Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
10 views30 Question
30 Question
Uploaded by
sohitThis document contains 30 multiple choice questions related to organic chemistry concepts like acid-base reactions, relative acidities of organic compounds, hyperconjugation effects, and identifying the strongest acids, bases, and reactions. The questions cover topics such as identifying the fastest reacting organic halide, strongest base, most acidic compound, and correct order of acid strength among given options.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocument38 pagesChemical Principles The Quest For Insight 7th Edition Atkins Test Bankgarrywolfelsjftl100% (18)
- Chemistry Model Question PaperDocument5 pagesChemistry Model Question PaperKevin Alexander100% (1)
- TCMSC4Document39 pagesTCMSC4Eco Defense100% (3)
- Analysis of Fats and OilsDocument19 pagesAnalysis of Fats and Oilsvishnoi1989% (9)
- Experiment #6Document11 pagesExperiment #6Tin-tin71% (7)
- D4952 1630083-1Document3 pagesD4952 1630083-1Asep TheaNo ratings yet
- AldehydesDocument22 pagesAldehydesjksiddharth24No ratings yet
- ChemDocument6 pagesChembighneshrath1No ratings yet
- Sample Paper: Time: 90 Minutes Max. Marks: 35Document9 pagesSample Paper: Time: 90 Minutes Max. Marks: 35Harsh PatelNo ratings yet
- Indian Association of Chemistry Teachers: National Standard Examination in Chemistry 2008-2009Document7 pagesIndian Association of Chemistry Teachers: National Standard Examination in Chemistry 2008-2009Rishabh PathakNo ratings yet
- C) Trigonal Planar: E-Pent-2-ene Z-Pent-2-ene Z-3-Methylpent-2-ene Z-2-Methylpent-2-eneDocument9 pagesC) Trigonal Planar: E-Pent-2-ene Z-Pent-2-ene Z-3-Methylpent-2-ene Z-2-Methylpent-2-eneJessicaNo ratings yet
- Organic ChemistryDocument45 pagesOrganic ChemistryAnubhav Sinha0% (1)
- Organic Chemistry: Daily Practice ProblemsDocument53 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Unit 11 MCQDocument7 pagesUnit 11 MCQJay VermaNo ratings yet
- Nsec 1999Document12 pagesNsec 1999CorneliaNo ratings yet
- NSEC Solved Paper 2010Document7 pagesNSEC Solved Paper 2010whatismyusername1947No ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument7 pagesAldehydes, Ketones and Carboxylic Acidskavitha2511977No ratings yet
- Aromatic CompoundsDocument16 pagesAromatic CompoundsadityaNo ratings yet
- Aldehydes & KetonesDocument23 pagesAldehydes & KetonesManthan JhaNo ratings yet
- Aldehydes, Ketons and Carboxylic Acid Unit 11 MCQDocument4 pagesAldehydes, Ketons and Carboxylic Acid Unit 11 MCQAbhinav TripathiNo ratings yet
- HC Docx1Document13 pagesHC Docx1ayushsekhariNo ratings yet
- Haloalkanes and Haloarenes Question BankDocument16 pagesHaloalkanes and Haloarenes Question BankBrown HustlerNo ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Term 2 Online Class Xi Chemistry 043Document4 pagesTerm 2 Online Class Xi Chemistry 043kumaryashxd07No ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- Orgsynth KeysDocument27 pagesOrgsynth KeysDebalina DassNo ratings yet
- 34 Alcohols & Ethers - Problems For Practice - Level 1Document14 pages34 Alcohols & Ethers - Problems For Practice - Level 1Abuturab MohammadiNo ratings yet
- Chemistry 22nd May 2024Document6 pagesChemistry 22nd May 2024Ayush GhatakNo ratings yet
- Kcet - Chemistry - 2019: Version Code: D-5Document7 pagesKcet - Chemistry - 2019: Version Code: D-5Manoj CNo ratings yet
- 11th - Chemistry MaterialDocument12 pages11th - Chemistry Materialprathiksha6660No ratings yet
- Carbonyl Compounds 12thDocument24 pagesCarbonyl Compounds 12thRaju SinghNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Aromatic Compounds (13th)Document24 pagesAromatic Compounds (13th)Raju SinghNo ratings yet
