Professional Documents
Culture Documents
The Parts of The Periodic Table
The Parts of The Periodic Table
Uploaded by
Danielle Loraine ElloOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Parts of The Periodic Table
The Parts of The Periodic Table
Uploaded by
Danielle Loraine ElloCopyright:
Available Formats
Atomic Numbers
1A 2A 3A 4A 5A 6A 7A 8A
(1) (2) (13) (14) (15) (16) (17) (18)
3B 4B 5B 6B 7B — 8B — 1B 2B
(3) (4) (5) (6) (7) (8) (9) (10) (11) (12)
1 1 2
2 3 4 5 6 7 8 9 10
3 11 12 13 14 15 16 17 18
4 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
5 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
6 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
7 87 88 89 104 105 106 107 108 109 110 111 112 — 114 — — — —
6 58 59 60 61 62 63 64 65 66 67 68 69 70 71
7 90 91 92 93 94 95 96 97 98 99 100 101 102 103
In the modern periodic table, the elements are listed in order of
increasing atomic number. The atomic number is the number of
protons in the nucleus of an atom. The number of protons define the
identity of an element (i.e., an element with 6 protons is a carbon atom,
no matter how many neutrons may be present). The number of protons
determines how many electrons surround the nucleus, and it is the
arrangement of these electrons that determines most of the chemical
behavior of an element.
In a periodic table arranged in order of increasing atomic number,
elements having similar chemical properties naturally line up in the
same column (group). For instance, all of the elements in Group 1A are
relatively soft metals, react violently with water, and form 1+ charges;
all of the elements in Group 8A are unreactive, monatomic gases at
room temperature, etc. In other words, there is a periodic repetition of
the properties of the chemical elements with increasing mass.
In the original periodic table published by Dimitri Mendeleev in 1869,
the elements were arranged according to increasing atomic mass — at
that time, the nucleus had not yet been discovered, and there was no
understanding at all of the interior structure of the atom, so atomic mass
was the only guide to use. Once the structure of the nucleus was
understood, it became clear that it was the atomic number that
governed the properties of the elements.
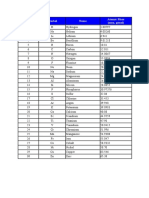
The Elements, sorted by Atomic Number
Atomic Atomic Mass
Symbol Name
Number (amu, g/mol)
1 H Hydrogen 1.00797
2 He Helium 4.00260
3 Li Lithium 6.941
4 Be Beryllium 9.01218
5 B Boron 10.81
6 C Carbon 12.011
7 N Nitrogen 14.0067
8 O Oxygen 15.9994
9 F Fluorine 18.998403
10 Ne Neon 20.179
11 Na Sodium 22.98977
12 Mg Magnesium 24.305
13 Al Aluminum 26.98154
14 Si Silicon 28.0855
15 P Phosphorus 30.97376
16 S Sulfur 32.06
17 Cl Chlorine 35.453
18 Ar Argon 39.948
19 K Potassium 39.0983
20 Ca Calcium 40.08
21 Sc Scandium 44.9559
22 Ti Titanium 47.90
23 V Vanadium 50.9415
24 Cr Chromium 51.996
25 Mn Manganese 54.9380
26 Fe Iron 55.847
27 Co Cobalt 58.9332
28 Ni Nickel 58.70
29 Cu Copper 63.546
30 Zn Zinc 65.38
31 Ga Gallium 69.72
32 Ge Germanium 72.59
33 As Arsenic 74.9216
34 Se Selenium 78.96
