Professional Documents
Culture Documents
Lecture 14b
Lecture 14b
Uploaded by
Dishant kumar yadav mhakhariyaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Lecture 14b
Lecture 14b
Uploaded by
Dishant kumar yadav mhakhariyaCopyright:
Available Formats
Structure Prediction-Comparative Model
Manu Madhavan
Lecture 14
Manu Madhavan ISC 211 Lecture 14 1 / 29
Outline
Homology Modeling
Refer: chapter 7 of Krane & Raymer [Kra02]
Manu Madhavan ISC 211 Lecture 14 2 / 29
Issues in existing models
None of the algorithms discussed so far is accurate in predicting exact
structures (with high accuracy) for long protein sequences
Applications such as drug discovery, protein ligand identification, etc
high accurate structure details are required
Use Homology: When the tertiary structure of one or more proteins
similar in primary structure to a target protein is known, the target
protein can be modeled using comparative modeling
Manu Madhavan ISC 211 Lecture 14 3 / 29
Issues in existing models
None of the algorithms discussed so far is accurate in predicting exact
structures (with high accuracy) for long protein sequences
Applications such as drug discovery, protein ligand identification, etc
high accurate structure details are required
Use Homology: When the tertiary structure of one or more proteins
similar in primary structure to a target protein is known, the target
protein can be modeled using comparative modeling
Manu Madhavan ISC 211 Lecture 14 4 / 29
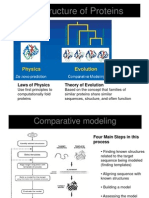
Comparative Model
aka Homology modeling
Predict the structure of the target protein via comparison with the
structures of related proteins.
Compare the known structure of homologous sequences
Manu Madhavan ISC 211 Lecture 14 5 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-1: Identify a set of protein structures related to the target
protein
BLAST and FASTA are used to identify related structures based on
sequence similarity
these structures serve as template for structure modeling
Step-2: Align the sequence of the target with the sequences of the
template proteins
Multiple sequence alignment- conserved regions of similarity can be
identified
CLUSTALW
Manual adjustment may be involved (Since MSA tools may generate
small percentage of error)
Manu Madhavan ISC 211 Lecture 14 6 / 29
Comparative Model-steps
Step-3: Construct the model
Superimpose the template structures and find the structurally conserve
regions
The backbone of the template structure is then aligned to these
conserved structures, forming a core of the model
Step-4: Model the loops
Select the best loop from a database of known loop conformation
Manu Madhavan ISC 211 Lecture 14 7 / 29
Comparative Model-steps
Step-3: Construct the model
Superimpose the template structures and find the structurally conserve
regions
The backbone of the template structure is then aligned to these
conserved structures, forming a core of the model
Step-4: Model the loops
Select the best loop from a database of known loop conformation
Manu Madhavan ISC 211 Lecture 14 7 / 29
Comparative Model-steps
Step-3: Construct the model
Superimpose the template structures and find the structurally conserve
regions
The backbone of the template structure is then aligned to these
conserved structures, forming a core of the model
Step-4: Model the loops
Select the best loop from a database of known loop conformation
Manu Madhavan ISC 211 Lecture 14 7 / 29
Comparative Model-steps
Step-3: Construct the model
Superimpose the template structures and find the structurally conserve
regions
The backbone of the template structure is then aligned to these
conserved structures, forming a core of the model
Step-4: Model the loops
Select the best loop from a database of known loop conformation
Manu Madhavan ISC 211 Lecture 14 7 / 29
Comparative Model-steps
Step-3: Construct the model
Superimpose the template structures and find the structurally conserve
regions
The backbone of the template structure is then aligned to these
conserved structures, forming a core of the model
Step-4: Model the loops
Select the best loop from a database of known loop conformation
Manu Madhavan ISC 211 Lecture 14 7 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
Comparative Model-steps
Step-5: Model the side chains
Identifying the positions of side chain atoms
Methods involves library search and computation based on molecular
dynamics
Step-6: Evaluate the model
PROCHECK, WHATCHECK, Verify-3D,....