- Sample Paper 3: ChemistryDocument13 pagesSample Paper 3: ChemistryPr SathishNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument7 pagesExercise-01 Check Your Grasp: O CH HO HOChesta MalhotraNo ratings yet
- Alcohol & EtherDocument217 pagesAlcohol & EtherAmitNo ratings yet
- Iit Questions On Carbonyl Compounds & Carboxylic Acid and Its DerivativeDocument12 pagesIit Questions On Carbonyl Compounds & Carboxylic Acid and Its DerivativeRaju SinghNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument29 pagesExercise-01 Check Your Grasp: O CH HO HOHet PrajapatiNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids: CHO H CH CH C CHDocument8 pagesAldehydes, Ketones and Carboxylic Acids: CHO H CH CH C CHUjjwal TomarNo ratings yet
- QP - Sol - NSEC 2012-13Document10 pagesQP - Sol - NSEC 2012-13Vardaan Bhatnagar100% (1)
- Chemistry Mock Test 1 PDFDocument9 pagesChemistry Mock Test 1 PDFVikas SinghNo ratings yet
- My Faculty Is Downloading Question Paper Alkyl HalideDocument4 pagesMy Faculty Is Downloading Question Paper Alkyl HalidesanskritiNo ratings yet
- HaloDocument17 pagesHaloadityakatariya157No ratings yet
- ALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestDocument6 pagesALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestPoorvaBakshiNo ratings yet
- Aldehydes and KetonesDocument29 pagesAldehydes and KetonesJiya singhNo ratings yet
- (a) mixture of oо and pоbromotoluenesDocument19 pages(a) mixture of oо and pоbromotoluenesmotikaviNo ratings yet
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thDocument16 pagesAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thAritra Lahiri100% (1)
- OrganicDocument9 pagesOrganicjitesh100kushwahaNo ratings yet
- P Block Elements QBDocument12 pagesP Block Elements QBRajeev KaushikNo ratings yet
- ch9 AlkynesDocument7 pagesch9 AlkynesApichat JunsodNo ratings yet
- A CIDITYDocument17 pagesA CIDITYApex InstituteNo ratings yet
- NEET 11 - Full TestDocument93 pagesNEET 11 - Full Testrs3369792No ratings yet
- Aromatic Compound SheetDocument71 pagesAromatic Compound Sheetadityavaish739No ratings yet
- Diwali Assignment 12thDocument19 pagesDiwali Assignment 12thNishantPlayz YtNo ratings yet
- Carbonyl Compounds SheetDocument133 pagesCarbonyl Compounds Sheetadityavaish739No ratings yet
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocument45 pagesChemical Principles The Quest For Insight 7th Edition Atkins Test Bankwadeperlid9d98k100% (32)
- Ebook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFDocument66 pagesEbook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFJaniceMarqueznxed100% (15)
- UnitTest - D09 Mar 2024Document28 pagesUnitTest - D09 Mar 2024NamraNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Haloalkanes and HaloarenesDocument5 pagesHaloalkanes and Haloareneskavitha2511977No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Erowid LSD (Acid) Vault - ChemistryDocument1 pageErowid LSD (Acid) Vault - ChemistryAdam BruhNo ratings yet
- Time Schedule of CINEST 2015Document9 pagesTime Schedule of CINEST 2015Yala ZiriNo ratings yet
- Ionization Energies of Atoms and Atomic Ions PDFDocument9 pagesIonization Energies of Atoms and Atomic Ions PDFFausto SalazarNo ratings yet
- Blabla ChemDocument5 pagesBlabla Chemfatima hahsmiNo ratings yet
- Corrosion and Corrosion ControlDocument43 pagesCorrosion and Corrosion ControlTEZ ANALYSIS AND STORIESNo ratings yet
- Microbiology 101: Laboratory Exercise #22: Carbohydrate MetabolismDocument16 pagesMicrobiology 101: Laboratory Exercise #22: Carbohydrate Metabolismmaraki998No ratings yet
- LIST OF REGISTERED DRUGS As of December 2012Document52 pagesLIST OF REGISTERED DRUGS As of December 2012Benjamin TantiansuNo ratings yet