35 Br Bromine 79.904
36 Kr Krypton 83.80
37 Rb Rubidium 85.4678
38 Sr Strontium 87.62
39 Y Yttrium 88.9059
40 Zr Zirconium 91.22
41 Nb Niobium 92.9064
42 Mo Molybdenum 95.94
43 Tc Technetium (98)
44 Ru Ruthenium 101.07
45 Rh Rhodium 102.9055
46 Pd Palladium 106.4
47 Ag Silver 107.868
48 Cd Cadmium 112.41
49 In Indium 114.82
50 Sn Tin 118.69
51 Sb Antimony 121.75
52 Te Tellurium 127.60
53 I Iodine 126.9045
54 Xe Xenon 131.30
55 Cs Cesium 132.9054
56 Ba Barium 137.33
57 La Lanthanum 138.9055
58 Ce Cerium 140.12
59 Pr Praseodymium 140.9077
60 Nd Neodymium 144.24
61 Pm Promethium (145)
62 Sm Samarium 150.4
63 Eu Europium 151.96
64 Gd Gadolinium 157.25
65 Tb Terbium 158.9254
66 Dy Dysprosium 162.50
67 Ho Holmium 164.9304
68 Er Erbium 167.26
69 Tm Thulium 168.9342
70 Yb Ytterbium 173.04
71 Lu Lutetium 174.967
72 Hf Hafnium 178.49
73 Ta Tantalum 180.9479
74 W Tungsten 183.85
75 Re Rhenium 186.207
76 Os Osmium 190.2
77 Ir Iridium 192.22
78 Pt Platinum 195.09
79 Au Gold 196.9665
80 Hg Mercury 200.59
81 Tl Thallium 204.37
82 Pb Lead 207.2
83 Bi Bismuth 208.9804
84 Po Polonium (209)
85 At Astatine (210)
86 Rn Radon (222)
87 Fr Francium (223)
88 Ra Radium 226.0254
89 Ac Actinium 227.0278
90 Th Thorium 232.0381
91 Pa Protactinium 231.0359
92 U Uranium 238.029
93 Np Neptunium 237.0482
94 Pu Plutonium (242)
95 Am Americium (243)
96 Cm Curium (247)
97 Bk Berkelium (247)
98 Cf Californium (251)
99 Es Einsteinium (252)
100 Fm Fermium (257)
101 Md Mendelevium (258)
102 No Nobelium (250)
103 Lr Lawrencium (260)
104 Rf Rutherfordium (261)
105 Db Dubnium (262)
106 Sg Seaborgium (263)
107 Bh Bohrium (262)
108 Hs Hassium (255)
109 Mt Meitnerium (256)
110 Ds Darmstadtium (269)
111 Rg Roentgenium (272)
112 Uub Ununbiium (277)
113 — —— ———
114 Uuq Ununquadium
You might also like
- Full Download Book Chemistry Chemical Reactivity PDFDocument41 pagesFull Download Book Chemistry Chemical Reactivity PDFmaureen.fine870100% (21)
- Sk025 - Chemistry 2 Student Version - 230414 - 085331Document197 pagesSk025 - Chemistry 2 Student Version - 230414 - 085331Maiha HarunaNo ratings yet
- Iso 17225 2 2021Document10 pagesIso 17225 2 2021Sabin CorchesNo ratings yet
- Gravity Filtration and Vacuum Filtration of Calcium Carbonate (CaCO3) SlurryDocument6 pagesGravity Filtration and Vacuum Filtration of Calcium Carbonate (CaCO3) SlurryElajah ZaragozaNo ratings yet
- Fundamental University Physics, Volume 1 (Mechanics) - Alonso, FinnDocument487 pagesFundamental University Physics, Volume 1 (Mechanics) - Alonso, Finnphoneee89% (18)
- BS en 1393 1997Document18 pagesBS en 1393 1997karthikkumar T RNo ratings yet
- Statins Stimulate Atherosclerosis and Heart Failure Pharmacological MechanismsDocument12 pagesStatins Stimulate Atherosclerosis and Heart Failure Pharmacological MechanismsSonata DaniatiekNo ratings yet
- Assignment 2 Database Mohammed Shalabi 201810028Document2 pagesAssignment 2 Database Mohammed Shalabi 201810028Mohammed ShalabiNo ratings yet
- Electronic Liquid Weigher 2005Document38 pagesElectronic Liquid Weigher 2005Gheorghe Claudiu100% (1)
- Atomic Weights 2013Document8 pagesAtomic Weights 2013LuisCastilloNo ratings yet
