Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
3 viewsSelected Answers From Atomic Structure
Selected Answers From Atomic Structure
Uploaded by
서연김The document contains 5 questions about atomic structure and periodic trends. Question 1 asks about how atomic radius changes as you move down the periodic table, question 2 asks about how first ionization energy is affected by atomic structure as you move down a group, question 3 asks about how atomic radius changes as you move across the periodic table, question 4 asks about what aspect of atomic structure affects atomic radius trends across a period, and question 5 asks how this aspect also affects trends in first ionization energy.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Periodic Trends GizmoDocument9 pagesPeriodic Trends GizmoGabe Itch67% (61)
- Syllabus Phy eDocument5 pagesSyllabus Phy eChhaylySrengNo ratings yet
- Atomic Charges and Trends On The Periodic TableDocument2 pagesAtomic Charges and Trends On The Periodic TablejnfurstNo ratings yet
- Introduction To The Modern Periodic TableDocument10 pagesIntroduction To The Modern Periodic Tablefrankycoc667No ratings yet
- CHM111E 1.1.1 - Periodic Table and Its Trends PDFDocument43 pagesCHM111E 1.1.1 - Periodic Table and Its Trends PDFanton petrovNo ratings yet
- Periodic Trends Guided NotesDocument4 pagesPeriodic Trends Guided NotesSamuel RobertsNo ratings yet
- Atomic RaduisDocument5 pagesAtomic Raduispeepee poopooNo ratings yet
- Worksheet On PTDocument3 pagesWorksheet On PTAlexander MalvarNo ratings yet
- 11 ChemDocument4 pages11 ChemRakeshNo ratings yet
- Inorganic ChemistryDocument88 pagesInorganic ChemistryFrancis HDNo ratings yet
- Module Three Lesson Two Guided NotesDocument4 pagesModule Three Lesson Two Guided NotesConnorCNo ratings yet
- Unit 1 - Atoms and The Periodic Table - Student VersionDocument28 pagesUnit 1 - Atoms and The Periodic Table - Student VersionAmadu sallieuNo ratings yet
- PtkeyconceptskeyDocument3 pagesPtkeyconceptskeyapi-287707344No ratings yet
- AP Chemistry - Trends in The Periodic TableDocument3 pagesAP Chemistry - Trends in The Periodic Tableilias1973No ratings yet
- Syllabus MEXT: PhysicsDocument2 pagesSyllabus MEXT: Physicswh atNo ratings yet
- Periodic Trends C12 2 07Document13 pagesPeriodic Trends C12 2 07Kuro NekoNo ratings yet
- Chem 11 ReviewDocument40 pagesChem 11 Reviewabdumari11No ratings yet
- Ib Chemistry: Topic 3 PeriodicityDocument60 pagesIb Chemistry: Topic 3 PeriodicityMichellycia AgathaNo ratings yet
- Chemistry ProjectDocument10 pagesChemistry ProjectIshan AggarwalNo ratings yet
- Chapter 2, 3Document35 pagesChapter 2, 3silvanyangelliong2No ratings yet
- Group 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Document66 pagesGroup 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Kristel Ann LaudeNo ratings yet
- Atoms and The Periodic TableDocument9 pagesAtoms and The Periodic TableShivaniNo ratings yet
- Guided Reading CH 6Document3 pagesGuided Reading CH 6api-310420482No ratings yet
- Periodic TrendsDocument11 pagesPeriodic TrendsFern HofileñaNo ratings yet
- STPDF2 Periodic Variations of Elements PDFDocument15 pagesSTPDF2 Periodic Variations of Elements PDFIcey DreiNo ratings yet
- CHM111 - Lecture Notes 3Document81 pagesCHM111 - Lecture Notes 3PES MASTER GAMEPLAYSNo ratings yet
- Unit 3 (Periodic Table) Test Review SheetDocument6 pagesUnit 3 (Periodic Table) Test Review SheetTheo ZhengNo ratings yet
- 2 CH 28 Nuclear Chemistry (Def Radioactivity)Document58 pages2 CH 28 Nuclear Chemistry (Def Radioactivity)Fatin IziantiNo ratings yet
- CHM012 - Module 3 (Part 3)Document9 pagesCHM012 - Module 3 (Part 3)haibaalisa00No ratings yet
- 1 A 1Document4 pages1 A 1mugadza.joseph86No ratings yet
- Amit Bikram Mishra - BSCH201Document10 pagesAmit Bikram Mishra - BSCH201Amit Bikram MishraNo ratings yet
