Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
17 viewsChemlab Project
Chemlab Project
Uploaded by
Xheena SarabiaOxalic acid is an organic compound found in many plants. It has the molecular formula C2H2O4 and is an alpha,omega-dicarboxylic acid with carboxyl groups at positions 1 and 2. Oxalic acid is mainly manufactured through the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. It is a strong poison that can cause renal damage, shock, and convulsions if ingested. Acetic acid is a synthetic carboxylic acid with the molecular formula C2H4O2. It has antibacterial and antifungal properties and is commonly used as a food additive and preservative. Both acids are corros
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Conquering Chemistry Module 2Document88 pagesConquering Chemistry Module 2Shayan Lahijanian0% (1)
- Dodigen 2808 TDSDocument1 pageDodigen 2808 TDSRashid SaleemNo ratings yet
- EsterificationDocument3 pagesEsterificationKhris Sy-HandumonNo ratings yet
- Ethanoic Acid ChemDocument14 pagesEthanoic Acid ChemjasmynefNo ratings yet
- Literature Survey of Oxalic Acid ProductionDocument8 pagesLiterature Survey of Oxalic Acid Productionvarun singhNo ratings yet
- Oxalic Acid Expt 5 Chem 34 DraftDocument4 pagesOxalic Acid Expt 5 Chem 34 DraftVanessaOlgaJ.Dagondon100% (2)
- Exp 10Document9 pagesExp 10ChantalDanaNo ratings yet
- Carboxylic Acids: Basic Properties Project by Student Ghita AntoniaDocument7 pagesCarboxylic Acids: Basic Properties Project by Student Ghita AntoniaAntonia GhitaNo ratings yet
- Vol 4 Ascorbic AcidDocument1 pageVol 4 Ascorbic AcidregdddNo ratings yet
- Volumetric Analysis of Ascorbic Acid Present in Citrus FruitsDocument19 pagesVolumetric Analysis of Ascorbic Acid Present in Citrus FruitsSanjeev BabbarNo ratings yet
- Carboxylic Acid and Derivatives PDFDocument12 pagesCarboxylic Acid and Derivatives PDFÏt's RîçkgãrçīäNo ratings yet
- Chemistry Project ANTACIDDocument17 pagesChemistry Project ANTACIDMAX MusicNo ratings yet
- Acetic Acid: 2 PropertiesDocument12 pagesAcetic Acid: 2 PropertiesGudang MyosiNo ratings yet
- Bihari INVESTIGATORY PROJECT PDFDocument17 pagesBihari INVESTIGATORY PROJECT PDFDeepa SinghNo ratings yet
- Acids Bases and SaltsDocument68 pagesAcids Bases and SaltsDarien Gale EgdaneNo ratings yet
- 2019 Ch104 Organic AcidsDocument111 pages2019 Ch104 Organic Acidsgenesis gengerNo ratings yet
- Carboxylic Acid 1Document14 pagesCarboxylic Acid 1Junaid KhanNo ratings yet
- Project On ChemistryDocument13 pagesProject On ChemistryBadhal PaudelNo ratings yet
- Chemistry of Ascorbic AcidDocument16 pagesChemistry of Ascorbic AcidGordon YapNo ratings yet
- Inorganic Compounds With Economic, Industrial, Environmental and Social ImpactDocument4 pagesInorganic Compounds With Economic, Industrial, Environmental and Social ImpactScribdTranslationsNo ratings yet
- Part 1Document5 pagesPart 1eljon18No ratings yet
- Acetic AcidDocument11 pagesAcetic AcidPushpa KaladeviNo ratings yet
- PHARCHEM1 Lec C3Document6 pagesPHARCHEM1 Lec C3qwertyuioppoiuewqNo ratings yet
- Ascorbic Acid - The Chemistry Underlying Its Antioxidant PropertiesDocument29 pagesAscorbic Acid - The Chemistry Underlying Its Antioxidant PropertiesfgonzalezNo ratings yet
- Carboxylic Acid - SPM ChemistryDocument11 pagesCarboxylic Acid - SPM ChemistryVinnySha SelvarajahNo ratings yet
- I. Experiment Title: Aldehyde, Ketone and Carboxylic AcidDocument10 pagesI. Experiment Title: Aldehyde, Ketone and Carboxylic AcidLaily SafitriNo ratings yet
- Acid Base PH Chemical Equations 33Document10 pagesAcid Base PH Chemical Equations 33Girish JoshiNo ratings yet