- Atomic MassesDocument5 pagesAtomic MassesJesús CastilloNo ratings yet
- 2019 Atomic WeightsDocument7 pages2019 Atomic WeightsMirella PopescuNo ratings yet
- Soalan Test 3 Semester 2 Sesi 2022 - 2023Document8 pagesSoalan Test 3 Semester 2 Sesi 2022 - 2023y15zrcdu1823No ratings yet
- Atomic Number Symbol Name Atomic Mass (Amu, G/mol)Document1 pageAtomic Number Symbol Name Atomic Mass (Amu, G/mol)fiza khanNo ratings yet
- Book 3Document3 pagesBook 3ayushdhardiwan27No ratings yet
- Chembuddy AnswerDocument67 pagesChembuddy AnswerNATASHA 'ALIA BINTI ZULKIFLINo ratings yet
- Guyp SK015 22-23Document7 pagesGuyp SK015 22-23Farena LazimNo ratings yet
- Learning Outcomes N FormulasDocument6 pagesLearning Outcomes N FormulaskalvenaNo ratings yet
- 4 Properties of Materials: Rs 12 Atomic and Molecular WeightsDocument4 pages4 Properties of Materials: Rs 12 Atomic and Molecular WeightsDilnesa EjiguNo ratings yet
- Ques & Ans Pka KMLDocument21 pagesQues & Ans Pka KMLMuganeshNo ratings yet
- Molar Mass ListDocument1 pageMolar Mass ListAmylia NatashaNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Relative Atomic Mass ConstantDocument2 pagesRelative Atomic Mass ConstantKhairul ZainuddinNo ratings yet
- Fundamental University Physics, Volume 3 (Quantum and Statistical Physics) - Alonso, FinnDocument611 pagesFundamental University Physics, Volume 3 (Quantum and Statistical Physics) - Alonso, Finnphoneee100% (7)
- Pra PSPM SK025 Set 3Document9 pagesPra PSPM SK025 Set 3catrineNo ratings yet
- pp1802fCOBALTO OOKKDocument52 pagespp1802fCOBALTO OOKKjsalamanca calderonNo ratings yet
- pp1802r Tellurium PDFDocument40 pagespp1802r Tellurium PDFDouglas AndersonNo ratings yet
- Rare Earth Elements USGSDocument44 pagesRare Earth Elements USGSMiguel HerreraNo ratings yet
- GENIUS Pre PSPM DK024 2223Document8 pagesGENIUS Pre PSPM DK024 2223isfaNo ratings yet
- 4 RamDocument1 page4 RamElda AldaNo ratings yet
- Chem-01-Atoms ElectronicStructure Lecture NotesDocument36 pagesChem-01-Atoms ElectronicStructure Lecture NotesSaraNo ratings yet
- Relative Atomic MassDocument1 pageRelative Atomic MassFATIN FARHANAH BINTI HALIDIN MoeNo ratings yet
- Unit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1Document7 pagesUnit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1aNo ratings yet
- pp1802d PDFDocument30 pagespp1802d PDFdicky saputraNo ratings yet
- History and Subatomic Particle Review Take Two KEYDocument5 pagesHistory and Subatomic Particle Review Take Two KEYAlliya DaymonNo ratings yet
- Antimony: Professional Paper 1802-CDocument30 pagesAntimony: Professional Paper 1802-CAndrew ChungNo ratings yet
- Pra PSPM KMS DK024 Sesi 22 - 23Document9 pagesPra PSPM KMS DK024 Sesi 22 - 23dilaylaNo ratings yet
- Problems in General Physics (Original)Document402 pagesProblems in General Physics (Original)Lehansh JaatNo ratings yet
- Abundance of Elements in EarthDocument6 pagesAbundance of Elements in EarthtaxxolNo ratings yet
- Titanium: Professional Paper 1802-TDocument16 pagesTitanium: Professional Paper 1802-THolmes's ApprenticeNo ratings yet