- 09 The Periodic Table SlidesDocument19 pages09 The Periodic Table Slidesjackoread28No ratings yet
- Nuclear Chemistry and EnergyDocument18 pagesNuclear Chemistry and EnergyJm EscobarNo ratings yet
- Idioma TecnicooDocument1 pageIdioma TecnicooAdrian CuculNo ratings yet
- Periodic Properties and Trends PDFDocument20 pagesPeriodic Properties and Trends PDFShivansh Awasthi XII B2No ratings yet
- Chemistry SOL Review: Part 2: Atomic Structure and Periodic RelationshipsDocument42 pagesChemistry SOL Review: Part 2: Atomic Structure and Periodic RelationshipshelperforeuNo ratings yet
- Lesson 1 The Structure of The AtomDocument8 pagesLesson 1 The Structure of The AtomsarahhannahghanyNo ratings yet
- A Level Chemistry GuideDocument34 pagesA Level Chemistry GuideRexton Jarvis100% (1)
- Screenshot 2023-11-04 at 4.12.18 PMDocument45 pagesScreenshot 2023-11-04 at 4.12.18 PMmalakbasahalNo ratings yet
- Micromechanics ApplicabilityDocument16 pagesMicromechanics ApplicabilityGraceNo ratings yet
- Lecture 1.1 Introduction To Materials Science Theory (1) (18 Files Merged)Document458 pagesLecture 1.1 Introduction To Materials Science Theory (1) (18 Files Merged)Danish SiddiquiNo ratings yet
- Atomic Structure and The Periodic TableDocument1 pageAtomic Structure and The Periodic Tableramghotra77No ratings yet
- Atomic Radii: Atomic Structure: Periodic TrendsDocument5 pagesAtomic Radii: Atomic Structure: Periodic TrendsRajat SharmaNo ratings yet
- Periodic Classification of The Element - Lesson - 2Document14 pagesPeriodic Classification of The Element - Lesson - 2samsonNo ratings yet
- Atomic SizeDocument2 pagesAtomic SizeFozia's Beauty Tips & Entertainment FoziaNo ratings yet
- Chapter 3 Periodic TableDocument30 pagesChapter 3 Periodic Tableanwarhs62100% (1)
- PeriodicityDocument6 pagesPeriodicityNetkoNo ratings yet
- Photoelectron SpectrosDocument15 pagesPhotoelectron Spectrosapi-182809945No ratings yet
- 8.22. Periodicity Student BookletDocument32 pages8.22. Periodicity Student BookletctyaxcgasvuhcaNo ratings yet
- Sch4uc Unit 1 Lesson 01 PDFDocument36 pagesSch4uc Unit 1 Lesson 01 PDFEmeka ChukwuNo ratings yet
- 1.7 Periodic Trends in Atomic PropertiesDocument5 pages1.7 Periodic Trends in Atomic PropertiesSilver AbdulahiNo ratings yet
- Periodicity NotesDocument5 pagesPeriodicity Notescgao30No ratings yet
- Atomic Radius and Electronegativity - QuestionsDocument4 pagesAtomic Radius and Electronegativity - QuestionsAnonymous Mj0pfScNo ratings yet
- Periodic TrendsDocument5 pagesPeriodic TrendsAnushkaNo ratings yet
- Booklet Periodic Trends PERIODICITY CLASS NOTESDocument34 pagesBooklet Periodic Trends PERIODICITY CLASS NOTESVed PatelNo ratings yet
- Upload - The Periodic Table and TrendsDocument14 pagesUpload - The Periodic Table and TrendsMbuyotiNo ratings yet
- Chemistry: Physical SettingDocument16 pagesChemistry: Physical SettingCRISTOFER FLORES REYESNo ratings yet
- Chemistry - Unit1Document20 pagesChemistry - Unit1Mahatma MurthiNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4No ratings yet
- Nuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1From EverandNuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1No ratings yet
Selected Answers From Atomic Structure
Selected Answers From Atomic Structure
Uploaded by
서연김0 ratings0% found this document useful (0 votes)
3 views3 pagesThe document contains 5 questions about atomic structure and periodic trends. Question 1 asks about how atomic radius changes as you move down the periodic table, question 2 asks about how first ionization energy is affected by atomic structure as you move down a group, question 3 asks about how atomic radius changes as you move across the periodic table, question 4 asks about what aspect of atomic structure affects atomic radius trends across a period, and question 5 asks how this aspect also affects trends in first ionization energy.