- GC 3 PDFDocument10 pagesGC 3 PDFs mvrht netNo ratings yet
- Chemical Constituents of PittosporumDocument10 pagesChemical Constituents of Pittosporumagnes alimboyoguenNo ratings yet
- 02 - Redox JEE PDFDocument60 pages02 - Redox JEE PDFmanasmehtaNo ratings yet
- Overview of The IFRA Standards - 48th AmendmentDocument16 pagesOverview of The IFRA Standards - 48th AmendmentDomitian PascaNo ratings yet
- Efka FA 4601: Technical InformationDocument4 pagesEfka FA 4601: Technical InformationMOEEN KHAN ASLAM KHAN RISALDARNo ratings yet
- Science Cheat SheetDocument6 pagesScience Cheat SheetAditi PandyaNo ratings yet
- IOCL Report 2011Document56 pagesIOCL Report 2011Ajay ShekhawatNo ratings yet
- AnaChem Titrimetry 3Document6 pagesAnaChem Titrimetry 3Jei HernandezNo ratings yet
- Waste Treatment and Disposal TechnologyDocument90 pagesWaste Treatment and Disposal TechnologybillNo ratings yet
- Chem September 2013Document84 pagesChem September 2013Orlando BarriosNo ratings yet
- CHM1 11 - 12 Q1 0603 FDDocument14 pagesCHM1 11 - 12 Q1 0603 FDガトゥラクラークキースNo ratings yet
- Nippon Steel Carbon Neutral Vision 2050: March 30, 2021Document54 pagesNippon Steel Carbon Neutral Vision 2050: March 30, 2021Kedar BhaveNo ratings yet
- On PreheatingDocument22 pagesOn PreheatingYYNo ratings yet
- Me Laboratory 1 Experiment No. 5A Orsat Apparatus: Answer/define The Following: 1. What Is Orsat Analysis?Document3 pagesMe Laboratory 1 Experiment No. 5A Orsat Apparatus: Answer/define The Following: 1. What Is Orsat Analysis?ANTHONETTE BERNABENo ratings yet
- How To Process Color and Black-And - White Reversal and Negative Films and PapersDocument20 pagesHow To Process Color and Black-And - White Reversal and Negative Films and PapersErden Sizgek100% (2)
- امتحان الشركة القابضة للبتروكيماوياتDocument4 pagesامتحان الشركة القابضة للبتروكيماوياتAhmed MansoorNo ratings yet
- VAI 2022 DEC Catalog (030 084)Document55 pagesVAI 2022 DEC Catalog (030 084)ihssanlabroujNo ratings yet
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioNo ratings yet
- Science Sample PaperDocument12 pagesScience Sample PaperKOMAL AGGARWALNo ratings yet
30 Question
30 Question
Uploaded by
sohit0 ratings0% found this document useful (0 votes)
10 views6 pagesThis document contains 30 multiple choice questions related to organic chemistry concepts like acid-base reactions, relative acidities of organic compounds, hyperconjugation effects, and identifying the strongest acids, bases, and reactions. The questions cover topics such as identifying the fastest reacting organic halide, strongest base, most acidic compound, and correct order of acid strength among given options.
Original Description:
Original Title
30 question ppt
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains 30 multiple choice questions related to organic chemistry concepts like acid-base reactions, relative acidities of organic compounds, hyperconjugation effects, and identifying the strongest acids, bases, and reactions. The questions cover topics such as identifying the fastest reacting organic halide, strongest base, most acidic compound, and correct order of acid strength among given options.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
10 views6 pages30 Question
30 Question
Uploaded by
sohitThis document contains 30 multiple choice questions related to organic chemistry concepts like acid-base reactions, relative acidities of organic compounds, hyperconjugation effects, and identifying the strongest acids, bases, and reactions. The questions cover topics such as identifying the fastest reacting organic halide, strongest base, most acidic compound, and correct order of acid strength among given options.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 6
1.