Check for validation anomalies (not-allowed ϕ, ψ) angles
Usually correct the problems identified by hand (if possible)
Research in automating many of the steps is trending
Manu Madhavan ISC 211 Lecture 14 8 / 29
RNA Secondary Structure Prediction
Manu Madhavan
Lecture 14b
Manu Madhavan ISC 211 Lecture 14b 9 / 29
Various types RNA
messenger RNA (mRNA)
transfer RNA (tRNA)
Ribosomal RNA (rRNA)
small interfering RNA (siRNA)
micro RNA (miRNA)
small nuclear RNA (snRNA)
small nucleolar RNA (snoRNA)
guide RNA (gRNA)
efference RNA(eRNA)
Manu Madhavan ISC 211 Lecture 14b 10 / 29
Non-coding RNA
RNA that isn’t translated into protein
Includes: tRNA, rRNA, snRNA, snoRNA, miRNA, gRNA, eRNA,
pRNA, tmRNA
mRNA contains untranslated regions (5’UTR, 3’UTR), but UTRs are
not considered ncRNA
Manu Madhavan ISC 211 Lecture 14b 11 / 29
RNA Basics
RNA bases: A, C, G, U
Watson-Crick Pair: Two hydrogen bonds
A-U ( 2 kcal/mol)
G-C ( 3 kcal/mol)
Wobble pair
G-U ( 1 kcal/mol)
Non-Canonical pairs (modified suitably)
Bases can only pair with one other base
Manu Madhavan ISC 211 Lecture 14b 12 / 29
RNA Structure
Manu Madhavan ISC 211 Lecture 14b 13 / 29
RNA Secondary Structure
Manu Madhavan ISC 211 Lecture 14b 14 / 29
RNA Motifs
Manu Madhavan ISC 211 Lecture 14b 15 / 29
RNA Motifs
Manu Madhavan ISC 211 Lecture 14b 16 / 29
Why Predict secondary structure
Knowing the shape of a biomolecule is invaluable in drug design and
understanding disease mechanisms
Current physical methods (X-Ray, NMR) are too expensive and
time-consuming
Predict shape from sequence of bases
Four basic structures: helices, loops, bulges and junctions
Manu Madhavan ISC 211 Lecture 14b 17 / 29
RNA Motifs
Manu Madhavan ISC 211 Lecture 14b 18 / 29
RNA Secondary structure representation
Manu Madhavan ISC 211 Lecture 14b 19 / 29
Definition
Manu Madhavan ISC 211 Lecture 14b 20 / 29
Base-pair maximization
Manu Madhavan ISC 211 Lecture 14b 21 / 29
DP- Table
Manu Madhavan ISC 211 Lecture 14b 22 / 29
DP- Table
Manu Madhavan ISC 211 Lecture 14b 23 / 29
DP- Table
Manu Madhavan ISC 211 Lecture 14b 24 / 29
DP- Table
Manu Madhavan ISC 211 Lecture 14b 25 / 29
DP- Table
Manu Madhavan ISC 211 Lecture 14b 26 / 29
Traceback
To determine the structure of the folded RNA by traceback, we first
create an empty list of pairs P. We initialize with i = 0, j = n Then,
we follow one of three scenarios.
1 If j ≤ i the procedure stops.
2 If M(i, j) = M(i, j − 1) then set i = i, j = j − 1 and continue.
3 Otherwise, ∀k : i ≤ k < j if Sk and Sj are complementary and
M(i, j) = M(i, k − 1) + M(k + 1, j − 1) + 1 append (k, j) P, then
traceback both with i = i, j = k − 1 and i = k + 1, j = j − 1
When the traceback finishes, P P contains all of the paired bases.
Manu Madhavan ISC 211 Lecture 14b 27 / 29
Limitation
Base pair maximization will not necessarily lead to the most stable
structure.
