Professional Documents
Culture Documents
Psa - Prostate - Juscheck
Psa - Prostate - Juscheck
Uploaded by
Vivian Gabriela Rojas Espinoza0 ratings0% found this document useful (0 votes)
7 views1 pageThis document provides information on a rapid test cassette for detecting prostate specific antigen (PSA) levels in whole blood, serum, or plasma. PSA is produced by the prostate gland and elevated levels can indicate prostate cancer. The test cassette uses gold conjugates and anti-PSA antibodies to detect total PSA. It has a detection limit of 3 ng/ml PSA and a reference value of 10 ng/ml for further clinical evaluation. The test procedure involves adding a blood, serum, or plasma specimen and buffer to the cassette for results within 10 minutes. Performance was validated against a commercial PSA ELISA test, with 99% correlation for sensitivity and specificity.
Original Description:
INSERTO PSA
Original Title
PSA_PROSTATE_ JUSCHECK
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides information on a rapid test cassette for detecting prostate specific antigen (PSA) levels in whole blood, serum, or plasma. PSA is produced by the prostate gland and elevated levels can indicate prostate cancer. The test cassette uses gold conjugates and anti-PSA antibodies to detect total PSA. It has a detection limit of 3 ng/ml PSA and a reference value of 10 ng/ml for further clinical evaluation. The test procedure involves adding a blood, serum, or plasma specimen and buffer to the cassette for results within 10 minutes. Performance was validated against a commercial PSA ELISA test, with 99% correlation for sensitivity and specificity.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
7 views1 pagePsa - Prostate - Juscheck
Psa - Prostate - Juscheck
Uploaded by
Vivian Gabriela Rojas EspinozaThis document provides information on a rapid test cassette for detecting prostate specific antigen (PSA) levels in whole blood, serum, or plasma. PSA is produced by the prostate gland and elevated levels can indicate prostate cancer. The test cassette uses gold conjugates and anti-PSA antibodies to detect total PSA. It has a detection limit of 3 ng/ml PSA and a reference value of 10 ng/ml for further clinical evaluation. The test procedure involves adding a blood, serum, or plasma specimen and buffer to the cassette for results within 10 minutes. Performance was validated against a commercial PSA ELISA test, with 99% correlation for sensitivity and specificity.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
PSA Rapid Test Cassette • Separate serum or plasma from blood as soon as possible to avoid hemolysis.
rom blood as soon as possible to avoid hemolysis. Use only 【LIMITATIONS】
clear non-hemolyzed specimens. 1. The PSA Rapid Test Cassette (Whole Blood/Serum/Plasma) is for in vitro diagnostic
(Whole Blood /Serum/Plasma) • Testing should be performed immediately after the specimens have been collected. Do not use only. This test should be used for the detection of PSA in whole blood, serum or plasma
leave the specimens at room temperature for prolonged periods. Serum and plasma specimen.
Package Insert specimens may be stored at 2-8°C for up to 3 days. For long term storage, specimens 2. The PSA Rapid Test Cassette (Whole blood /Serum /Plasma) will only indicate the semi
A rapid test for the qualitative detection of Prostate Specific Antigen (PSA) in whole blood, should be kept below -20°C. Whole blood collected by venipuncture should be stored at quantitative level of PSA in the specimen and should not be used as the sole criteria for the
serum or plasma. 2-8°C if the test is to be run within 2 days of collection. Do not freeze whole blood diagnosis of Prostate Cancer.
For professional in vitro diagnostic use only. specimens. Whole blood collected by fingerstick should be tested immediately. 3. A significant numbers of patients with BPH (more that 15%) and less than 1% of healthy
【INTENDED USE】 • Bring specimens to room temperature prior to testing. Frozen specimens must be individuals have elevated PSA. Even if the test results are positive, further clinical
The PSA Prostate Specific Antigen Semi-Quantitative Rapid Test Cassette (Whole Blood completely thawed and mixed well prior to testing. Specimens should not be frozen and evaluation should be considered with other clinical information available to the physician.
/Serum /Plasma) is a rapid chromatographic immunoassay for semi-quantitative detection of thawed repeatedly. 4. PSA levels may be unreliable in patients who receive hormone therapy or prostate gland
Prostate Specific Antigen in whole blood, serum or plasma. • If specimens are to be shipped, they should be packed in compliance with local regulations manipulation.
