Professional Documents
Culture Documents
PKM Betoambari
PKM Betoambari
Uploaded by
nur liylaCopyright:
Available Formats
You might also like
- TIR-A15-14 Design Wind Load DeterminationDocument31 pagesTIR-A15-14 Design Wind Load Determinationjay kimNo ratings yet
- Research Methods For Architecture Ebook - Lucas, Ray - Kindle Store PDFDocument1 pageResearch Methods For Architecture Ebook - Lucas, Ray - Kindle Store PDFMohammed ShriamNo ratings yet
- Mitsubishi Triton l200 Specifications Specs PDFDocument28 pagesMitsubishi Triton l200 Specifications Specs PDFngulumi82100% (2)
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Hasil Running Amplirun Di Alat PCRDocument8 pagesHasil Running Amplirun Di Alat PCRandi takwaNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Sample CoaDocument20 pagesSample Coasales.karpschemNo ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- Variant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesDocument6 pagesVariant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesKetevan MigriauliNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- 6MD61xx KEMA-Certificate IEC61850 V4Document2 pages6MD61xx KEMA-Certificate IEC61850 V4Nguyễn Tuấn ViệtNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- FCC Test Report: BTL IncDocument70 pagesFCC Test Report: BTL IncLizardo Francisco Da SilvaNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- Npe SD Sqe Pip Ax4303 0009 Pad Leak TestDocument7 pagesNpe SD Sqe Pip Ax4303 0009 Pad Leak TestYulian Adhriansyah100% (1)
- SAR Test ReportDocument81 pagesSAR Test ReportEngenheiro Stefan ObermarkNo ratings yet
- Car Charger CE-EMC ReportDocument21 pagesCar Charger CE-EMC Reportgqg9sw6kbgNo ratings yet
- Certificado FCCDocument23 pagesCertificado FCCAline FernandesNo ratings yet
- Gradient Performance ReportDocument3 pagesGradient Performance ReportRiad ManamanniNo ratings yet
- Jytszb-R12-2100610 en 300328 WifiDocument77 pagesJytszb-R12-2100610 en 300328 WifionallpelinNo ratings yet
- Installation Qualification 2023.09.04 09.36.41Document2 pagesInstallation Qualification 2023.09.04 09.36.41Satriawan SyahNo ratings yet
- Asset 1731 QA ReportDocument5 pagesAsset 1731 QA Reportrubyhall bio-medicalNo ratings yet
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- Ochnaflavone 4'-Methyl ether-COA-PRF21083044Document2 pagesOchnaflavone 4'-Methyl ether-COA-PRF21083044DhavalNo ratings yet
- Tuv Report: Sample InformationDocument1 pageTuv Report: Sample InformationBiotomy LifesciencesNo ratings yet
- ODS HTQ CB Certificates Reports 62368 1 60950 1Document345 pagesODS HTQ CB Certificates Reports 62368 1 60950 1technical.managerNo ratings yet
- LabUPlus SerialconnectDocument7 pagesLabUPlus SerialconnectJose Perez PerezNo ratings yet
- Martor V.W.2 PDFDocument3 pagesMartor V.W.2 PDFViorel PopNo ratings yet
- Martor V.W.2 PDFDocument3 pagesMartor V.W.2 PDFViorel PopNo ratings yet
- EF-Flex-400 IEC Report IEC TS 63163 IEC61215-2Document37 pagesEF-Flex-400 IEC Report IEC TS 63163 IEC61215-2cesar gaiborNo ratings yet
- DocumentationDocument4 pagesDocumentationPradeep Kumar BowmarajuNo ratings yet
- Genexpert® DX System Installation Qualification ReportDocument3 pagesGenexpert® DX System Installation Qualification Reportdar zipNo ratings yet
- Gradient RMDocument6 pagesGradient RMRiad ManamanniNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- 10-1982 SJM Certificate PCS-900Document2 pages10-1982 SJM Certificate PCS-900anon_238578985No ratings yet