It may create structure with many interior loops or hairpins which are
energetically unfavorable.
Results comparable to aligning sequences with scattered matches—
not biologically reasonable.
Manu Madhavan ISC 211 Lecture 14b 28 / 29
References I
Dan E Krane, Fundamental concepts of bioinformatics, Pearson Education India,
2002.
Manu Madhavan ISC 211 Lecture 14b 29 / 29
You might also like
- (Main) Genetic Engineering Lab Report Part 2 - Group 2Document17 pages(Main) Genetic Engineering Lab Report Part 2 - Group 2catarina alexandriaNo ratings yet
- Dr. Qudsia YousafiDocument30 pagesDr. Qudsia YousafiMalaika RehmanNo ratings yet
- Protein3dstructureprediction 161109084221Document30 pagesProtein3dstructureprediction 161109084221Jhanvi SNo ratings yet
- Homology Modelling Notes PDFDocument30 pagesHomology Modelling Notes PDFkamleshNo ratings yet
- Comparative Protein Structure Modeling Using ModellerDocument47 pagesComparative Protein Structure Modeling Using ModellerAlexNo ratings yet
- Bioinformatics Notes - 17Bt54: Module - 4Document48 pagesBioinformatics Notes - 17Bt54: Module - 4Samuel PrinceNo ratings yet
- Pieper NucleicAcidsRes 2004Document6 pagesPieper NucleicAcidsRes 2004ctak2022No ratings yet
- Genome Sequencing Projects: Increase in The Number of Protein SequencesDocument27 pagesGenome Sequencing Projects: Increase in The Number of Protein SequencesBiju ThomasNo ratings yet
- Homolgy ModelingDocument19 pagesHomolgy ModelingJainendra JainNo ratings yet
- Eswar MethodsMolBiol 2008Document25 pagesEswar MethodsMolBiol 2008huouinkyoumaNo ratings yet
- (Methods in Molecular Biology, 2231) Kazutaka Katoh - Multiple Sequence Alignment - Methods and Protocols-Humana (2020)Document322 pages(Methods in Molecular Biology, 2231) Kazutaka Katoh - Multiple Sequence Alignment - Methods and Protocols-Humana (2020)Diego AlmeidaNo ratings yet
- Homology Modeling: Ref: Structural Bioinformatics, P.E Bourne Molecular Modeling, FolkersDocument16 pagesHomology Modeling: Ref: Structural Bioinformatics, P.E Bourne Molecular Modeling, FolkersPallavi VermaNo ratings yet
- Protein Structure Prediction Using Homology ModelingDocument11 pagesProtein Structure Prediction Using Homology Modelingthelmamapangela64No ratings yet
- Multiple Sequence AlignmentDocument6 pagesMultiple Sequence AlignmentGeovani Gregorio Rincon NavasNo ratings yet
- HomolgyDocument21 pagesHomolgySatish SahuNo ratings yet
- Competitive Learning Neural NetworkDocument62 pagesCompetitive Learning Neural Networktoon townNo ratings yet
- A Study On Protein Sequence Clustering With OPTICSDocument6 pagesA Study On Protein Sequence Clustering With OPTICSJournal of ComputingNo ratings yet
- Re Comb 2003Document16 pagesRe Comb 2003mustafaNo ratings yet
- 04-Alinemiento Múltiple de SecuenciasDocument14 pages04-Alinemiento Múltiple de SecuenciasAlexander Ccahuana IgnacioNo ratings yet
- Homology Modeling, Also Known As Comparative Modeling ofDocument19 pagesHomology Modeling, Also Known As Comparative Modeling ofManoharNo ratings yet
- Bif501 AssDocument3 pagesBif501 Asssana 568aNo ratings yet
- Tertiary Structure Prediction Methods: Any Given Protein SequenceDocument29 pagesTertiary Structure Prediction Methods: Any Given Protein Sequencesurrender003No ratings yet