【SUMMARY】 covering the transportation of etiologic agents. 5. High concentrations of PSA may produce a dose hook effect, resulting in false negative
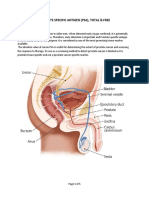
Prostate specific antigen (PSA) is produced by prostate glandular and endothelial cells. It is a 【MATERIALS】 results. High dose hook effect has not been observed with this test up to 30,000ng/ml PSA.
single chain glycoprotein with a molecular weight of approximate 34 kDa. 1 PSA exists in three Materials provided 【EXPECTED VALUES】
major forms circulating in the serum. These forms are free PSA, PSA bound to α1 – Test cassettes Droppers Buffer Package insert The minimum indicative level of PSA for Prostate Cancer is generally agreed to be 3ng/ml and
Antichymotrypsin (PSA-ACT) and PSA complexed with α2–macroglobulin (PSA-MG).2 Materials required but not provided the warning level is generally agreed to be 10ng/ml.3 The PSA Rapid Test Cassette (Whole
PSA has been detected in various tissues of the male urogenital system but only prostate Specimen collection containers Centrifuge blood /Serum /Plasma) has been compared with a leading commercial PSA ELISA test. The
glandular and endothelial cells secrete it. The PSA level in serum of healthy men is between Lancets (for fingerstick whole blood only) Timer correlation between these two results is more than 99%.
0.1 ng/mL and 2.6 ng/mL. It can be elevated in malignant conditions such as prostate cancer, Heparinized capillary tubes and dispensing bulb (for fingerstick whole blood only) 【PERFORMANCE CHARACTERISTICS】
and in benign condition such as benign prostatic hyperplasia and prostatitis. A PSA level of 3 【DIRECTIONS FOR USE】 Sensitivity and Specificity
to 10ng/ml is considered to be in the “gray-zone” and levels above 10ng/ml are highly Allow the test, specimen, buffer and/or controls to reach room temperature (15-30°C) The PSA Rapid Test Cassette (Whole blood /Serum /Plasma) has been tested with a leading
indicative of cancer.3 Patients with PSA values between 3-10ng/ml should undergo further prior to testing. commercial PSA EIA Test using clinical samples.
analysis of the prostate by biopsy. 1. Bring the pouch to room temperature before opening it. Remove the test cassette from the
The prostate specific antigen test is the most valuable tool available for the diagnosis of early Method EIA
sealed pouch and use it as soon as possible. Twist off the tab of the buffer vial without Total Results
prostate cancer. Many studies have confirmed that the presence of PSA is the most useful and Results Positive Negative
squeezing. Then place it on a clean and level surface. PSA Rapid
meaningful tumor marker known for prostate cancer and prostate infection of Benign Prostatic Positive 205 3 208
2. Place the cassette on a clean and level surface. Test Cassette
Hyperplasia (BPH).4 Negative 2 351 353
For Serum,Plasma or Venipuncture Whole Blood specimens:
The PSA Prostate Specific Antigen Semi-Quantitative Rapid Test Device (Whole blood /Serum Total Results 207 354 561
• Hold the dropper vertically and transfer 1 drop of serum or plasma (approximately 40L)
/Plasma) utilizes a combination of colloidal gold conjugate and anti-PSA antibodies to Relative Sensitivity: 99.0% (95%CI:*96.6%-99.9%) *Confidence Intervals
or 2 drops of venipuncture whole blood (approximately 80l) to the specimen well (S) of
selectively detect total PSA in whole blood, serum or plasma. The test has a cut-off value of Relative Specificity: 99.2% (95%CI:*97.5%-99.8%)
test cassette, then add 1 drop of buffer (approximately 40L) and start the timer. See
3ng/ml and a reference value of 10ng/ml. Overall accuracy: 99.1% (95%CI:*97.9%-99.7%)
illustration below.