- Test Report: Applicant AddressDocument9 pagesTest Report: Applicant AddressEnzo AscañoNo ratings yet
- QP-209-18 NDT ProcedureDocument80 pagesQP-209-18 NDT ProcedurewildanmuhammadnajmiNo ratings yet
- Mapa de Concessões 1 1Document1 pageMapa de Concessões 1 1Venkat PachaNo ratings yet
- Sisymposiumharrisburgpaworkshoptraceyvincent PDFDocument76 pagesSisymposiumharrisburgpaworkshoptraceyvincent PDFNancyNo ratings yet
- Mac25 Maintenance Section 2Document27 pagesMac25 Maintenance Section 2Wahyu SriharjaNo ratings yet
- Primus Overview Catalogue ANGDocument8 pagesPrimus Overview Catalogue ANGpesumasinad0% (1)
- Eng/Npd Girish Comprehen Sive Charan Marketing: Internal Quality Audit Schedule No:Qms Ia-1Document1 pageEng/Npd Girish Comprehen Sive Charan Marketing: Internal Quality Audit Schedule No:Qms Ia-1DhinakaranNo ratings yet
- C161 Occupational Health Services ConventionDocument7 pagesC161 Occupational Health Services ConventionKeith RhodesNo ratings yet
- Buddy SystemDocument7 pagesBuddy SystemJahangir SiddikiNo ratings yet
- 2018 Trial 1 Biology Questions and Marking SchemeDocument11 pages2018 Trial 1 Biology Questions and Marking SchemeKodhekNo ratings yet
- FSM 2000Document52 pagesFSM 2000aram_hNo ratings yet
- Subarachnoid Haemorrhage:Pathology, Clinical Features and ManagementDocument48 pagesSubarachnoid Haemorrhage:Pathology, Clinical Features and Managementesene1100% (1)
- Hydro-Distillation Process in ExtractingDocument9 pagesHydro-Distillation Process in ExtractingFarhan PhaanzNo ratings yet
- ANH 8D OnlineDocument6 pagesANH 8D OnlineisserHsl 'v'No ratings yet
- What Is A Real Estate Investment Trust?: AreitisaDocument45 pagesWhat Is A Real Estate Investment Trust?: AreitisakoosNo ratings yet
- Hexply 8552: Mid-Toughened, High Strength, Damage-Resistant, Structural Epoxy MatrixDocument6 pagesHexply 8552: Mid-Toughened, High Strength, Damage-Resistant, Structural Epoxy MatrixshaxahNo ratings yet
- PHD Pharma 23 IdDocument1 pagePHD Pharma 23 Idos krishnaNo ratings yet
- Edwin Maturino - Benchmark Reviving The Professional CultureDocument9 pagesEdwin Maturino - Benchmark Reviving The Professional Cultureapi-693631580No ratings yet
- Implementation of Restructuring of The NcrpoDocument1 pageImplementation of Restructuring of The Ncrpojames antonioNo ratings yet
- Diploma in Business: Assignment BriefDocument10 pagesDiploma in Business: Assignment Brief陈肇远No ratings yet
- Ongc V Saw PipesDocument9 pagesOngc V Saw PipesManisha SinghNo ratings yet
- Science 7 q3 Module 3 Week3Document23 pagesScience 7 q3 Module 3 Week3Mary Cila TingalNo ratings yet
- SHELL100 12pDocument2 pagesSHELL100 12pLuizABastosNo ratings yet
- Geotechnical Properties of Dublin Boulder ClayDocument18 pagesGeotechnical Properties of Dublin Boulder ClayBLPgalwayNo ratings yet
- Summary of Maximum Load and Energy Consumption of Kwara State Goevrnment Mdas in Ilorin MetropolisDocument61 pagesSummary of Maximum Load and Energy Consumption of Kwara State Goevrnment Mdas in Ilorin MetropolisAbdulyekini AhmaduNo ratings yet
- A G.709 Optical Transport Network Tutorial: White PaperDocument12 pagesA G.709 Optical Transport Network Tutorial: White PapersumitNo ratings yet
- O-Levels Pure Mathematics ExemplarDocument36 pagesO-Levels Pure Mathematics Exemplartanatswarunganga7No ratings yet
- Cambridge Ordinary Level: Cambridge Assessment International EducationDocument20 pagesCambridge Ordinary Level: Cambridge Assessment International EducationJack KowmanNo ratings yet
