Professional Documents
Culture Documents
DPP2 GOC Advan45637102346-20220803123932439228
DPP2 GOC Advan45637102346-20220803123932439228
Uploaded by
Drushya SalunkeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DPP2 GOC Advan45637102346-20220803123932439228
DPP2 GOC Advan45637102346-20220803123932439228
Uploaded by
Drushya SalunkeCopyright:
Available Formats
ORGANIC CHEMISTRY
(JEE-ADVANCED)
GENERAL ORGANIC CHEMISTRY (GOC) DPP-2
Single Correct :
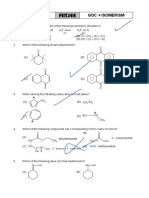
1. Which of the following is correct order of stability of given carbocations?
⊕
⊕ CH2 ⊕
(I) (II) (III) (IV)
⊕
O
(A) II > I > III > IV (B) II > IV > III > I (C) I > II > III > IV (D) I > IV > II > III
2. Which one is correct order for resonance energy?
(A) < (B) Θ> Θ
⊕ ⊕
(C) < (D) <
O
3. Statement-1: is less basic than
N N
H H
Statement-2: There are three lone pairs of electrons in second compound as compared to
only one in first compound.
(A) Statement-1 is true, statement-2 is true and statement-2 is correct explanation for
statement-1.
(B) Statement-1is true, statement-2 is true andstatement-2isNOTthe correct explanation for
statement-1.
(C) Statement-1 is true, statement-2 is false.
(D) Statement-1 is false, statement-2 is true.
N
4. (a) Et3N (b) N (c) N
Compare basic strength of following:
(A) a > b > c (B) b > a > c (C) b > c > a (D) c > b > a
Download ATP STAR APP Page No.1
And Practice For Free
5. Select the correct statement.
NH2 NH2
CH3
(A) is more basic than
COOH COOH
CH3
(B) is less acidic than
NMe2 NMe2
CH3
(C) is more basic than
NO2 NO2
CH3
(D) has shorter C–N bond length than
6. The correct order of acidity for the following compounds is
CO2H CO2H CO2H
CO2H OH
I. HO OH II. III. IV.
OH
OH
(A) I > II > III > IV (B) III > I > II > IV (C) III > IV > II > I (D) I > III > IV > II
7. The order of basicity among the following compounds is
NH NH2
I. H3C NH2 II. N NH III. HN N IV. H2N NH
(A) II > I > IV > III (B) IV > II > III > I (C) IV > I > II > III (D) I > IV > III > II
8. The correct order of acid strength of the following carboxylic acid is
O
O H OH
I. H II.
OH H
O OH
III. MeO IV. H3C
OH O
(A) III > II > I > IV (B) I > II > III > IV (C) I > III > II > IV (D) II > I > IV > III
One or more than one correct :
9. Which of the following is/are aromatic species.
(A) (B) (C) (D)
N ⊕
O
H
Download ATP STAR APP Page No.2
And Practice For Free
10. Which of the following represent correct order of acidic strength
CH3 Cl Br
(A) CH ≡ CH > CH2 = CH2 > CH3 – CH3 (B) OH > OH > OH
COOH COOH COOH
(C) > > (D) Me2CH–OH > Me3CH–OH > MeCH2–OH
COOH COOH COOH
11. With respect to the compounds I-V, choose the correct statement(s).
H
H
H
H-CH3 H H
I II III IV V
(A) The acidity of compound I is due to delocalization in the conjugate base.
(B) The conjugate base of compound IV is aromatic
(C) Compounds II becomes more acidic, when it has a-NO2 substituent.
(D) The acidity of compounds follows the order I > IV > V > II > III.
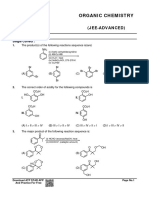
12. Consider the following four compounds I, II, III and IV.
H3C CH3
H3C CH3 NH2 N
NH2 N
O2N NO2 O2N NO2
NO2 NO2
I II III I
Choose the correct statement(s).