【PRINCIPLE】 Precision
For Fingerstick Whole Blood specimen:
The PSA Rapid Test Cassette (Whole Blood /Serum /Plasma) is a semi-quantitative,
• To use a capillary tube: Fill the capillary tube and transfer approximately 80L of Intra-Assay
membrane based immunoassay for the detection of PSA in whole blood, serum or plasma. Assays were carried out to determine assay reproducibility using replicates of 10 tests in three
fingerstick whole blood specimen to the specimen area of test cassette, then add 1
The membrane is pre-coated with PSA antibodies on the test line region. During testing, the
drop of buffer (approximately 40 L) and start the timer. See illustration below. different runs for each of three lots using PSA specimen levels at 0ng/ml, 2ng/ml, 3ng/ml,
specimen reacts with the particle coated with anti-PSA antibody. The mixture migrates upward
• To use hanging drops: Allow 2 hanging drops of fingerstick whole blood specimen 10ng/ml and 20ng/ml. The specimens were correctly identified >99% of the time.
on the membrane chromatographically by capillary action to react with anti-PSA antibodies on
(approximately 80 L) to fall into the specimen area of test cassette, then add 1 drop of Inter-Assay
the membrane and generate a colored line. A test line (T) intensity weaker than the reference
line (R) indicates that the PSA level in the specimen is between 3-10ng/ml. A test line (T) buffer (approximately 40 L) and start the timer. See illustration below. Between-run precision has been determined by using the five PSA specimen levels at 0ng/ml,
3. Wait for the colored line(s) to appear*. Read results at 5 minutes. Do not interpret the 2 ng/ml, 3 ng/ml, 10 ng/ml and 20 ng/ml of PSA in 3 independent assays. Three different lots
intensity equal or close to the reference line (R) indicates that the PSA level in the specimen is of the PSA Rapid Test Cassette (Whole Blood /Serum /Plasma) have been tested using these
approximately 10ng/ml. A test line (T) intensity stronger than the reference line (R) indicates result after 10 minutes.
*Note: if migration is not observed in the result window after 30 seconds, add one or two specimens. The specimens were correctly identified >99% of the time.
that the PSA level in the specimen is above 10ng/ml. To serve as a procedural control, a Interfering Substances
colored line will always appear in the control line region (C) indicating that proper volume of extra drops of buffer.
The following substances do not interfere with the test results at the indicated concentrations:
specimen has been added and membrane wicking has occurred. Ascorbic Acid at 200mg/l, Hemoglobin at 10g/l, Triglyceride at 30g/l, Bilirubin at 1,000mg/dl,
【REAGENTS】 Uric Acid at 200mg/l.
The cassette contains PSA monoclonal antibody particles and PSA monoclonal antibody
coated on the membrane. 【BIBLIOGRAPHY】
【PRECAUTIONS】 1. Wang MC, Valenzuela LA, Murphy GP, et al., Purification of human prostate specificity
Please read all the information in this package insert before performing the test. antigen. Invest Urol 1979; 17: 159-163.
1. For professional in vitro diagnostic use only. Do not use after the expiration date. 2. Christens A, Laurell CB, Lilja H. Enzymatic activity of prostate –specific antigen and its
2. The test should remain in the sealed pouch or closed canister until ready to use. reaction with extracellular serine proteinase Inhibitors. Eur J Biochem 1990;
3. Do not eat, drink or smoke in the area where the specimens or kits are handled. 194:755-763.
4. Do not use the test if the pouch is damaged. 3. Catalona WJ, Southurick PC, Slawin KM, et al., Comparison of percent free PSA, PSA
5. All specimens should be considered potentially hazardous and handled in the same density and age-specific PSA cut-offs for prostate cancer detection and staging. Urology
manner as an infectious agent. 2000 Aug 1:56(2):255-60.
6. Wear protective clothing such as laboratory coats, disposable gloves or eye protection 4. Vancangh PJ, De Nayer P, Sauvage P, et al., Free to total prostate-specific antigen (PSA)
when specimens are being tested. ratio is superior to total PSA in differentially benign prostate hypertrophy from prostate
7. The used test should be discarded according to local regulations. cancer. Prostate Supplement, 1996, 7:30-34.
8. Humidity and temperature can adversely affect results. Index of Symbols
【STORAGE AND STABILITY】
Attention, see instructions Authorized
Store as packaged at room temperature or refrigerated (2-30°C). The test is stable through the Tests per kit
expiration date printed on the sealed pouch or label of the closed canister. The test must for use Representative
remain in the sealed pouch or closed canister until use. DO NOT FREEZE. Do not use beyond For in vitro
the expiration date. Use by Do not reuse
diagnostic use only
【SPECIMEN COLLECTION AND PREPARATION】 【INTERPRETATION OF RESULTS】
• The PSA Rapid Test Cassette (Whole blood /Serum /Plasma) can be performed using (Please refer to the illustration above) Store between 2-30°C Lot Number REF Catalog #
whole blood (from venipuncture or fingerstick) serum or plasma. POSITIVE:* Three distinct colored lines appear.