- Pre-Assessment QuestionsDocument18 pagesPre-Assessment Questionsk.sachinNo ratings yet
- Conclusions and Future WorkDocument12 pagesConclusions and Future WorkashishkkrNo ratings yet
- (Art) Meta-Cognitive Neural Network For Classification Problems in A Sequential Learning FrameworkDocument11 pages(Art) Meta-Cognitive Neural Network For Classification Problems in A Sequential Learning FrameworkPrissTinkNo ratings yet
- Proteins: Benchmarking Template Selection and Model Quality Assessment For High-Resolution Comparative ModelingDocument10 pagesProteins: Benchmarking Template Selection and Model Quality Assessment For High-Resolution Comparative ModelingLavanya Priya SathyanNo ratings yet
- Protein Modelling ToolDocument6 pagesProtein Modelling ToolShovana DeyNo ratings yet
- Comparison of FRF and Modal Methods For CombiningDocument20 pagesComparison of FRF and Modal Methods For CombiningCaio CesarNo ratings yet
- 10 21105 Joss 05057Document5 pages10 21105 Joss 05057Arpanet ArpanetNo ratings yet
- Praline 3Document18 pagesPraline 3CRISTIAN GABRIEL ZAMBRANO VEGANo ratings yet
- Homologymodeling 150123025144 Conversion Gate01Document27 pagesHomologymodeling 150123025144 Conversion Gate01Jhanvi SNo ratings yet
- 3-D Structure of Proteins: Laws of Physics Theory of EvolutionDocument9 pages3-D Structure of Proteins: Laws of Physics Theory of EvolutionladykikyoyunaNo ratings yet
- Metamotifs - A Generative Model For Building Families of Nucleotide Position Weight MatricesDocument16 pagesMetamotifs - A Generative Model For Building Families of Nucleotide Position Weight Matricesanon_174376942No ratings yet
- Protein Classification Using Hybrid Feature Selection TechniqueDocument9 pagesProtein Classification Using Hybrid Feature Selection TechniqueSudhakar TripathiNo ratings yet
- Supervised Machine Learning Techniques in Bioinformatics: Protein ClassificationDocument109 pagesSupervised Machine Learning Techniques in Bioinformatics: Protein ClassificationJoynul Abedin ZubairNo ratings yet
- Pi Is 0969212699801774Document14 pagesPi Is 0969212699801774Sahil8No ratings yet
- BIF101_II_Spring 2024 (1)Document8 pagesBIF101_II_Spring 2024 (1)My OpinionNo ratings yet
- tmp5573 TMPDocument11 pagestmp5573 TMPFrontiersNo ratings yet
- 1 s2.0 S136759312100051X MainDocument10 pages1 s2.0 S136759312100051X MainSumaiya Sadia BristyNo ratings yet
- Machine Learning For ChemistryDocument4 pagesMachine Learning For ChemistryLoga NathanNo ratings yet
- Unit 3Document9 pagesUnit 3document3580No ratings yet
- Vtu ML Lab ManualDocument47 pagesVtu ML Lab ManualNigar67% (3)
- ML Lab ManualDocument47 pagesML Lab Manualharshit gargNo ratings yet
- Homology ModellingDocument21 pagesHomology ModellingJerome FrancisNo ratings yet
- Protein ModellingDocument53 pagesProtein ModellingiluvgermieNo ratings yet
- Empirical Analysis of Performance Parameters For Consistency in Distributed DatabaseDocument29 pagesEmpirical Analysis of Performance Parameters For Consistency in Distributed DatabaseMona AbdelaleemNo ratings yet
- Unit-5 BioinformaticsDocument13 pagesUnit-5 Bioinformaticsp vmuraliNo ratings yet
- MsaDocument28 pagesMsaMausam KumravatNo ratings yet