Fast reaction with KOH(aq) is of
(a) CH3SH
(b) CH3I
(c) CH3Cl
(d) CH3NH2
2. Find out strongest base
(a) NH3
(b) CH3NH2
(c) CH3NHCH3
(d) NI3
3. Stability order is correct for given:
(a) B>C>A
(b) A>B>C
(c) C>A>B
(d) A>C>B
4. Maximum pH value is of
(a) CH3COOH
(b) CH3CH2COOH
(c) CH3SO3H
(d) CH3CH2SO3H
5. Find out most acidic
(a) CH3SH
(b) CH3NH2
(c) CH3OH
(d) CH4
6. Find out most acidic among the given
(a) CH3OH
(b) CH3COOH
(c) CH3SO3H
7. Most basic is
(a) NH2–
(b) CH3–
(c) SH–
(d) OH–
8. Find out the most acidic among the following :
(a) HC CH
(b) NH3
(c) CH4
(d) H2O
9. Acid gives H+ with metal then find out H+ which are removed by metal on
the reaction.
(a) α, β (b) β, ƴ (c) ƴ, α (d) any one
10 Find out the most basic
(a) NCl3
(b) NI3
(c) NH3
(d) NF3
11. Most acidic is
(a) CF3COOH
(b) CCl3COOH
(c) CH3COOH
(d) C2H5COOH
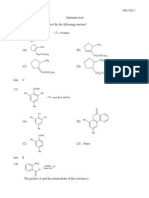
12. Most acidic is
13. Find out the pH value (increasing order)
(A) CCl3COOH
(B) CF3COOH
(C) CH3COOH
(D) H2O
(a) B < A < C < D
(b) A < B < C < D
(c) B < C < D < A
(d) C < D < A < B
14. Which has Fast reaction with
(a) CH3—Cl
(b) CH3—I
(c) CH3—Br
(d) CH3—F
15. Correct the basic order of the given
16. Find out the most acidic among the following :
(a.) CCl3COOH
(b.) CH3COOH
(c.) CF3COOH
(d.) C2H5COOH
17. Find out the most acidic among the following :
(a.) H2S
(b.) H2O
(c.) NH3
(d.) HCl
18. Find out the most basic
(a.) NCl3
(b.) NI3
(c.) NH3
(d.) NF3
19. Most acidic is
(a.) CH3COOH
(b.) CH3CH2COOH
(c.) CCl3COOH
(d.) HCOOH
20. Most acidic is
(a.) HCOOH
(b.) NO2COOH
(c.) CH3COOH
(d.) ClCOOH
21. Find out the pH value (increasing order)
(A) H2O (B) CH3COOH (C) HCOOH
(D) C2H5COOH
(a.) B < A < C < D
(b.) A < B < C < D
(c.) B < C < D < A
(d.) C < B < D < A
22. Find out the pH value (increasing order)
(A) CH3OH (B) HC º CH (C) NH3
(D) H2O
(a.) B < A < C < D
(b.) A < B < C < D
(c.) B < C < D < A
(d.) D < A < B < C
23. Which has Fast reaction with
(a.) CH3—SH
(b.) CH3—NH2
(c.) CH3—OH
(d.) CH3—Cl
24. Which has Fast reaction with
25. Find out possible reaction
(a.) CH3—Cl + NH2–
(b.) CH3—NH2 + OH–
(c.) CH3—NH2 + SH–
(d.) CH3—OH + SH–
26. Hyperconjugation is
(a.) σ-π conjugation.
(b.) noticed due to delocalisation of σ and π bonds.
(c.) no bond resonance.
(d.) All the above.
27. Hyperconjugation effect involves :
(a.) Delocalization of lone pair into an adjacent π bond.