• To collect Fingerstick Whole Blood specimens: • A test line (T) intensity weaker than the reference line (R) indicates a PSA level between Do not use if package is
• Wash the patient’s hand with soap and warm water or clean with an alcohol swab. Allow 3-10 ng/ml.
damaged
to dry. • A test line (T) intensity equal or close to the reference line (R) indicates a PSA level of
• Massage the hand without touching the puncture site by rubbing down the hand towards approximately 10ng/ml.
the fingertip of the middle or ring finger. • A test line (T) intensity stronger than the reference line (R) indicates a PSA level more
• Puncture the skin with a sterile lancet. Wipe away the first sign of blood. than 10ng/ml.
• Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the NEGATIVE: Color lines appear in both the control (C) and reference (R) regions. No
puncture site. apparent colored line appears in the test line region (T). This indicates a PSA level below
• Add the Fingerstick Whole Blood specimen to the test by using a capillary tube: 3ng/ml.
• Touch the end of the capillary tube to the blood until filled to approximately 80 L. INVALID: Control line (C) or reference line (R) fails to appear. Insufficient specimen
Avoid air bubbles. volume or incorrect procedural techniques are the most likely reasons for control line failure. Number: 145601200
• Place the bulb onto the top end of the capillary tube, then squeeze the bulb to Review the procedure and repeat the test with a new test cassette. If the problem persists, Effective date: 2017-03-03
dispense the whole blood to the specimen area of the test cassette. discontinue using the test kit immediately and contact your local distributor.
• Add the Fingerstick Whole Blood specimen to the test by using hanging drops: 【QUALITY CONTROL】
• Position the patient’s finger so that the drop of blood is just above the specimen area A procedural control is included in the test. The appearance of colored lines in the control line
of the test cassette. region (C) and reference line region (R) is considered a procedural control. It confirms
• Allow 2 hanging drops of fingerstick whole blood to fall into the center of the specimen sufficient specimen volume, adequate membrane wicking and correct procedural technique.
area on the test cassette, or move the patient’s finger so that the hanging drop Control standards are not supplied with this kit; however, it is recommended that positive and
touches the center of the specimen area. Avoid touching the finger directly to the negative controls be tested as a good laboratory practice to confirm the test procedure and to
specimen area. verify proper test performance.
You might also like
- 1128500P WPC2000 Manual Extranet PDFDocument250 pages1128500P WPC2000 Manual Extranet PDFjuan estradaNo ratings yet
- Sta. Rosa Community Hospital, Sta. Rosa City, Laguna: Actual DeliveryDocument1 pageSta. Rosa Community Hospital, Sta. Rosa City, Laguna: Actual DeliveryAnastasya Gishella RorongNo ratings yet
- PDF Thors Power Program Load Calculator Microsoft Excelgsheet PDFDocument4 pagesPDF Thors Power Program Load Calculator Microsoft Excelgsheet PDFSxf KtmNo ratings yet
- Free Psa PDFDocument4 pagesFree Psa PDFJimboreanu György PaulaNo ratings yet
- TPSA-ML Flex InsertDocument16 pagesTPSA-ML Flex InsertisitalamasbonitaNo ratings yet
- Prostrate Specific Antigen-1Document23 pagesProstrate Specific Antigen-1Mayowa Gbotesho100% (1)
- 1 AparnaDocument4 pages1 AparnaKie Amelia SuwantoNo ratings yet