Original Description:
Original Title
Selected Answers from Atomic Structure
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains 5 questions about atomic structure and periodic trends. Question 1 asks about how atomic radius changes as you move down the periodic table, question 2 asks about how first ionization energy is affected by atomic structure as you move down a group, question 3 asks about how atomic radius changes as you move across the periodic table, question 4 asks about what aspect of atomic structure affects atomic radius trends across a period, and question 5 asks how this aspect also affects trends in first ionization energy.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
3 views3 pagesSelected Answers From Atomic Structure
Selected Answers From Atomic Structure
Uploaded by
서연김The document contains 5 questions about atomic structure and periodic trends. Question 1 asks about how atomic radius changes as you move down the periodic table, question 2 asks about how first ionization energy is affected by atomic structure as you move down a group, question 3 asks about how atomic radius changes as you move across the periodic table, question 4 asks about what aspect of atomic structure affects atomic radius trends across a period, and question 5 asks how this aspect also affects trends in first ionization energy.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 3
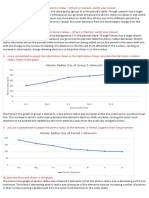
Selected Answers from Atomic Structure & Periodic Trends work:
1. What is happening to the atom’s size (atomic radius) as you move downward on the
periodic table?
2. What aspect of atomic structure most likely affects the First Ionization Energy trend
down a group (the energy required to remove a valence electron)?
3. What is happening to the atom’s size (atomic radius) as you move to the right on the
periodic table?
4. What aspect of atomic structure most likely affects the atomic radius trend across a
period?
5. How does this aspect of atomic structure affect the First Ionization Energy trend (the
energy required to remove a valence electron)?
You might also like
- Periodic Trends GizmoDocument9 pagesPeriodic Trends GizmoGabe Itch67% (61)
- Syllabus Phy eDocument5 pagesSyllabus Phy eChhaylySrengNo ratings yet
- Atomic Charges and Trends On The Periodic TableDocument2 pagesAtomic Charges and Trends On The Periodic TablejnfurstNo ratings yet
- Introduction To The Modern Periodic TableDocument10 pagesIntroduction To The Modern Periodic Tablefrankycoc667No ratings yet
- CHM111E 1.1.1 - Periodic Table and Its Trends PDFDocument43 pagesCHM111E 1.1.1 - Periodic Table and Its Trends PDFanton petrovNo ratings yet
- Periodic Trends Guided NotesDocument4 pagesPeriodic Trends Guided NotesSamuel RobertsNo ratings yet
- Atomic RaduisDocument5 pagesAtomic Raduispeepee poopooNo ratings yet
- Worksheet On PTDocument3 pagesWorksheet On PTAlexander MalvarNo ratings yet
- 11 ChemDocument4 pages11 ChemRakeshNo ratings yet
- Inorganic ChemistryDocument88 pagesInorganic ChemistryFrancis HDNo ratings yet
- Module Three Lesson Two Guided NotesDocument4 pagesModule Three Lesson Two Guided NotesConnorCNo ratings yet
- Unit 1 - Atoms and The Periodic Table - Student VersionDocument28 pagesUnit 1 - Atoms and The Periodic Table - Student VersionAmadu sallieuNo ratings yet
- PtkeyconceptskeyDocument3 pagesPtkeyconceptskeyapi-287707344No ratings yet
- AP Chemistry - Trends in The Periodic TableDocument3 pagesAP Chemistry - Trends in The Periodic Tableilias1973No ratings yet
- Syllabus MEXT: PhysicsDocument2 pagesSyllabus MEXT: Physicswh atNo ratings yet
- Periodic Trends C12 2 07Document13 pagesPeriodic Trends C12 2 07Kuro NekoNo ratings yet
- Chem 11 ReviewDocument40 pagesChem 11 Reviewabdumari11No ratings yet
- Ib Chemistry: Topic 3 PeriodicityDocument60 pagesIb Chemistry: Topic 3 PeriodicityMichellycia AgathaNo ratings yet
- Chemistry ProjectDocument10 pagesChemistry ProjectIshan AggarwalNo ratings yet
- Chapter 2, 3Document35 pagesChapter 2, 3silvanyangelliong2No ratings yet
- Group 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Document66 pagesGroup 3: Periodic Relationship: Wendell Bandiola (THE PERIODIC TABLE)Kristel Ann LaudeNo ratings yet