(A) The order of basicity is II > I > III > IV.
(B) The magnitude of pKb difference between I and II is more than that between III and IV.
(C) Resonance effect is more in III than in IV.
(D) Steric effect makes compound IV more basic than III.
13. Select the correct order among the following:
(A) Order of heat of hydrogenation < <
(B) Order of heat of combustion < <
(C) Order of resonance energy < <
NH NH NH
(D) Order of basic strength HN NH < NH <
Download ATP STAR APP Page No.3
And Practice For Free
Paragraph :
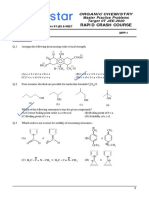
Comprehension # 1
(a)
OH
(x) (q)
H NH–Me
(w)
H(y) (b)
(p) H
H2N COOH
NH(r)
H2N NH2
(d)
H C NH2
(z) (s)
C
H
(c)
14. Which of the following is correct order of acidic strength.
(A) b > a > d > c (B) b > a > c > d (C) b > d > a > c (D) a > b > d > c
15. Which of the following is correct order of basic strength.
(A) q > s > p > r (B) r > s > q > p (C) r > q > s > p (D) q > r > s > p
Comprehension # 2
(a)
H2C–H3C CH3
(c) (e)
H NH–CH3
(f)
N
H (d)NH2 CH3
(b)
O–H C≡CH
(h) (g)
16. Which of the following is correct order of acidic strength.
(A) h > d > g (B) h > g > d (C) g > d > h (D) d > h > g
17. Which of the following is correct order of basic strength.
(A) f > e > d (B) d > f > e (C) e > f > d (D) f > d > e
Numerical value :
18. Among the following, the number of aromatic compound(s) is
⊕
Θ ⊕
⊕ Θ
Download ATP STAR APP Page No.4
And Practice For Free
19. Among the following compounds:
(i) (ii) C8H8−2 (iii) (iv)
N
(v) (vi) (vii) (viii)
N N N
N
OH
⊕
(ix) C3H3+1 (x) (xi)
N
(a) Number of compounds which are aromatic = w
(b) Number of compounds which are non-aromatic = x
(c) Number of compounds which are anti-aromatic = y
(d) Number of compounds which readily undergo Dimerization at room temperature= z.
What is the sum of w + x + y + z.
Match the Column
20. Match the column-I with column-II. Note that column-I may have more than one matching
options in column-II.
Column-I Column-II
(stability) (Reason)
H3C ⊕ CH3
C
+
(A) > CH3 (P) Inductive effect
CH3
– –
(B) H3C–C < CH3 (Q) Resonance
CH3
CH3
+ +
(C) H3C–C > H3C–CH2 (R) Hyperconjugation
CH3
H3C
CH2 CH2
(D) > CH3 (S) Steric hindrance
ANSWER KEY
1. (C) 2. (C) 3. (D) 4. (C) 5. (C)
6. (A) 7. (C) 8. (A) 9. (ABC) 10. (AC)
11. (ABC) 12. (CD) 13. (BCD) 14. (B) 15. (C)
16. (B) 17. (A) 18. 5 19. 13
20. (A) → P, Q, R; (B) → P; (C) → P, R; (D) → Q, R
Download ATP STAR APP Page No.5
And Practice For Free
You might also like
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- GOC - Acid - Base QDocument11 pagesGOC - Acid - Base Qphokatka0No ratings yet
- General Organc Chemistry & IsomerismDocument14 pagesGeneral Organc Chemistry & IsomerismHarsh MeenaNo ratings yet
- For More Material Join: @jeeadvanced - 2024: Level 2Document47 pagesFor More Material Join: @jeeadvanced - 2024: Level 2anuragrana12345678No ratings yet
- Goc Question Bank: Complete Course On Organic Chemistry For JEE 2020Document8 pagesGoc Question Bank: Complete Course On Organic Chemistry For JEE 2020Vishvas Ranjan SrivastavaNo ratings yet