- Engleza Maritima 4: 2a-SMCP External ROUTINE Communication - READING Comprehension-Book1Document5 pagesEngleza Maritima 4: 2a-SMCP External ROUTINE Communication - READING Comprehension-Book1dark959595No ratings yet
PKM Betoambari
PKM Betoambari
Uploaded by
nur liylaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PKM Betoambari
PKM Betoambari
Uploaded by
nur liylaCopyright:
Available Formats
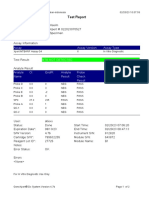
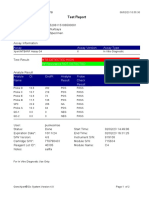
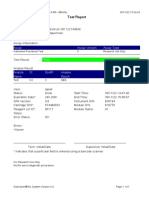
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
Test Report
Patient ID: 7472066905550001
Patient ID 2: 0128
Sample ID: 230703727821
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 2 NEG PASS
Probe C 0.0 2 NEG PASS
Probe E 0.0 1 NEG PASS
Probe B 0.0 3 NEG PASS
SPC 23.9 237 PASS PASS
Probe A 0.0 -5 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: PKM Katobengke
Status: Done Start Time: 24/02/23 10:14:17
Expiration Date*: 27/08/23 End Time: 24/02/23 11:55:39
S/W Version: 4.8 Instrument S/N: 807107
Cartridge S/N*: 5094497689 Module S/N: 210061047
Reagent Lot ID*: 27917 Module Name: B1
Notes: wd. maulidia ( pkm betoambari)
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 1 of 6
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 2 of 6
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
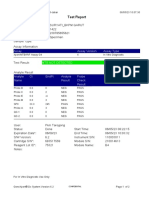
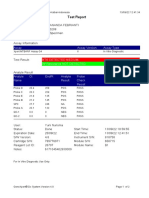
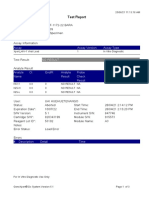
Test Report
Patient ID: 7472067007950005
Patient ID 2: 0127
Sample ID: 230692878721
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 -3 NEG PASS
Probe C 0.0 -2 NEG PASS
Probe E 0.0 3 NEG PASS
Probe B 0.0 2 NEG PASS
SPC 31.1 212 PASS PASS
Probe A 0.0 -6 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: PKM Katobengke
Status: Done Start Time: 24/02/23 09:55:34
Expiration Date*: 27/08/23 End Time: 24/02/23 11:36:37
S/W Version: 4.8 Instrument S/N: 807107
Cartridge S/N*: 5094497706 Module S/N: 210061093
Reagent Lot ID*: 27917 Module Name: B3
Notes: dian nur fadhilah ( pkm betoambari )
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 3 of 6
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 4 of 6
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
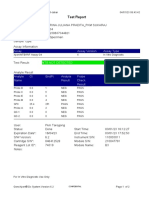
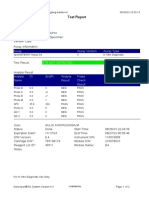
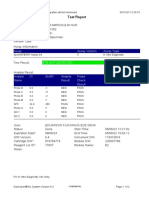
Test Report
Patient ID: 7472060107600039
Patient ID 2: 0126
Sample ID: 230703695121
Test Type: Specimen
Sample Type:
Assay Information
Assay Assay Version Assay Type
Xpert MTB-RIF Assay G4 6 In Vitro Diagnostic
Test Result: MTB NOT DETECTED
-
Analyte Result
Analyte Ct EndPt Analyte Probe
Name Result Check
Result
Probe D 0.0 -2 NEG PASS
Probe C 0.0 -6 NEG PASS
Probe E 0.0 -6 NEG PASS
Probe B 0.0 4 NEG PASS
SPC 23.8 228 PASS PASS
Probe A 0.0 3 NEG PASS
QC-1 0.0 0 NEG PASS
QC-2 0.0 0 NEG PASS
User: PKM Katobengke
Status: Done Start Time: 24/02/23 09:51:54
Expiration Date*: 27/08/23 End Time: 24/02/23 11:33:28
S/W Version: 4.8 Instrument S/N: 807107
Cartridge S/N*: 5094497818 Module S/N: 210068117
Reagent Lot ID*: 27917 Module Name: B2
Notes: samsuria ( PKM Betoambari )
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 5 of 6
807107-PKM Katobengke-Baubau-Indonesia 24/02/23 14:39:51
Test Report
Error Status: OK
-
Errors
<None>
Tech. Initial/Date Supervisor Initial/Date
* indicates that a particular field is entered using a barcode scanner
For In Vitro Diagnostic Use Only.