- Note JJ JJDocument23 pagesNote JJ JJms9216974No ratings yet
- Carboxylic AcidsDocument15 pagesCarboxylic Acidsmadhu bonamNo ratings yet
- Uses of Laccases in The Food IndustryDocument9 pagesUses of Laccases in The Food Industrymayaalouane450No ratings yet
- Number of Atoms in Na2CO3Document3 pagesNumber of Atoms in Na2CO3Floila Jane YmasNo ratings yet
- Baqai J Health Sci 2011 14 2 33 40Document8 pagesBaqai J Health Sci 2011 14 2 33 40Cristina ElenaNo ratings yet
- Alcohols, Phenols and EthersDocument99 pagesAlcohols, Phenols and EthersLesterwin UdarbeNo ratings yet
- Acetic Acid-Properties and Its UsesDocument4 pagesAcetic Acid-Properties and Its UseskarthikeyanNo ratings yet
- Acids & Bases: Neha Kapil Scientific Officer NitraDocument33 pagesAcids & Bases: Neha Kapil Scientific Officer NitraNeha KapilNo ratings yet
- Analitik PresenDocument4 pagesAnalitik PresenMuhammad IrsyadNo ratings yet
- The Alkaline Hydrolysis ProcessDocument6 pagesThe Alkaline Hydrolysis ProcessNemanja MartinovićNo ratings yet
- Oxalic AcidDocument12 pagesOxalic AcidAnwaar KhanNo ratings yet
- Glycerol: Inorganic Chemical Compound Salt K Mno Oxidizing AgentDocument6 pagesGlycerol: Inorganic Chemical Compound Salt K Mno Oxidizing AgentfilchibuffNo ratings yet
- Carboxylic Acid byDocument38 pagesCarboxylic Acid byAuroraNo ratings yet
- Carboxylic Acid: From Wikipedia, The Free EncyclopediaDocument11 pagesCarboxylic Acid: From Wikipedia, The Free EncyclopediaDipti BagdeNo ratings yet
- Carboxylic AcidDocument13 pagesCarboxylic AcidJeremy NayagamNo ratings yet
- Agsam Shiela May R MODULE 8 Learning ActivitiesDocument3 pagesAgsam Shiela May R MODULE 8 Learning ActivitiesSecretNo ratings yet
- Lecture 3 - CarbohydratesDocument91 pagesLecture 3 - CarbohydratesAsyraf Arshat0% (1)
- AVCL 9A Properties of Carboxylic Acids and EstersDocument8 pagesAVCL 9A Properties of Carboxylic Acids and EstersGiane MadrigalNo ratings yet
- Chem Project Class 12 With Investigatory Project On ' Antacids'Document15 pagesChem Project Class 12 With Investigatory Project On ' Antacids'SHAHBAN55550% (1)
- Carboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeDocument9 pagesCarboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeFighter_ace_97No ratings yet
- Text 10.1Document6 pagesText 10.1Ashley MessamNo ratings yet
- Calcium OxalateDocument1 pageCalcium Oxalatekishe50No ratings yet
- 65 Ess16016 Dikonversi DikonversiDocument6 pages65 Ess16016 Dikonversi DikonversiMuhammad Ilham TaufiqNo ratings yet
- A. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentDocument27 pagesA. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentKeyvir AulinzNo ratings yet
- LayoutDocument55 pagesLayoutHenok Moges KassahunNo ratings yet
- Practical BiochemistryDocument108 pagesPractical BiochemistryCindy Nona100% (1)
- Names: Bryle Kristiann CamaroteDocument18 pagesNames: Bryle Kristiann CamaroteKateNo ratings yet
- Chem Project Class 12 With Investigatory Project OnDocument23 pagesChem Project Class 12 With Investigatory Project OnshashankNo ratings yet
- Chapter No.02 Carbohydrates IIDocument56 pagesChapter No.02 Carbohydrates IIsolutionsexpert70059165No ratings yet
- Jawahar Navodaya Vidhyalaya Angul: Analytical Project Chemistr YDocument16 pagesJawahar Navodaya Vidhyalaya Angul: Analytical Project Chemistr YCY CYMONNo ratings yet
- CHEMISTRY Project For Class 12Document19 pagesCHEMISTRY Project For Class 12Naveen Doss100% (1)
- Biochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingFrom EverandBiochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingRating: 4 out of 5 stars4/5 (1)