- Atoms and The Periodic TableDocument9 pagesAtoms and The Periodic TableShivaniNo ratings yet
- Guided Reading CH 6Document3 pagesGuided Reading CH 6api-310420482No ratings yet
- Periodic TrendsDocument11 pagesPeriodic TrendsFern HofileñaNo ratings yet
- STPDF2 Periodic Variations of Elements PDFDocument15 pagesSTPDF2 Periodic Variations of Elements PDFIcey DreiNo ratings yet
- CHM111 - Lecture Notes 3Document81 pagesCHM111 - Lecture Notes 3PES MASTER GAMEPLAYSNo ratings yet
- Unit 3 (Periodic Table) Test Review SheetDocument6 pagesUnit 3 (Periodic Table) Test Review SheetTheo ZhengNo ratings yet
- 2 CH 28 Nuclear Chemistry (Def Radioactivity)Document58 pages2 CH 28 Nuclear Chemistry (Def Radioactivity)Fatin IziantiNo ratings yet
- CHM012 - Module 3 (Part 3)Document9 pagesCHM012 - Module 3 (Part 3)haibaalisa00No ratings yet
- 1 A 1Document4 pages1 A 1mugadza.joseph86No ratings yet
- Amit Bikram Mishra - BSCH201Document10 pagesAmit Bikram Mishra - BSCH201Amit Bikram MishraNo ratings yet
- 09 The Periodic Table SlidesDocument19 pages09 The Periodic Table Slidesjackoread28No ratings yet
- Nuclear Chemistry and EnergyDocument18 pagesNuclear Chemistry and EnergyJm EscobarNo ratings yet
- Idioma TecnicooDocument1 pageIdioma TecnicooAdrian CuculNo ratings yet
- Periodic Properties and Trends PDFDocument20 pagesPeriodic Properties and Trends PDFShivansh Awasthi XII B2No ratings yet
- Chemistry SOL Review: Part 2: Atomic Structure and Periodic RelationshipsDocument42 pagesChemistry SOL Review: Part 2: Atomic Structure and Periodic RelationshipshelperforeuNo ratings yet
- Lesson 1 The Structure of The AtomDocument8 pagesLesson 1 The Structure of The AtomsarahhannahghanyNo ratings yet
- A Level Chemistry GuideDocument34 pagesA Level Chemistry GuideRexton Jarvis100% (1)
- Screenshot 2023-11-04 at 4.12.18 PMDocument45 pagesScreenshot 2023-11-04 at 4.12.18 PMmalakbasahalNo ratings yet
- Micromechanics ApplicabilityDocument16 pagesMicromechanics ApplicabilityGraceNo ratings yet
- Lecture 1.1 Introduction To Materials Science Theory (1) (18 Files Merged)Document458 pagesLecture 1.1 Introduction To Materials Science Theory (1) (18 Files Merged)Danish SiddiquiNo ratings yet
- Atomic Structure and The Periodic TableDocument1 pageAtomic Structure and The Periodic Tableramghotra77No ratings yet
- Atomic Radii: Atomic Structure: Periodic TrendsDocument5 pagesAtomic Radii: Atomic Structure: Periodic TrendsRajat SharmaNo ratings yet
- Periodic Classification of The Element - Lesson - 2Document14 pagesPeriodic Classification of The Element - Lesson - 2samsonNo ratings yet
- Atomic SizeDocument2 pagesAtomic SizeFozia's Beauty Tips & Entertainment FoziaNo ratings yet
- Chapter 3 Periodic TableDocument30 pagesChapter 3 Periodic Tableanwarhs62100% (1)
- PeriodicityDocument6 pagesPeriodicityNetkoNo ratings yet
- Photoelectron SpectrosDocument15 pagesPhotoelectron Spectrosapi-182809945No ratings yet
- 8.22. Periodicity Student BookletDocument32 pages8.22. Periodicity Student BookletctyaxcgasvuhcaNo ratings yet
- Sch4uc Unit 1 Lesson 01 PDFDocument36 pagesSch4uc Unit 1 Lesson 01 PDFEmeka ChukwuNo ratings yet
- 1.7 Periodic Trends in Atomic PropertiesDocument5 pages1.7 Periodic Trends in Atomic PropertiesSilver AbdulahiNo ratings yet
- Periodicity NotesDocument5 pagesPeriodicity Notescgao30No ratings yet
- Atomic Radius and Electronegativity - QuestionsDocument4 pagesAtomic Radius and Electronegativity - QuestionsAnonymous Mj0pfScNo ratings yet
- Periodic TrendsDocument5 pagesPeriodic TrendsAnushkaNo ratings yet
- Booklet Periodic Trends PERIODICITY CLASS NOTESDocument34 pagesBooklet Periodic Trends PERIODICITY CLASS NOTESVed PatelNo ratings yet
- Upload - The Periodic Table and TrendsDocument14 pagesUpload - The Periodic Table and TrendsMbuyotiNo ratings yet
- Chemistry: Physical SettingDocument16 pagesChemistry: Physical SettingCRISTOFER FLORES REYESNo ratings yet
- Chemistry - Unit1Document20 pagesChemistry - Unit1Mahatma MurthiNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4No ratings yet
- Nuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1From EverandNuclear Fusion Enhanced by Negative Mass – A Proposed Method and Device – (Part 1): Nuclear Fusion Enhanced by Negative Mass, #1No ratings yet