GeneXpert® Dx System Version 4.8 Page 6 of 6
You might also like
- TIR-A15-14 Design Wind Load DeterminationDocument31 pagesTIR-A15-14 Design Wind Load Determinationjay kimNo ratings yet
- Research Methods For Architecture Ebook - Lucas, Ray - Kindle Store PDFDocument1 pageResearch Methods For Architecture Ebook - Lucas, Ray - Kindle Store PDFMohammed ShriamNo ratings yet
- Mitsubishi Triton l200 Specifications Specs PDFDocument28 pagesMitsubishi Triton l200 Specifications Specs PDFngulumi82100% (2)
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay Typeyossy aprillyaNo ratings yet
- IWA 29TH PKM TRG 2021.12.01 08.35.17 DetailsDocument4 pagesIWA 29TH PKM TRG 2021.12.01 08.35.17 Detailsakreditasi tarogong 2023No ratings yet
- Hasim. S1Document2 pagesHasim. S1pkmsilo1No ratings yet
- NANI K 46TH PASUNDAN 2021.12.01 08.36.02 DetailsDocument4 pagesNANI K 46TH PASUNDAN 2021.12.01 08.36.02 Detailsakreditasi tarogong 2023No ratings yet
- 2023.05.06 08.22.15 DetailsDocument2 pages2023.05.06 08.22.15 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 15.25.33 DetailsDocument2 pages2023.01.03 15.25.33 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 08.06.58 DetailsDocument2 pages2023.01.03 08.06.58 Detailsakreditasi tarogong 2023No ratings yet
- 2023.01.03 16.12.27 DetailsDocument2 pages2023.01.03 16.12.27 Detailsakreditasi tarogong 2023No ratings yet
- APIHDocument2 pagesAPIHpuskesmas cigeulisNo ratings yet
- Cihideung TCM DetailsDocument10 pagesCihideung TCM Detailsmulyadi diningrumNo ratings yet
- Nurbaya 2023.02.02 14.49.06 DetailsDocument2 pagesNurbaya 2023.02.02 14.49.06 DetailsRahmatul LailiNo ratings yet
- 2023.01.03 11.48.53 DetailsDocument2 pages2023.01.03 11.48.53 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypesetyawanankesNo ratings yet
- SUKARMINI-DPM DR ABDUL GHOFIRDocument2 pagesSUKARMINI-DPM DR ABDUL GHOFIRlaboratorium mitra utamaNo ratings yet
- SUHARDI 61 TAROGONG 2021.12.01 14.18.16 DetailsDocument2 pagesSUHARDI 61 TAROGONG 2021.12.01 14.18.16 Detailsakreditasi tarogong 2023No ratings yet
- Test Report: Assay Assay Version Assay TypeDocument6 pagesTest Report: Assay Assay Version Assay TypeJose F. Ramirez MendozaNo ratings yet
- Bungga Hobrouw (R.anak)Document2 pagesBungga Hobrouw (R.anak)lukas mansnandifuNo ratings yet
- 3Document6 pages3Jose F. Ramirez MendozaNo ratings yet
- Ananda FebriantiDocument2 pagesAnanda FebriantiInternis RsuyarsiNo ratings yet
- Tamron IDocument2 pagesTamron IEri OiNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument2 pagesTest Report: Assay Assay Version Assay TypeMelania Dessy Savitri SunarjadiNo ratings yet
- Hasil Running Amplirun Di Alat PCRDocument8 pagesHasil Running Amplirun Di Alat PCRandi takwaNo ratings yet
- 2023.01.03 09.59.10 DetailsDocument2 pages2023.01.03 09.59.10 Detailsakreditasi tarogong 2023No ratings yet
- Rsi Sa 12-10-2023Document6 pagesRsi Sa 12-10-2023Laboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- M.SAIDI 2023.09.13 09.58.13 DetailsDocument2 pagesM.SAIDI 2023.09.13 09.58.13 DetailsLaboratorium RSI Sultan Agung BanjarbaruNo ratings yet
- Instrum 301122144638 2022.11.30 14.47.40 DetailsDocument1 pageInstrum 301122144638 2022.11.30 14.47.40 DetailsLabovida RoraimaNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument3 pagesTest Report: Assay Assay Version Assay TypeAlex MoralesNo ratings yet
- Sample CoaDocument20 pagesSample Coasales.karpschemNo ratings yet