- Blast 2 Sequences, A New Tool For Comparing Protein and Nucleotide SequencesDocument17 pagesBlast 2 Sequences, A New Tool For Comparing Protein and Nucleotide SequencesXâlmañ KháñNo ratings yet
- Comparison and Evaluation of Multiple Sequence Alignment Tools in BininformaticsDocument6 pagesComparison and Evaluation of Multiple Sequence Alignment Tools in Bininformaticsprashant_kaushal_2No ratings yet
- Sequence AnalysisDocument6 pagesSequence Analysisharrison9No ratings yet
- Effective Navigation of Query Results Based On Concept HierarchiesDocument17 pagesEffective Navigation of Query Results Based On Concept Hierarchiesakuthota6029No ratings yet
- 2013 J Theor Biol 328 77-88Document12 pages2013 J Theor Biol 328 77-88mbrylinskiNo ratings yet
- Theory: Sequence Alignment Is A Process of Aligning Two Sequences To Achieve Maximum Levels ofDocument5 pagesTheory: Sequence Alignment Is A Process of Aligning Two Sequences To Achieve Maximum Levels ofGopi ShankarNo ratings yet
- Lecture 101Document43 pagesLecture 101Jean ReneNo ratings yet
- Chapter 4 After ModfiyDocument4 pagesChapter 4 After Modfiyfatmahelawden000No ratings yet
- Machine Learning Laboratory SylbDocument1 pageMachine Learning Laboratory SylbVijay MahalingamNo ratings yet
- Combining Machine Learning and Agent-Based Modeling To Study Biomedical SystemsDocument33 pagesCombining Machine Learning and Agent-Based Modeling To Study Biomedical SystemsMarcelo Marcy Majstruk CimilloNo ratings yet
- DATA MINING AND MACHINE LEARNING. PREDICTIVE TECHNIQUES: REGRESSION, GENERALIZED LINEAR MODELS, SUPPORT VECTOR MACHINE AND NEURAL NETWORKSFrom EverandDATA MINING AND MACHINE LEARNING. PREDICTIVE TECHNIQUES: REGRESSION, GENERALIZED LINEAR MODELS, SUPPORT VECTOR MACHINE AND NEURAL NETWORKSNo ratings yet
- Class AssignmentDocument8 pagesClass AssignmentDishant kumar yadav mhakhariyaNo ratings yet
- Decision TreeDocument4 pagesDecision TreeDishant kumar yadav mhakhariyaNo ratings yet
- Lecture 9Document19 pagesLecture 9Dishant kumar yadav mhakhariyaNo ratings yet
- Lecture 12Document27 pagesLecture 12Dishant kumar yadav mhakhariyaNo ratings yet
- Lecture 13Document17 pagesLecture 13Dishant kumar yadav mhakhariyaNo ratings yet
- Lecture 15Document28 pagesLecture 15Dishant kumar yadav mhakhariyaNo ratings yet
- Lecture 2 - Barriers To CommunicationDocument11 pagesLecture 2 - Barriers To CommunicationDishant kumar yadav mhakhariyaNo ratings yet
- Kalasalingam Academy of Research and Education (Deemed To Be University) Anand Nagar, Krishnankoil - 626126Document60 pagesKalasalingam Academy of Research and Education (Deemed To Be University) Anand Nagar, Krishnankoil - 626126Dishant kumar yadav mhakhariyaNo ratings yet
- Lecture 3 - Non - Verbal CommunicationDocument26 pagesLecture 3 - Non - Verbal CommunicationDishant kumar yadav mhakhariyaNo ratings yet
- Certificate For COVID-19 Vaccination: Beneficiary DetailsDocument1 pageCertificate For COVID-19 Vaccination: Beneficiary DetailsNiranjan WarakeNo ratings yet
- Bacterialand Archael InclusionsDocument15 pagesBacterialand Archael InclusionsXimena AlcántaraNo ratings yet
- Laboratory Techniques in Rabies: Fourth EditionDocument494 pagesLaboratory Techniques in Rabies: Fourth EditionKimjhee Yang WongNo ratings yet