(b.) Delocalisation of π electrons into an adjacent double bond.
(c.) Delocalization of σ electrons into an adjacent π bond.
(d.) All are true
28. Which of the following has hyperconjugation effect ?
29. Maximum hyperconugation is present in:
(a) Tert-butylbenzene
(b) ethylbenzene
(c) isopropyl benzene
(d) toluene.
30. Which one of the following does not exhibit hyperconjugation ?
(a) Ethanal
(b) Allylene
(c) Isobutylene
(d) Trifluro acetaldehyde
You might also like
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocument38 pagesChemical Principles The Quest For Insight 7th Edition Atkins Test Bankgarrywolfelsjftl100% (18)
- Chemistry Model Question PaperDocument5 pagesChemistry Model Question PaperKevin Alexander100% (1)
- TCMSC4Document39 pagesTCMSC4Eco Defense100% (3)
- Analysis of Fats and OilsDocument19 pagesAnalysis of Fats and Oilsvishnoi1989% (9)
- Experiment #6Document11 pagesExperiment #6Tin-tin71% (7)
- D4952 1630083-1Document3 pagesD4952 1630083-1Asep TheaNo ratings yet
- AldehydesDocument22 pagesAldehydesjksiddharth24No ratings yet
- ChemDocument6 pagesChembighneshrath1No ratings yet
- Sample Paper: Time: 90 Minutes Max. Marks: 35Document9 pagesSample Paper: Time: 90 Minutes Max. Marks: 35Harsh PatelNo ratings yet
- Indian Association of Chemistry Teachers: National Standard Examination in Chemistry 2008-2009Document7 pagesIndian Association of Chemistry Teachers: National Standard Examination in Chemistry 2008-2009Rishabh PathakNo ratings yet
- C) Trigonal Planar: E-Pent-2-ene Z-Pent-2-ene Z-3-Methylpent-2-ene Z-2-Methylpent-2-eneDocument9 pagesC) Trigonal Planar: E-Pent-2-ene Z-Pent-2-ene Z-3-Methylpent-2-ene Z-2-Methylpent-2-eneJessicaNo ratings yet
- Organic ChemistryDocument45 pagesOrganic ChemistryAnubhav Sinha0% (1)
- Organic Chemistry: Daily Practice ProblemsDocument53 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Unit 11 MCQDocument7 pagesUnit 11 MCQJay VermaNo ratings yet
- Nsec 1999Document12 pagesNsec 1999CorneliaNo ratings yet
- NSEC Solved Paper 2010Document7 pagesNSEC Solved Paper 2010whatismyusername1947No ratings yet
- Aldehydes, Ketones and Carboxylic AcidsDocument7 pagesAldehydes, Ketones and Carboxylic Acidskavitha2511977No ratings yet
- Aromatic CompoundsDocument16 pagesAromatic CompoundsadityaNo ratings yet
- Aldehydes & KetonesDocument23 pagesAldehydes & KetonesManthan JhaNo ratings yet
- Aldehydes, Ketons and Carboxylic Acid Unit 11 MCQDocument4 pagesAldehydes, Ketons and Carboxylic Acid Unit 11 MCQAbhinav TripathiNo ratings yet
- HC Docx1Document13 pagesHC Docx1ayushsekhariNo ratings yet
- Haloalkanes and Haloarenes Question BankDocument16 pagesHaloalkanes and Haloarenes Question BankBrown HustlerNo ratings yet
- 12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS QDocument20 pages12TH CBSE DPP 37. Aldehydes, Ketones and Carboxylic Acids MCQ ASSERTION REASON CS Q123No ratings yet
- Term 2 Online Class Xi Chemistry 043Document4 pagesTerm 2 Online Class Xi Chemistry 043kumaryashxd07No ratings yet
- CadDocument8 pagesCadRamesh Babu GarlapatiNo ratings yet
- Orgsynth KeysDocument27 pagesOrgsynth KeysDebalina DassNo ratings yet
- 34 Alcohols & Ethers - Problems For Practice - Level 1Document14 pages34 Alcohols & Ethers - Problems For Practice - Level 1Abuturab MohammadiNo ratings yet