- Client: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Document60 pagesClient: Pt. STBC Location: Autoclave 3 & 4 Report No.: 001-PAUT/RBT-STBC/XI/2022Rizal HidayatullahNo ratings yet
- Variant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesDocument6 pagesVariant Ii Turbo Hemoglobin Testing System: Touseasanaidin The Diagnosis of DiabetesKetevan MigriauliNo ratings yet
- Test Report: Assay Assay Version Assay TypeDocument7 pagesTest Report: Assay Assay Version Assay TyperosmayaniimutzNo ratings yet
- 6MD61xx KEMA-Certificate IEC61850 V4Document2 pages6MD61xx KEMA-Certificate IEC61850 V4Nguyễn Tuấn ViệtNo ratings yet
- Variant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesDocument6 pagesVariant Ii Hemoglobin Testing System For Hba: Bio-Rad LaboratoriesBhageshwar ChaudharyNo ratings yet
- FCC Test Report: BTL IncDocument70 pagesFCC Test Report: BTL IncLizardo Francisco Da SilvaNo ratings yet
- VARGA ResultsDocument3 pagesVARGA ResultsCocos MirelNo ratings yet
- Npe SD Sqe Pip Ax4303 0009 Pad Leak TestDocument7 pagesNpe SD Sqe Pip Ax4303 0009 Pad Leak TestYulian Adhriansyah100% (1)
- SAR Test ReportDocument81 pagesSAR Test ReportEngenheiro Stefan ObermarkNo ratings yet
- Car Charger CE-EMC ReportDocument21 pagesCar Charger CE-EMC Reportgqg9sw6kbgNo ratings yet
- Certificado FCCDocument23 pagesCertificado FCCAline FernandesNo ratings yet
- Gradient Performance ReportDocument3 pagesGradient Performance ReportRiad ManamanniNo ratings yet
- Jytszb-R12-2100610 en 300328 WifiDocument77 pagesJytszb-R12-2100610 en 300328 WifionallpelinNo ratings yet
- Installation Qualification 2023.09.04 09.36.41Document2 pagesInstallation Qualification 2023.09.04 09.36.41Satriawan SyahNo ratings yet
- Asset 1731 QA ReportDocument5 pagesAsset 1731 QA Reportrubyhall bio-medicalNo ratings yet
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- Ochnaflavone 4'-Methyl ether-COA-PRF21083044Document2 pagesOchnaflavone 4'-Methyl ether-COA-PRF21083044DhavalNo ratings yet
- Tuv Report: Sample InformationDocument1 pageTuv Report: Sample InformationBiotomy LifesciencesNo ratings yet
- ODS HTQ CB Certificates Reports 62368 1 60950 1Document345 pagesODS HTQ CB Certificates Reports 62368 1 60950 1technical.managerNo ratings yet
- LabUPlus SerialconnectDocument7 pagesLabUPlus SerialconnectJose Perez PerezNo ratings yet
- Martor V.W.2 PDFDocument3 pagesMartor V.W.2 PDFViorel PopNo ratings yet
- Martor V.W.2 PDFDocument3 pagesMartor V.W.2 PDFViorel PopNo ratings yet
- EF-Flex-400 IEC Report IEC TS 63163 IEC61215-2Document37 pagesEF-Flex-400 IEC Report IEC TS 63163 IEC61215-2cesar gaiborNo ratings yet
- DocumentationDocument4 pagesDocumentationPradeep Kumar BowmarajuNo ratings yet
- Genexpert® DX System Installation Qualification ReportDocument3 pagesGenexpert® DX System Installation Qualification Reportdar zipNo ratings yet
- Gradient RMDocument6 pagesGradient RMRiad ManamanniNo ratings yet
- A-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportDocument4 pagesA-Star Testing & Inspection (S) Pte LTD: Magnetic Particle Testing ReportHari KarthickNo ratings yet
- 10-1982 SJM Certificate PCS-900Document2 pages10-1982 SJM Certificate PCS-900anon_238578985No ratings yet
- Test Report: Applicant AddressDocument9 pagesTest Report: Applicant AddressEnzo AscañoNo ratings yet
- QP-209-18 NDT ProcedureDocument80 pagesQP-209-18 NDT ProcedurewildanmuhammadnajmiNo ratings yet
- Mapa de Concessões 1 1Document1 pageMapa de Concessões 1 1Venkat PachaNo ratings yet
