Professional Documents
Culture Documents
Bonding Practice
Bonding Practice
Uploaded by
Aditya Verma0 ratings0% found this document useful (0 votes)
21 views29 pagesOriginal Title
Bonding practice
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
21 views29 pagesBonding Practice
Bonding Practice
Uploaded by
Aditya VermaCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 29
DPP No. 8
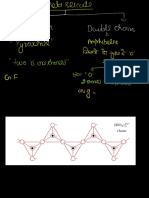
‘What is the nature of chemical bonding between Cs and F ?
(A) lonic (®) Covalent (©) Coordinate (0) Metallic
‘The lattice energy of sodium chloride crystal is the energy released when one mole of NaClis) is formed
from:
(A) Na(g) and Cig) atoms (®) Na‘@) and Cg) ions
(C) Na(s) and Cl,(@) (0) crystallization from aqueous solution of sodium chlo-
ride.
‘Which does not favour the formation of ionic compound
(A) the ionization energy of the metal atom should be low.
(©) the lattice energy ofthe compound formed must be love
(©) the electron afity ofthe non-metal should be high
(©) the lattice energy of the compound formed must be high.
Cctet configuration cannot be achieved through
(A) loss of electrons (8) gain of electrons
(©) sharing of electrons (0) exchange of electrons
In which of the following molecules, bonding is not taking place in excited state :
(A) CH, (8) BF; OIF, (0) PCI,
The bonds present in N,O are
(A) only ionic (8) covalent and co-ordinate
(C)only covalent (©) covalent and ionic
Which of the following species does not obey octet rule
(A) SIF, (8) PCI, (Ici
Answer the following
() Whats the covalency of Carbon in C,H, ?
(ii) What types of bonds and how many of each are present in NH,* ion?
DPP No. #8
3, ®) 4. (0) 5.
8 (i) four (i three covalent and one coordinate
DPP No. 9
The correct representation of Lewis dot structure of HNO, is
i 9 8
(ay Hoe (8) H-O-N=0 () H-O-N=0 H-w—0
Oo 6 }
2 ‘Species not obeying octet rule is/are
(A) coz” (8) BF, (©) NOZ (0) PCI,
3. PCI, exists but NCI, does not, because
(A) Nitrogen has no vacant 2¢-orbitals (©) N and Cihave almost same EN
(C) N-atom is much smaller than P-atom (D) Nitrogen is highly inert
4, The molecular without any lone pair around the centred atom is
(A) Xeo, ©) xe0, (©) XeF, (0) Xe0,F,
‘Match the species in column (I) with their characteristics in column (Il)
Column-1 Column-iL
(P) BH, (1) 2 bond pair and 3 lone pair on central atom
icy, (2) 4 bond pair and no lone pair on central atom
®) IC. (8) 3 bond pair and 1 lone pair on central atom
SIC (4) 2 bond pair and 2 lone pair on central atom.
(6) 4 bond pair and 2 lone pair on central atom
“ (@)P=2;Q=4:R=1;
(0) P=2,Q=4;R=5;S=4 (D)P=2,Q=4;R=3;S=4
‘The odd electron molecules among the following is/are
(NO, (8) NO ©Clo, (co
Assign formal charges to all atoms in the given species,
©: H
i
- HeN-H
@ Ay © §-a-3 ©
& t y 4
Explain on the basis of formal charge, which ofthe following is a more appropriate structure for ©," ion
be f or fe=c-é]
DPP No. #9
(cy . 3 (A)
(ABC)
(a) All zero
(b) All have zero except single bonded oxygen (-1)
(All have zero except nitrogen (+1)
(@) Both single bonded O-atoms have (~1), N-atom has (+1) and double bonded O-atom has zero.
[e=c=e]
DPP No. 10
Resonating structures have different
(A) Atomic arrangements (B) Electronic arrangements
(C) Functional groups (©) Sigma bond
Resonance hybrid of nitrate ion is
(A) (8)
o-%
(D)
I, Onn= Rhee O-%
The correct order of C-N bond length in the given compounds is
P| CH,CN Q:HNCO R= CH,CONH,
(A)P>Q>R ()P=Q=R (C)R>Q>P (O)R>P>Q
Correct order of bond length is
(A) CO. > CO, > CO (B)CO, > CO >CO,* (C) CO > CO, > CO,* (D) None of these.