- CPP (GOC-2) RBooster ResDocument10 pagesCPP (GOC-2) RBooster RessahilNo ratings yet
- Quiz-General Organic Chemistry & Isomerism-Snd - SNDDocument4 pagesQuiz-General Organic Chemistry & Isomerism-Snd - SNDayesha sheikhNo ratings yet
- BocheemistryDocument9 pagesBocheemistryponveeraventhanpNo ratings yet
- Acidity (General Organic Chemistry)Document7 pagesAcidity (General Organic Chemistry)prashant sharmaNo ratings yet
- Sheet-5 (Ftp-S & Zenith) 29 AprilDocument13 pagesSheet-5 (Ftp-S & Zenith) 29 Apriluser19.tv.lgNo ratings yet
- Goc-II Ex e SiamrpnDocument22 pagesGoc-II Ex e SiamrpnArchit KhemaniNo ratings yet
- Daily Practice Problems: Organic Chemistry (GOC-I) - DPP-3 - BATCH: DROPPER'S 2021 (MAINS & ADV)Document8 pagesDaily Practice Problems: Organic Chemistry (GOC-I) - DPP-3 - BATCH: DROPPER'S 2021 (MAINS & ADV)Prayash dashNo ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Goc 6002eec9ca05fDocument14 pagesGoc 6002eec9ca05fAyush TiwariNo ratings yet
- SECTION-I (Single Choice Questions) : IIT - JEE: 2016 Crash Course (C 7 - A 1) Date: Topic: Iupac, Nomenclature, GocDocument11 pagesSECTION-I (Single Choice Questions) : IIT - JEE: 2016 Crash Course (C 7 - A 1) Date: Topic: Iupac, Nomenclature, GocSachin DedhiaNo ratings yet
- Final 100 Question by SY SirDocument29 pagesFinal 100 Question by SY Sirv k (venkat da)No ratings yet
- Exercise 12Document29 pagesExercise 12Soumya AgrawalNo ratings yet
- First Year - GOC, ISOMERISM - Revision - CPP - CKHDocument3 pagesFirst Year - GOC, ISOMERISM - Revision - CPP - CKHSeaN GabrielNo ratings yet
- @bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDDocument5 pages@bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDxkryxxzNo ratings yet
- Most Important Jee Pyqs Organic Chemistry-Some Basic Principles and TechniquesDocument9 pagesMost Important Jee Pyqs Organic Chemistry-Some Basic Principles and Techniquesyouanpal7835No ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- Organic 11th - Revision (GOC & Hydrocarbon) - DPPsDocument20 pagesOrganic 11th - Revision (GOC & Hydrocarbon) - DPPsNeelam KumariNo ratings yet
- Mixed DPP 8 - 20 (Isomerism)Document37 pagesMixed DPP 8 - 20 (Isomerism)shresthgaur19No ratings yet
- NHC HDocument4 pagesNHC HBhavya JainNo ratings yet
- Indian Association of Chemistry Teachers: National Standard Examination in Chemistry 2007-2008Document10 pagesIndian Association of Chemistry Teachers: National Standard Examination in Chemistry 2007-2008Srinivas VenkataramanNo ratings yet
- GOCDocument15 pagesGOCprashant sharmaNo ratings yet
- AromaticDocument29 pagesAromaticgoswamiaddNo ratings yet
- Rapid Crash Course: Single CorrectDocument8 pagesRapid Crash Course: Single CorrectHudsun HornetNo ratings yet
- Goc Basic Strength Assignment-10Document17 pagesGoc Basic Strength Assignment-10Ananta MurugeshanNo ratings yet
- Reactive Intermediate TestDocument6 pagesReactive Intermediate TestDhruv patelNo ratings yet
- Acid - BasesDocument34 pagesAcid - Basesshafique khanNo ratings yet
