A Series 71P11AĐình Sơn HoàngNo ratings yet
- TDS 0033 FlexoTop 202003Document3 pagesTDS 0033 FlexoTop 202003Mearg NgusseNo ratings yet
- X PPT CH 12 ElectricityDocument12 pagesX PPT CH 12 ElectricityAakriti100% (1)
- Psychoanalythic TheoryDocument1 pagePsychoanalythic TheorySilver BroochNo ratings yet
- Mec R2018Document227 pagesMec R2018Kishore Kumar RNo ratings yet
- Functional Specification For Deck CraneDocument31 pagesFunctional Specification For Deck Craneaiyubi20% (1)