5. The strength of bonds by S~8, p—p, S—p overlap is generally in the order
(A) s-s>s-p>p-p (B) s-s>p-p>s-p (©) s-p>s-s>p-p (0) p-p>s-s>s-p
6. Which of the following atomic orbital overlappings are not allowed
(A)All (8) (i) (i) ii) (C) (i Gil) (v) (0) Gi) only
Indicate the wrong statement according to VBT
(A) A sigma bond has no free rotation about the inter-nuclear axis.
(B) p-orbitals always have only sidewise overlapping.
(C) s-orbitals never form x - bonds
(D) There can be more than one sigma bond between two atoms.
Which of the following overlaps is/are incorrect [assuming X-axis to be the internuclear axis]
(a) 2p, + 2p, > x (b) 2p, + 2p, 96 (© 2p, + 2p, > 2 (A) 18 + 2p, > x
(e) 2p, + 2p, > x () 1s+28 30
(A) ‘a & (8) bad
Cat (0)'e'&'e
DPP No. #10
3 «(C)
8." (BD)
Hybridization of orbitals of carbon in CH, is necessary to explain which of the following
(A) Equality of strength of all C-H bonds (8) Methane is non-polar
(©) Tetravalency of Carbon () Carbon has complete octet
2. In which of the following, 'N’ atom is sp? hybridised
(A) NH, (8) NH, (C) NH; (2) Noc!
432 4
3. The hybridization of carbon atoms in C, - C, single bond of HC = C-CH=CH, is
(A) sp* - sp’ (B) sp’ - sp (C) sp - sp’ (0) sp°- sp
4. In C,0,, the hybridization state of Carbon is :
(A) sp (B) sp? (©) sp* (D) Both sp and sp?
5. Shape of NH, is very similar to
(BF, (@) CH> (©) so, © cH,
6." Which starred carbon atom in the following molecules show sp’ hybridisation
(A) Cg SHO (8) CHg Coc! (©) (CHg)gN>O — (D) CHyCOCHySOOCzHs
z In how many of the following species, the central atoms have two lone pairs of electrons ?
XeF, Xer5- F,Se0,
XeF,* XeOF, ClOF,
IC SCI, OsF,
Match the following :
Column (I)
Species
(A) IBr,-
Column (II)
Characteristics of central atom
(p) sp°d?, 2 lone pairs
(q) sp°d, 1 lone pair
(r) sp3d?, 1 lone pair
(s) sp°d°, 2 lone pair
(t) sp°d, 3 lone pairs
DPP No. #11
3 8)
8, (A-1); (8-3); C-p);@-n).
DPP No. 12
Carbon atoms in C,(CN), are
(A) All sp-hybridised (6) All sp*-hybridised —_(C) All sp’-hybridised (0) sp and sp*-hybridised.
BF, + F > BF,
What is the hybridiation state of B in BF, and BF
(A) 5p, sp’ (8) sp’, sp’ (©) sp*, sp (©) sp°, sp'd
The hybridisation of P in phosphate ion (PO,») is the same as :
A) Lin ICe, (©) S in so. (C) Nin NO, (©) Sin So.
Specify the hybridisations of central atom in the following species respectively {N,-, NOCI, N,O}
(A) sp, sp*, SP (8) sp, sp, sp’ (C) sp*, sp, sp (O) sp* , sp? sp.
The correct order of increasing s character (in percentage) in the hybrid orbitals in below molecules / ions
is (assume all hybrid orbitals are exactly equivalent)
co. XeF, I NCI, BeCl, (9)
1 ul 1 IV Vv
() (p— bp > bp — bp
(ep = lone pair electrons, bp = bond pair electrons)
(B) Lone pair and double bond occupy equitorial position in trigonal bipyramidal structure.
(C) More electronegative atoms occupy axial position in trigonal bipyramidal structure.
() Bigger atoms occupy axial positions in trigonal bipyramidal structure
In which of the following species, one of bond angle is expected to be more than 120°.
(A) N,O (B)NO- (Cc) NO,* (D) XeF,*
Match the isostructural pairs :
(a) SF, () IF"
(b) PCI, (i) CIF
(c) ICl, (iii) SnCl-
(Ly (iv) CIF,
(e) Ic (v) CIF
() PCle (vi) XeF,
DPP No. #13
3. ©) 4. oO)
8 (aii) (beill) (civ) -v) (e-vi) (F).