- CPP2 GOC Advan5222948788824536134Document4 pagesCPP2 GOC Advan5222948788824536134Drushya SalunkeNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet-10 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-2 Exact DurationDocument2 pagesDate Planned: - / - / - Daily Tutorial Sheet-10 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-2 Exact DurationVIDYA SRI GANESHNo ratings yet
- Model QP 7Document3 pagesModel QP 7Swarnabha BiswasNo ratings yet
- Acid - BasesDocument35 pagesAcid - BasesSachin SinghalNo ratings yet
- GOC Revision Assignment-1 SCQDocument15 pagesGOC Revision Assignment-1 SCQbhatianilay21No ratings yet
- RP RP CL CL CL RP CL PRDocument8 pagesRP RP CL CL CL RP CL PRJAIMIN PATELNo ratings yet
- GocDocument20 pagesGocSuyog SardaNo ratings yet
- IIT-JAM 2016 With SolutionDocument25 pagesIIT-JAM 2016 With SolutiongauravNo ratings yet
- Oc PT 2 - Student Copy - (Eng)Document6 pagesOc PT 2 - Student Copy - (Eng)Ramkumar SundaramNo ratings yet
- Nsec Solved Past Paper 2007Document10 pagesNsec Solved Past Paper 2007TEJA SINGHNo ratings yet
- NSEC Solved Paper 2007 PDFDocument10 pagesNSEC Solved Paper 2007 PDFMd MasoodNo ratings yet
- As Mhy FG 9 SVGy 5 M Qo KC2 oDocument52 pagesAs Mhy FG 9 SVGy 5 M Qo KC2 osingharyendra175No ratings yet
- Goc 1Document3 pagesGoc 1Twisha ViraniNo ratings yet
- Carbanion and Other Intermediate DPPDocument9 pagesCarbanion and Other Intermediate DPPJeetNo ratings yet
- Chemistry Test Series 02.01.2024 Question Paper & SolutionDocument11 pagesChemistry Test Series 02.01.2024 Question Paper & Solutionseemarai298035No ratings yet
- Atp Mega RevDocument14 pagesAtp Mega RevSURAKSHA PATELNo ratings yet
- Goc Stereo PDFDocument32 pagesGoc Stereo PDFDeepak GargNo ratings yet
- Practice Paper - Phase 3Document6 pagesPractice Paper - Phase 3Dhairya VinayakNo ratings yet
- Haloalkanes and Haloarenes _ DPP 02 __ Lakshya NEET 2025Document4 pagesHaloalkanes and Haloarenes _ DPP 02 __ Lakshya NEET 2025aashish.tskNo ratings yet
- DPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)Document2 pagesDPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)ADARSH KUMAR BEHERANo ratings yet
- GOC DPPDocument35 pagesGOC DPPRayNo ratings yet
- Iupac 1Document37 pagesIupac 1shodhan shettyNo ratings yet
- Aromaticity Assingment PDFDocument10 pagesAromaticity Assingment PDFGaurav YadavNo ratings yet
- CAC Glorifire T2 Ques.+Key 18-05-2024Document9 pagesCAC Glorifire T2 Ques.+Key 18-05-2024jerupatianjaliNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- Day - 28 Goc-2 (27-12-2022) ChemistryDocument4 pagesDay - 28 Goc-2 (27-12-2022) ChemistryVIKRANTH KUMAR JAKKOJUNo ratings yet
- DPP1 SBlock Advan6264893396548698825Document4 pagesDPP1 SBlock Advan6264893396548698825Drushya SalunkeNo ratings yet
- S Block ChemhaackDocument13 pagesS Block ChemhaackDrushya SalunkeNo ratings yet
- CPP2 GOC Advan5222948788824536134Document4 pagesCPP2 GOC Advan5222948788824536134Drushya SalunkeNo ratings yet
- Complete S Block ElementsDocument108 pagesComplete S Block ElementsDrushya SalunkeNo ratings yet