- Chemistry 22nd May 2024Document6 pagesChemistry 22nd May 2024Ayush GhatakNo ratings yet
- Kcet - Chemistry - 2019: Version Code: D-5Document7 pagesKcet - Chemistry - 2019: Version Code: D-5Manoj CNo ratings yet
- 11th - Chemistry MaterialDocument12 pages11th - Chemistry Materialprathiksha6660No ratings yet
- Carbonyl Compounds 12thDocument24 pagesCarbonyl Compounds 12thRaju SinghNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Aromatic Compounds (13th)Document24 pagesAromatic Compounds (13th)Raju SinghNo ratings yet
- Sample Paper 3: ChemistryDocument13 pagesSample Paper 3: ChemistryPr SathishNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument7 pagesExercise-01 Check Your Grasp: O CH HO HOChesta MalhotraNo ratings yet
- Alcohol & EtherDocument217 pagesAlcohol & EtherAmitNo ratings yet
- Iit Questions On Carbonyl Compounds & Carboxylic Acid and Its DerivativeDocument12 pagesIit Questions On Carbonyl Compounds & Carboxylic Acid and Its DerivativeRaju SinghNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument29 pagesExercise-01 Check Your Grasp: O CH HO HOHet PrajapatiNo ratings yet
- Aldehydes, Ketones and Carboxylic Acids: CHO H CH CH C CHDocument8 pagesAldehydes, Ketones and Carboxylic Acids: CHO H CH CH C CHUjjwal TomarNo ratings yet
- QP - Sol - NSEC 2012-13Document10 pagesQP - Sol - NSEC 2012-13Vardaan Bhatnagar100% (1)
- Chemistry Mock Test 1 PDFDocument9 pagesChemistry Mock Test 1 PDFVikas SinghNo ratings yet
- My Faculty Is Downloading Question Paper Alkyl HalideDocument4 pagesMy Faculty Is Downloading Question Paper Alkyl HalidesanskritiNo ratings yet
- HaloDocument17 pagesHaloadityakatariya157No ratings yet
- ALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestDocument6 pagesALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestPoorvaBakshiNo ratings yet
- Aldehydes and KetonesDocument29 pagesAldehydes and KetonesJiya singhNo ratings yet
- (a) mixture of oо and pоbromotoluenesDocument19 pages(a) mixture of oо and pоbromotoluenesmotikaviNo ratings yet
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thDocument16 pagesAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 12thAritra Lahiri100% (1)
- OrganicDocument9 pagesOrganicjitesh100kushwahaNo ratings yet
- P Block Elements QBDocument12 pagesP Block Elements QBRajeev KaushikNo ratings yet
- ch9 AlkynesDocument7 pagesch9 AlkynesApichat JunsodNo ratings yet
- A CIDITYDocument17 pagesA CIDITYApex InstituteNo ratings yet
- NEET 11 - Full TestDocument93 pagesNEET 11 - Full Testrs3369792No ratings yet
- Aromatic Compound SheetDocument71 pagesAromatic Compound Sheetadityavaish739No ratings yet
- Diwali Assignment 12thDocument19 pagesDiwali Assignment 12thNishantPlayz YtNo ratings yet
- Carbonyl Compounds SheetDocument133 pagesCarbonyl Compounds Sheetadityavaish739No ratings yet
- Chemical Principles The Quest For Insight 7th Edition Atkins Test BankDocument45 pagesChemical Principles The Quest For Insight 7th Edition Atkins Test Bankwadeperlid9d98k100% (32)
- Ebook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFDocument66 pagesEbook Chemical Principles The Quest For Insight 7Th Edition Atkins Test Bank Full Chapter PDFJaniceMarqueznxed100% (15)