- Cardiac Troponin IDocument1 pageCardiac Troponin IPABRIK SEPULUHNo ratings yet
- Yuan 1992Document5 pagesYuan 1992Residen Urologi RSCMNo ratings yet
- Urine-Based Tests: UrinalysisDocument31 pagesUrine-Based Tests: Urinalysisshuvam sanatiNo ratings yet
- Finals 1Document20 pagesFinals 1Jey TeeNo ratings yet
- Prostate Specific Antigen (PSA) : Enzyme Immunoassay Test Kit Catalog Number: 10109Document4 pagesProstate Specific Antigen (PSA) : Enzyme Immunoassay Test Kit Catalog Number: 10109yousrazeidan19790% (1)
- My Final SeminarDocument40 pagesMy Final SeminartimiiniyiiNo ratings yet
- PSA Elisa: Specimen Collection and PreparationDocument3 pagesPSA Elisa: Specimen Collection and PreparationAsesor Bioquímico de Licitaciones BiotecNo ratings yet
- Insert - Elecsys Total PSA - Ms - 04641655190.V13.EnDocument5 pagesInsert - Elecsys Total PSA - Ms - 04641655190.V13.Entawfiq MohammadNo ratings yet
- 03 ON 2020 Prostate WBDocument43 pages03 ON 2020 Prostate WBabdullahNo ratings yet
- Laprak Imser HBsAGDocument6 pagesLaprak Imser HBsAGashaarul97No ratings yet
- PsaDocument4 pagesPsaEddy TubónNo ratings yet
- PSA Pathway: Patient Requests A PSA TestDocument1 pagePSA Pathway: Patient Requests A PSA Testaveen rasulNo ratings yet
- Role of Tumour Marker in UrologyDocument5 pagesRole of Tumour Marker in UrologyAung Ko HtetNo ratings yet
- Hoch Meister LDocument2 pagesHoch Meister LMuhd Idris Aizat AdamNo ratings yet
- Dr. Jad Alsmadi, MD., Assistant Professor, Faculty of Medicine, The Hashemite UniversityDocument15 pagesDr. Jad Alsmadi, MD., Assistant Professor, Faculty of Medicine, The Hashemite UniversityAnas HamadNo ratings yet
- IFU 1110001731 Fastep D-SYP-42 CE英文说明书 041619Document1 pageIFU 1110001731 Fastep D-SYP-42 CE英文说明书 041619BPG ServiceNo ratings yet
- Free PSADocument4 pagesFree PSANIGHT tubeNo ratings yet
- Immulite 1000Document12 pagesImmulite 1000Syazmin KhairuddinNo ratings yet
- NG/ML Benign: Prostate of and ProstateDocument4 pagesNG/ML Benign: Prostate of and ProstateSubhasis PandaNo ratings yet
- BPH - PsaDocument1 pageBPH - PsaDeshan AdikariNo ratings yet
- 30 - Evaluation of Prostate-Specific Antigen (PSA) Membrane Test Assays For The Forensic Identification of Seminal Fluid PDFDocument4 pages30 - Evaluation of Prostate-Specific Antigen (PSA) Membrane Test Assays For The Forensic Identification of Seminal Fluid PDFMN IrshadNo ratings yet
- Prostate Cancer Screening With Prostate-Specific Antigen (PSA) Test: A Clinical Practice GuidelineDocument12 pagesProstate Cancer Screening With Prostate-Specific Antigen (PSA) Test: A Clinical Practice GuidelinePatriana Puspaningrat Anak AgungNo ratings yet
- Prostate CancerDocument4 pagesProstate CancerNadia SalwaniNo ratings yet
- (TPSA) : Summary and Explanation PrincipleDocument7 pages(TPSA) : Summary and Explanation PrinciplecassNo ratings yet
- 158 Iajps158012020Document4 pages158 Iajps158012020Muhammad Irfan AnwarNo ratings yet
- Serum Free Prostate Specific Antigen in Indian Female Breast Cancer Patients 150 154Document5 pagesSerum Free Prostate Specific Antigen in Indian Female Breast Cancer Patients 150 154Pavithra VNo ratings yet
- H322-Cea Elisa-2.0-Ht-1.0Document2 pagesH322-Cea Elisa-2.0-Ht-1.0Frank DelgadoNo ratings yet
- Psa PresentationDocument38 pagesPsa PresentationPavithra VNo ratings yet
- Diagnosis of Prostate CancerDocument8 pagesDiagnosis of Prostate CancerAntonio asdNo ratings yet
- Pa29.4Document15 pagesPa29.4mathewsymssNo ratings yet