- Basic Atomic Structure WorksheetDocument4 pagesBasic Atomic Structure WorksheetTrisha GolesNo ratings yet
- Gallium: Professional Paper 1802-HDocument48 pagesGallium: Professional Paper 1802-HPaula GeorgianaNo ratings yet
- Jadual Berkala UnsurDocument1 pageJadual Berkala Unsurkhadijah madhadzirNo ratings yet
- 1 Q Ready Form PSPM 1 Sk015Document14 pages1 Q Ready Form PSPM 1 Sk015WAN NUR ALEEYA TASNIM BINTI WAN MOHAMED HAZMAN MoeNo ratings yet
- Fundamental University Physics. (Second Edition), Volume 2 (Fields and Waves) - Alonso, FinnDocument665 pagesFundamental University Physics. (Second Edition), Volume 2 (Fields and Waves) - Alonso, Finnphoneee100% (16)
- Chemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDF ScribdDocument67 pagesChemistry Chemical Reactivity 11E 11Th Edition John C Kotz Full Chapter PDF Scribdjessica.carter247100% (10)
- Atomic Struct 2ADocument2 pagesAtomic Struct 2AVictoria FuenmayorNo ratings yet
- Periodic Trends AssignmentDocument4 pagesPeriodic Trends AssignmentFarhan HabibzaiNo ratings yet
- The Parts of The Periodic TableDocument4 pagesThe Parts of The Periodic TableS.packialakshmiNo ratings yet
- Ebook PDF Chemistry A Molecular Approach Third 3rd Canadian Edition PDFDocument41 pagesEbook PDF Chemistry A Molecular Approach Third 3rd Canadian Edition PDFkathleen.williams876100% (37)
- Read Each Question Underline The Key Words. Then, Scan The Following Table of Atomic Weights To Find The Correct Answer. Work QuicklyDocument1 pageRead Each Question Underline The Key Words. Then, Scan The Following Table of Atomic Weights To Find The Correct Answer. Work QuicklyRasyidahNo ratings yet
- Ebook Chemistry Chemical Reactivity 11E PDF Full Chapter PDFDocument67 pagesEbook Chemistry Chemical Reactivity 11E PDF Full Chapter PDFsandy.wicker653100% (33)
- Periodic TableDocument1 pagePeriodic Tablemarjorie Villanueva100% (1)
- Specific Heat of MetalDocument6 pagesSpecific Heat of MetalJohn ChenNo ratings yet
- The Main Postulates of Dalton's Atomic Theory AreDocument6 pagesThe Main Postulates of Dalton's Atomic Theory ArePriyanshu PalNo ratings yet
- Periodic Table A LevelDocument24 pagesPeriodic Table A Leveltechibu252No ratings yet
- Set 3Document5 pagesSet 3MOHAMAD AIMAN MOHAMAD ZAKINo ratings yet
- Aug 20Document7 pagesAug 20Renz D' MadNo ratings yet
- Periodic TableDocument2 pagesPeriodic TableSaheed KalliyadanpoyilNo ratings yet
- Rata-Rata Kandungan Unsur Pada Kerak BumiDocument4 pagesRata-Rata Kandungan Unsur Pada Kerak BumiolgaNo ratings yet
- 1986 Bookmatter HeatTransferDocument69 pages1986 Bookmatter HeatTransferYng Dmb Broke GuyNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- Tabla Periódica PDFDocument1 pageTabla Periódica PDFVicente Oliva VarelaNo ratings yet
- Che 1000 Periodic Table 2020 Academic YearDocument1 pageChe 1000 Periodic Table 2020 Academic YearMwinde SimbezaNo ratings yet
- Textbook Ebook Chemistry Chemical Reactivity 10Th Edition John C Kotz All Chapter PDFDocument43 pagesTextbook Ebook Chemistry Chemical Reactivity 10Th Edition John C Kotz All Chapter PDFdebra.lee709100% (7)