Correct order of bond lenath is
(A) SO, > SO, > SO, (B) SO2->S02->S0,
(C) SO, > SO0> S02 (D) None of these.
Which of the following molecule contains shortest N-O bond ?
(A) NOF (B) NO,- (C) NO,- (0) NH,OH
How many types of bond length are there in SO, ?
(A) one (B) two (C) three (D) four
Select the correct order for bond angle.
(A) PH, < ASH, < NH, < SbH, (B) F,0 BCI, > BBr,
Select the correct order of bond angle of the following species
C10; BrO;,|05
(A) BrO, > 10; > CIO, (B) ClO, > BrO, > 10,
©) 10, >BrO, > ClO, () 10, ClO,
Which of the following order is/are correct about the bond angle.
(A) OF, PF. () KiF, < SF, < NH.
CO, anion has which of the following charcteristics
(A) Bonds of unequal length (8) sp? hybridisation of C atom
(©) Resonance stabilization (©) Same bond angles.
Compare bond angles in the following pairs
(a) F,0 and HO (b) NH, and PH, SO, and SO, (@) No, and NO>
DPP No. #15
1 A) 2
6* (ABD) 7’ (BCD)
8. (@)F,OPH, (¢)SO, NO
ee DPP No. 21
Choose the compounds of maximum and minimum ionic character from LiCl, RbCl, BeC!, and MgCl,
(A) LiCl and RbCI (B)RbClandBeCl, — (C) RbCland MgCl, —_(D) MgCl, and BeC,
2 An ion without pseudo-inert gas configuration is :
(A) Ag" (8) Ca* (©) Zn* (0) Fe
3. Correct order of increasing solubility in water of RbI, Cdl, and PbO, is
(A) PbO,, CdI,,RbI_ (8) RI, Cal, PLO, —(C) Cd, PbO, RBI. (D) PbO, RbI, Cl,
AQCI is colourless whereas AgI is yellow, because
(A) Ag* possesses 18 electrons in shell to screen the nuclear charge
(B) Ag’ shows pseudo inert gas configuration,
(©) Distortion of I- is more pronounced than CI-ion.
(D) Existence of d-d transition.
Arrange the following in decreasing order of melting point : BeCl,, MgCl,, CaCI, and BaCl,
(A) BeCl, > MgCl, > CaCl, > BaCl, (8) BaCl, > MgCl, > CaCl, > BeCl,
(©) BeCi, > CaCl; > MgCl, > BaCl, (D) BaCl, > CaCl, > MgCl, > BeCl,
Which among the following has maximum covalent character
(A) NaCl (8) MgCl, (C)AICI, (0) Cacl,
Which of the following statements is INCORRECT
(A) Agl is less soluble in water than AgF due to more polarisation of I in comparison to F~ ion.
(©) Lil is less soluble in water than LiF due to more polarisation of I- in comparison to F- ion.
(C) Colour of some compounds can also be explained on the basis of polarisation of anion
(0) The greater covalent character of AgCI as compared to NaCl can be explained on the basis of Fajan’s rule.
Answer the following question
(a) Among LiF and Lil, which has more covalent character ?
(©) Lil is soluble in water but LiF is not. Why ?
® ©
() 7.
(a) Electronegativity difference Li and iodine is less than Li and F. Thus, Lil is more covalent.
(©) Although Li is same in both the compounds yet difference in the size of F~ and I” is not same
F” is smaller than I" hence lattice energy of LiF is more than that of Lil. Similarly heat of hydration of F—
more than that of I”. But the decrease of L.E. from LiF to Lil is much more than the decrease in heat of
hydration from LiF to Lil. Hence solubility increases from LiF to Lil
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5822)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Coding DecodingDocument21 pagesCoding DecodingAditya VermaNo ratings yet
- Mathmatics Basic Sample Paper 10Document13 pagesMathmatics Basic Sample Paper 10Aditya VermaNo ratings yet
- SilicateDocument31 pagesSilicateAditya VermaNo ratings yet
- हिंदी व्याकरण वर्णमाला Class 12thDocument2 pagesहिंदी व्याकरण वर्णमाला Class 12thAditya VermaNo ratings yet
- Reagent ListDocument5 pagesReagent ListAditya VermaNo ratings yet
- CBSE Class 10 Social Science Agriculture MCQsDocument3 pagesCBSE Class 10 Social Science Agriculture MCQsAditya VermaNo ratings yet