- PSA Testing For Prostate Cancer in Asymptomatic Men: Information For Health PractitionersDocument4 pagesPSA Testing For Prostate Cancer in Asymptomatic Men: Information For Health PractitionersDalibor CetojevicNo ratings yet
- Free Prostate-Specific Antigen (PSA)Document15 pagesFree Prostate-Specific Antigen (PSA)OngNo ratings yet
- Babaian 2001Document4 pagesBabaian 2001Benny KurniawanNo ratings yet
- Screening Prostate Web AlgorithmDocument5 pagesScreening Prostate Web AlgorithmFernanda OliveiraNo ratings yet
- Prostat Tumor MarkerDocument20 pagesProstat Tumor MarkerChristian SihiteNo ratings yet
- Articulo - PsaDocument9 pagesArticulo - PsaGrace Pinto AlvarezNo ratings yet
- HCG Strips 2017Document3 pagesHCG Strips 2017Enrique SaLoNo ratings yet
- Prostate Cancer Clinical Pathway - July 2020Document4 pagesProstate Cancer Clinical Pathway - July 2020VincentEguzoNo ratings yet
- Prostate Specific Antigen Total FreeDocument5 pagesProstate Specific Antigen Total Freeडा. सत्यदेव त्यागी आर्यNo ratings yet
- IFU Pruebas Embarazo Placa MonlabtestDocument4 pagesIFU Pruebas Embarazo Placa MonlabtestTurner TenneyNo ratings yet
- C86001 13.04.04.068405 iFlash-Free PSA CLIA V10.0 2020-04-07Document5 pagesC86001 13.04.04.068405 iFlash-Free PSA CLIA V10.0 2020-04-07Adams QuintanillaNo ratings yet
- Proteins As Tumour MarkersDocument14 pagesProteins As Tumour MarkersEma MalNo ratings yet
- HCV (Cassette)Document1 pageHCV (Cassette)عائش العموديNo ratings yet
- Prostate CancerDocument61 pagesProstate CancerChris FrenchNo ratings yet
- Pca ScreeningDocument6 pagesPca ScreeningNariska CooperNo ratings yet
- Establishing The Heparin Therapeutic Range Using aPTT and Anti-XaDocument4 pagesEstablishing The Heparin Therapeutic Range Using aPTT and Anti-Xaimran ahmed siddiquiNo ratings yet
- FMF Certification of Biochemical Laboratories - NewDocument4 pagesFMF Certification of Biochemical Laboratories - Newhoa1405No ratings yet
- H321-Afp Elisa-2.0ê+ À¿ HT-1.0Document2 pagesH321-Afp Elisa-2.0ê+ À¿ HT-1.0Frank DelgadoNo ratings yet
- (PSA) Level in Patients With Symptomatic Benign Prostatic HyperplasiaDocument4 pages(PSA) Level in Patients With Symptomatic Benign Prostatic HyperplasiaЕленко ПоповNo ratings yet
- C86000 13.04.04.068305 iFlash-Total PSA CLIA V7.0 2020-04-07Document4 pagesC86000 13.04.04.068305 iFlash-Total PSA CLIA V7.0 2020-04-07Adams QuintanillaNo ratings yet
- INS PS - E EN Ichroma PSA Plus - Rev.00 - 170719Document4 pagesINS PS - E EN Ichroma PSA Plus - Rev.00 - 170719RizoreNo ratings yet
- Prostate-Specific Antigen (PSA) Isoform p2PSA in Prostate Cancer Screening: Systematic Review of Current Evidence and Further PerspectivesDocument8 pagesProstate-Specific Antigen (PSA) Isoform p2PSA in Prostate Cancer Screening: Systematic Review of Current Evidence and Further PerspectivesLacazette AubameyangNo ratings yet
- FPSA DonnéeDocument6 pagesFPSA DonnéebilogbaNo ratings yet
- PSA Package InsertDocument2 pagesPSA Package InsertCasa anfa LaboratoireNo ratings yet
- B15631MDocument13 pagesB15631ManwitramakshayNo ratings yet
- 69th AACC Annual Scientific Meeting Abstract eBookFrom Everand69th AACC Annual Scientific Meeting Abstract eBookNo ratings yet
- Policloropreno FDADocument4 pagesPolicloropreno FDAAlejandro ZagalNo ratings yet
- Advantages and Disadvantages of Literature ReviewDocument7 pagesAdvantages and Disadvantages of Literature Reviewea59j0hqNo ratings yet
- Airfit-N20 Disinfection-Sterilization-Guide Amer Eng PDFDocument3 pagesAirfit-N20 Disinfection-Sterilization-Guide Amer Eng PDFhorse888No ratings yet