- Sisymposiumharrisburgpaworkshoptraceyvincent PDFDocument76 pagesSisymposiumharrisburgpaworkshoptraceyvincent PDFNancyNo ratings yet
- Mac25 Maintenance Section 2Document27 pagesMac25 Maintenance Section 2Wahyu SriharjaNo ratings yet
- Primus Overview Catalogue ANGDocument8 pagesPrimus Overview Catalogue ANGpesumasinad0% (1)
- Eng/Npd Girish Comprehen Sive Charan Marketing: Internal Quality Audit Schedule No:Qms Ia-1Document1 pageEng/Npd Girish Comprehen Sive Charan Marketing: Internal Quality Audit Schedule No:Qms Ia-1DhinakaranNo ratings yet
- C161 Occupational Health Services ConventionDocument7 pagesC161 Occupational Health Services ConventionKeith RhodesNo ratings yet
- Buddy SystemDocument7 pagesBuddy SystemJahangir SiddikiNo ratings yet
- 2018 Trial 1 Biology Questions and Marking SchemeDocument11 pages2018 Trial 1 Biology Questions and Marking SchemeKodhekNo ratings yet
- FSM 2000Document52 pagesFSM 2000aram_hNo ratings yet
- Subarachnoid Haemorrhage:Pathology, Clinical Features and ManagementDocument48 pagesSubarachnoid Haemorrhage:Pathology, Clinical Features and Managementesene1100% (1)
- Hydro-Distillation Process in ExtractingDocument9 pagesHydro-Distillation Process in ExtractingFarhan PhaanzNo ratings yet
- ANH 8D OnlineDocument6 pagesANH 8D OnlineisserHsl 'v'No ratings yet
- What Is A Real Estate Investment Trust?: AreitisaDocument45 pagesWhat Is A Real Estate Investment Trust?: AreitisakoosNo ratings yet
- Hexply 8552: Mid-Toughened, High Strength, Damage-Resistant, Structural Epoxy MatrixDocument6 pagesHexply 8552: Mid-Toughened, High Strength, Damage-Resistant, Structural Epoxy MatrixshaxahNo ratings yet
- PHD Pharma 23 IdDocument1 pagePHD Pharma 23 Idos krishnaNo ratings yet
- Edwin Maturino - Benchmark Reviving The Professional CultureDocument9 pagesEdwin Maturino - Benchmark Reviving The Professional Cultureapi-693631580No ratings yet
- Implementation of Restructuring of The NcrpoDocument1 pageImplementation of Restructuring of The Ncrpojames antonioNo ratings yet
- Diploma in Business: Assignment BriefDocument10 pagesDiploma in Business: Assignment Brief陈肇远No ratings yet
- Ongc V Saw PipesDocument9 pagesOngc V Saw PipesManisha SinghNo ratings yet
- Science 7 q3 Module 3 Week3Document23 pagesScience 7 q3 Module 3 Week3Mary Cila TingalNo ratings yet
- SHELL100 12pDocument2 pagesSHELL100 12pLuizABastosNo ratings yet
- Geotechnical Properties of Dublin Boulder ClayDocument18 pagesGeotechnical Properties of Dublin Boulder ClayBLPgalwayNo ratings yet
- Summary of Maximum Load and Energy Consumption of Kwara State Goevrnment Mdas in Ilorin MetropolisDocument61 pagesSummary of Maximum Load and Energy Consumption of Kwara State Goevrnment Mdas in Ilorin MetropolisAbdulyekini AhmaduNo ratings yet
- A G.709 Optical Transport Network Tutorial: White PaperDocument12 pagesA G.709 Optical Transport Network Tutorial: White PapersumitNo ratings yet
- O-Levels Pure Mathematics ExemplarDocument36 pagesO-Levels Pure Mathematics Exemplartanatswarunganga7No ratings yet
- Cambridge Ordinary Level: Cambridge Assessment International EducationDocument20 pagesCambridge Ordinary Level: Cambridge Assessment International EducationJack KowmanNo ratings yet
- Engleza Maritima 4: 2a-SMCP External ROUTINE Communication - READING Comprehension-Book1Document5 pagesEngleza Maritima 4: 2a-SMCP External ROUTINE Communication - READING Comprehension-Book1dark959595No ratings yet