- UnitTest - D09 Mar 2024Document28 pagesUnitTest - D09 Mar 2024NamraNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Haloalkanes and HaloarenesDocument5 pagesHaloalkanes and Haloareneskavitha2511977No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Erowid LSD (Acid) Vault - ChemistryDocument1 pageErowid LSD (Acid) Vault - ChemistryAdam BruhNo ratings yet
- Time Schedule of CINEST 2015Document9 pagesTime Schedule of CINEST 2015Yala ZiriNo ratings yet
- Ionization Energies of Atoms and Atomic Ions PDFDocument9 pagesIonization Energies of Atoms and Atomic Ions PDFFausto SalazarNo ratings yet
- Blabla ChemDocument5 pagesBlabla Chemfatima hahsmiNo ratings yet
- Corrosion and Corrosion ControlDocument43 pagesCorrosion and Corrosion ControlTEZ ANALYSIS AND STORIESNo ratings yet
- Microbiology 101: Laboratory Exercise #22: Carbohydrate MetabolismDocument16 pagesMicrobiology 101: Laboratory Exercise #22: Carbohydrate Metabolismmaraki998No ratings yet
- LIST OF REGISTERED DRUGS As of December 2012Document52 pagesLIST OF REGISTERED DRUGS As of December 2012Benjamin TantiansuNo ratings yet
- GC 3 PDFDocument10 pagesGC 3 PDFs mvrht netNo ratings yet
- Chemical Constituents of PittosporumDocument10 pagesChemical Constituents of Pittosporumagnes alimboyoguenNo ratings yet
- 02 - Redox JEE PDFDocument60 pages02 - Redox JEE PDFmanasmehtaNo ratings yet
- Overview of The IFRA Standards - 48th AmendmentDocument16 pagesOverview of The IFRA Standards - 48th AmendmentDomitian PascaNo ratings yet
- Efka FA 4601: Technical InformationDocument4 pagesEfka FA 4601: Technical InformationMOEEN KHAN ASLAM KHAN RISALDARNo ratings yet
- Science Cheat SheetDocument6 pagesScience Cheat SheetAditi PandyaNo ratings yet
- IOCL Report 2011Document56 pagesIOCL Report 2011Ajay ShekhawatNo ratings yet
- AnaChem Titrimetry 3Document6 pagesAnaChem Titrimetry 3Jei HernandezNo ratings yet
- Waste Treatment and Disposal TechnologyDocument90 pagesWaste Treatment and Disposal TechnologybillNo ratings yet
- Chem September 2013Document84 pagesChem September 2013Orlando BarriosNo ratings yet
- CHM1 11 - 12 Q1 0603 FDDocument14 pagesCHM1 11 - 12 Q1 0603 FDガトゥラクラークキースNo ratings yet
- Nippon Steel Carbon Neutral Vision 2050: March 30, 2021Document54 pagesNippon Steel Carbon Neutral Vision 2050: March 30, 2021Kedar BhaveNo ratings yet
- On PreheatingDocument22 pagesOn PreheatingYYNo ratings yet
- Me Laboratory 1 Experiment No. 5A Orsat Apparatus: Answer/define The Following: 1. What Is Orsat Analysis?Document3 pagesMe Laboratory 1 Experiment No. 5A Orsat Apparatus: Answer/define The Following: 1. What Is Orsat Analysis?ANTHONETTE BERNABENo ratings yet
- How To Process Color and Black-And - White Reversal and Negative Films and PapersDocument20 pagesHow To Process Color and Black-And - White Reversal and Negative Films and PapersErden Sizgek100% (2)
- امتحان الشركة القابضة للبتروكيماوياتDocument4 pagesامتحان الشركة القابضة للبتروكيماوياتAhmed MansoorNo ratings yet
- VAI 2022 DEC Catalog (030 084)Document55 pagesVAI 2022 DEC Catalog (030 084)ihssanlabroujNo ratings yet
- Tabla de PotencialesDocument6 pagesTabla de PotencialesLuis AntonioNo ratings yet
- Science Sample PaperDocument12 pagesScience Sample PaperKOMAL AGGARWALNo ratings yet