- Dezinfeqcia SterilizaciaDocument29 pagesDezinfeqcia SterilizaciaLuka KhvichiaNo ratings yet
- NSTP FeatureDocument2 pagesNSTP FeatureCharisseNo ratings yet
- Acidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)Document29 pagesAcidifiers and Antiseptics Lecture Notes-Dr - Jibachha Sah, Lecturer, M.V.SC (Pharmacology)jibachha sahNo ratings yet
- Fundamentals of Oriental MedicineDocument72 pagesFundamentals of Oriental Medicinemd_corona62No ratings yet
- Section 1 - Chemical Product and Company Information: Safety Data Sheet - Sulfur Hexafluoride (SF)Document8 pagesSection 1 - Chemical Product and Company Information: Safety Data Sheet - Sulfur Hexafluoride (SF)sufizamaniNo ratings yet
- Keselamatan Dan Kesihatan PekerjaanDocument15 pagesKeselamatan Dan Kesihatan PekerjaanOsman EsaNo ratings yet
- School Forms (Sf2) June-March 2015Document109 pagesSchool Forms (Sf2) June-March 2015Kristoffer Alcantara RiveraNo ratings yet
- Alison Morton-Cooper Health Care and The Autism Spectrum A Guide For Health Professionals, Parents and Carers 2004Document130 pagesAlison Morton-Cooper Health Care and The Autism Spectrum A Guide For Health Professionals, Parents and Carers 2004lillouana100% (1)
- Home Remedies Using Mustard Seeds Prophet666Document2 pagesHome Remedies Using Mustard Seeds Prophet666Hussainz AliNo ratings yet
- Pgdwash021 - Sparsh Hospital - Sanitation and Public HealthDocument9 pagesPgdwash021 - Sparsh Hospital - Sanitation and Public HealthMadhumithaa muralieNo ratings yet
- Mammoth Eureka Utah Mine Closures MapsDocument22 pagesMammoth Eureka Utah Mine Closures MapsRussell HartillNo ratings yet
- Chromium, Hexavalent, Method 8023, 02-2009, 9th EdDocument6 pagesChromium, Hexavalent, Method 8023, 02-2009, 9th EdTicha MaharaniNo ratings yet
- Charlotte Sharrad: Personal StatementDocument3 pagesCharlotte Sharrad: Personal Statementapi-428492384No ratings yet
- Nursing: Why I Want To Become A NurseDocument4 pagesNursing: Why I Want To Become A Nurseabraham lilyNo ratings yet
- ENGLISH (Précis & Composition) : Mock TestDocument3 pagesENGLISH (Précis & Composition) : Mock Testshahid mehmoodNo ratings yet
- Historical Phases of CSRDocument12 pagesHistorical Phases of CSRHIMANSHU RAWATNo ratings yet
- Fehling's Test: Comparative Test Reactions of CarbohydratesDocument33 pagesFehling's Test: Comparative Test Reactions of CarbohydratesTom Anthony Tonguia100% (1)
- My Chevening Journey, Part 1 - Leadership EssayDocument8 pagesMy Chevening Journey, Part 1 - Leadership EssayBroker KhanNo ratings yet
- Essay Lang IV AphasiaDocument39 pagesEssay Lang IV AphasiaAnLu PietroboniNo ratings yet
- AO2021-0066 Guidelines On The Issuance of Certificate On Inclusion (COI) in The Blood Services Network (BSN)Document17 pagesAO2021-0066 Guidelines On The Issuance of Certificate On Inclusion (COI) in The Blood Services Network (BSN)gravadorceciliaNo ratings yet
- The ST Kitts Eye StudyDocument50 pagesThe ST Kitts Eye StudyPaul H ArtesNo ratings yet
- Government of Gujarat and Family Welfare Department Resolution No - Gau/102019/Sfs-84/Chhl Sa Chiv Alaya, Gandhinagar Date:8/7/2021Document6 pagesGovernment of Gujarat and Family Welfare Department Resolution No - Gau/102019/Sfs-84/Chhl Sa Chiv Alaya, Gandhinagar Date:8/7/2021NghgghjhNo ratings yet
- Medical Ethics/Ethical PrinciplesDocument20 pagesMedical Ethics/Ethical PrinciplesPALATTAO, AUBRIE L. BSMT2-8No ratings yet
- LAS 3 Sample 3Document34 pagesLAS 3 Sample 3Mary Jean MagdayNo ratings yet