- Molecules of Life - TransesDocument3 pagesMolecules of Life - TransesJenny Ruth TubanNo ratings yet
- Enhanced Foreign Protein Accumulation in Nicotiana Benthamiana Leaves Co-Infiltrated With A TMV Vector and Plant Cell Cycle Regulator GenesDocument7 pagesEnhanced Foreign Protein Accumulation in Nicotiana Benthamiana Leaves Co-Infiltrated With A TMV Vector and Plant Cell Cycle Regulator Geneszhaoyue12112001No ratings yet
- Thesis For Research Paper On Genetic EngineeringDocument8 pagesThesis For Research Paper On Genetic Engineeringcnowkfhkf100% (1)
- Gmo Research Paper OutlineDocument7 pagesGmo Research Paper Outlinewtdcxtbnd100% (1)
- Li Et Al. 2022 - Untangling The Web of Intratumour HeterogeneityDocument10 pagesLi Et Al. 2022 - Untangling The Web of Intratumour HeterogeneitysodiogoesNo ratings yet
- Rivera-Laboratory Exercise No. 2-AnaphyDocument3 pagesRivera-Laboratory Exercise No. 2-AnaphyRivera, Trishia PilonNo ratings yet
- Nucleic Acid - An Overview - ScienceDirect TopicsDocument10 pagesNucleic Acid - An Overview - ScienceDirect TopicsBauza, Stephanie Dawn T.No ratings yet
- Flow Cytometry: Meroj A. JasemDocument58 pagesFlow Cytometry: Meroj A. Jasemahmad100% (1)
- Bms 07Document110 pagesBms 07Sabyasachi DasNo ratings yet
- Pseudo-Serendipity by Way of Random Variation: DNADocument7 pagesPseudo-Serendipity by Way of Random Variation: DNALeon LaupNo ratings yet
- Bio NotesDocument2 pagesBio NotesBobNo ratings yet
- The Discovery of CRISPR in Archaea and BacteriaDocument17 pagesThe Discovery of CRISPR in Archaea and BacteriaLightspeedNo ratings yet
- Cell Biology & BiochemistryDocument320 pagesCell Biology & BiochemistryVai SanNo ratings yet
- Red Blood Cell Membrane-Coated Functionalized Au Nanocage AsDocument12 pagesRed Blood Cell Membrane-Coated Functionalized Au Nanocage AsMuhammad Shehr YarNo ratings yet
- HIS-Select Technology GuideDocument6 pagesHIS-Select Technology GuideSigma-AldrichNo ratings yet
- Lab 8 - Transcription-Translation-ONLINE VERSION - 2021Document11 pagesLab 8 - Transcription-Translation-ONLINE VERSION - 2021thesoccerprince.10No ratings yet
- Alexander Et Al-2018-British Journal of Pharmacology PDFDocument113 pagesAlexander Et Al-2018-British Journal of Pharmacology PDFEduard José Martinez CamacaroNo ratings yet
- Purification of EnzymeDocument29 pagesPurification of Enzymeplastioid4079No ratings yet
- MIMM 211 MidtermDocument8 pagesMIMM 211 MidtermIpshitanandi100% (1)
- Mendel S Genes Molecular Characterization - 2011Document8 pagesMendel S Genes Molecular Characterization - 2011MARIA DE LOS ANGELES ORTIZ CRUZNo ratings yet
- G12 DR BiologyDocument196 pagesG12 DR BiologyAli RahimiNo ratings yet
- Post Graduate Degree Standard Paper - I Code:017Document2 pagesPost Graduate Degree Standard Paper - I Code:017Mk.jeyaNo ratings yet
- Neet Code C Question Paper PDFDocument41 pagesNeet Code C Question Paper PDFMagnifestoNo ratings yet
- Cell Resp and PhotosynDocument12 pagesCell Resp and Photosynhtb495No ratings yet
- Summer Training BrochureDocument1 pageSummer Training BrochureAbhilashRayaguruNo ratings yet
- Cell StructuresDocument54 pagesCell StructuresWilliam DC RiveraNo ratings yet