- Anodizing Ver 1Document14 pagesAnodizing Ver 1aditya_welekarNo ratings yet
- Dynamics of Protein and Mixed Protein Rsurfactant Adsorption Layers at The Water Rfluid InterfaceDocument44 pagesDynamics of Protein and Mixed Protein Rsurfactant Adsorption Layers at The Water Rfluid InterfaceqaeszNo ratings yet
- Prof Dr. Zhari - Halal Pharma IngredientsDocument82 pagesProf Dr. Zhari - Halal Pharma IngredientsHisyamuddin Kamarudin100% (1)
- Is 6396 - 2000 De-CarburisationDocument10 pagesIs 6396 - 2000 De-CarburisationDheeraj Chavan100% (1)
- Project Report PDFDocument30 pagesProject Report PDFsaateh100% (3)
- Physics Section - I (Single Correct Choice Type)Document18 pagesPhysics Section - I (Single Correct Choice Type)FTRIBUTO CLOZZONo ratings yet
- Alfa Laval AQ4: Gasketed Plate Heat Exchanger For HVAC ApplicationsDocument2 pagesAlfa Laval AQ4: Gasketed Plate Heat Exchanger For HVAC ApplicationsNacer KhaosNo ratings yet
- MCAT Full Length3Document75 pagesMCAT Full Length3AliNo ratings yet
- Modeling of Lithium-Ion Battery For Charging/Discharging Characteristics Based On Circuit ModelDocument10 pagesModeling of Lithium-Ion Battery For Charging/Discharging Characteristics Based On Circuit ModelJeannot MpianaNo ratings yet
- Alkanes ClassDocument27 pagesAlkanes ClassRyan JamesNo ratings yet
- "BEST": Biochemical Engineering Simulation Technology: NRE /MPDocument23 pages"BEST": Biochemical Engineering Simulation Technology: NRE /MPSachini SarthchandraNo ratings yet
- Biochem Lab Report - Habaradas Exp#3Document9 pagesBiochem Lab Report - Habaradas Exp#3Acel Anne Espino HabaradasNo ratings yet
- NIST Typefaces For Symbols in Scientific ManuscriptsDocument2 pagesNIST Typefaces For Symbols in Scientific ManuscriptsAdromarNo ratings yet
- Identification of Synthetic Cannabinoids 5F-Adb and Xlr-11 in Seized Sample in Penang, MalaysiaDocument6 pagesIdentification of Synthetic Cannabinoids 5F-Adb and Xlr-11 in Seized Sample in Penang, Malaysia049 VIMAL CHANDERNo ratings yet
- Alkana-1Document61 pagesAlkana-1ayundhaNo ratings yet
- 2010 10 05-Guentner GHN Manual2009 ENDocument45 pages2010 10 05-Guentner GHN Manual2009 ENThanh Trung Nguyen Phan100% (1)
- Last Exams Questions Papers 2018 Dr. Gopika PDFDocument111 pagesLast Exams Questions Papers 2018 Dr. Gopika PDFrhea100% (1)
- Catalogo Juntas MicartaDocument32 pagesCatalogo Juntas MicartaMemoNo ratings yet
- BSB - Series New PDFDocument47 pagesBSB - Series New PDFSenghy MaoNo ratings yet
- ITP 4 NOV PresentationR1-Dr. ArvindDocument17 pagesITP 4 NOV PresentationR1-Dr. ArvindAfuriata ZebuaNo ratings yet
- Chinese Herbal Medicines: Pei-Ling Tang, Er-Wei Hao, Jia-Gang Deng, Xiao-Tao Hou, Zuo-Hui Zhang, Jin-Ling XieDocument6 pagesChinese Herbal Medicines: Pei-Ling Tang, Er-Wei Hao, Jia-Gang Deng, Xiao-Tao Hou, Zuo-Hui Zhang, Jin-Ling Xiemetha anandaNo ratings yet
- Cocoa ButterDocument4 pagesCocoa ButterelisiaNo ratings yet
- Tranexamic AcidDocument5 pagesTranexamic Acidiabureid7460No ratings yet
- Cytopreparatory Technique: Ama AfrahDocument49 pagesCytopreparatory Technique: Ama Afrahreuben kwotaNo ratings yet