- Activity 2Document8 pagesActivity 2Xheena SarabiaNo ratings yet
- Answer Sheet For Activity 1 Lab Group 3Document5 pagesAnswer Sheet For Activity 1 Lab Group 3Xheena SarabiaNo ratings yet
- Act 3 J Gen ChemDocument5 pagesAct 3 J Gen ChemXheena SarabiaNo ratings yet
- Genchem Lec Act1Document5 pagesGenchem Lec Act1Xheena SarabiaNo ratings yet
- Genchem LecDocument2 pagesGenchem LecXheena SarabiaNo ratings yet
- GEN CHEM ACT 2, SerevoDocument12 pagesGEN CHEM ACT 2, SerevoXheena SarabiaNo ratings yet
- ReflectionLABPaperOnGenChem - XheenaSarabia IIIDDocument5 pagesReflectionLABPaperOnGenChem - XheenaSarabia IIIDXheena SarabiaNo ratings yet
- GenChem Activity 3Document3 pagesGenChem Activity 3Xheena SarabiaNo ratings yet
- Batomalaki, JelynT Activity1Document8 pagesBatomalaki, JelynT Activity1Xheena SarabiaNo ratings yet
- Dahdahchemlab ProjDocument3 pagesDahdahchemlab ProjXheena SarabiaNo ratings yet
- AbibibbibibibibibDocument1 pageAbibibbibibibibibXheena SarabiaNo ratings yet
- Abibi Chemlab ProjDocument2 pagesAbibi Chemlab ProjXheena SarabiaNo ratings yet
- Technicalwriting ReqsDocument6 pagesTechnicalwriting ReqsXheena SarabiaNo ratings yet
- SARABIA, XHEENA - Lesson 15 Properties of GasesDocument3 pagesSARABIA, XHEENA - Lesson 15 Properties of GasesXheena SarabiaNo ratings yet
- Part3 DRUGSDocument3 pagesPart3 DRUGSXheena SarabiaNo ratings yet
- Excuse LetterDocument1 pageExcuse LetterXheena SarabiaNo ratings yet
- Dictionary of Terms in CriminologyDocument27 pagesDictionary of Terms in CriminologyXheena Sarabia100% (1)
- DisputeRes XSDocument2 pagesDisputeRes XSXheena SarabiaNo ratings yet
- ANSI-IsO Turning Inserts 1Document5 pagesANSI-IsO Turning Inserts 1Parameshwaran PraveenNo ratings yet
- Pricelist Wef. 01112022 TRADEDocument5 pagesPricelist Wef. 01112022 TRADEGarena Free fireNo ratings yet
- Ds Chopped Strand Mat PBDocument2 pagesDs Chopped Strand Mat PBbasanth babuNo ratings yet
- Cost - Effective HousingDocument17 pagesCost - Effective HousingpriyankaNo ratings yet
- ChemistryTestfinal - LASTDocument30 pagesChemistryTestfinal - LASTpurpleasma64No ratings yet
- Silk Manufacturing ProcessDocument2 pagesSilk Manufacturing ProcessAjay Krishna0% (1)
- Product Data Sheet LT 80P: Siegling - Total Belting SolutionsDocument2 pagesProduct Data Sheet LT 80P: Siegling - Total Belting SolutionsGuilherme AugustoNo ratings yet
- Steel SlagDocument15 pagesSteel SlagNaveen B R 4AI19CV037No ratings yet
- Technical SpecificationDocument211 pagesTechnical SpecificationVitali MihalachiNo ratings yet
- Ovoid Pipe Profiles-SimonaDocument12 pagesOvoid Pipe Profiles-SimonaSankar CdmNo ratings yet
- Application Notes Welding (English)Document8 pagesApplication Notes Welding (English)metallurgist100% (6)
- Thurmalox 8200 Painting SpecificationDocument2 pagesThurmalox 8200 Painting SpecificationFreddy Carl FredricksenNo ratings yet
- Omv Comp VDL S 100Document2 pagesOmv Comp VDL S 100Robert IsacNo ratings yet
- The Headband PatternDocument4 pagesThe Headband PatternrarrinNo ratings yet
- Waterwall - Fireside CorrosionDocument8 pagesWaterwall - Fireside CorrosionwahonodNo ratings yet
- Penguard Tie Coat 100Document3 pagesPenguard Tie Coat 100Mohamed FarhanNo ratings yet
- List of Boiler ManufacturerDocument28 pagesList of Boiler ManufacturerGp MishraNo ratings yet
- F 3204 - 16Document4 pagesF 3204 - 16jose floresNo ratings yet
- 6.chemical Precipitation (07.04.2020)Document70 pages6.chemical Precipitation (07.04.2020)HARI PRASATHNo ratings yet
- Hydropalat WE 3650Document28 pagesHydropalat WE 3650APEX SONNo ratings yet
- Powder Production Techniques: AtomizationDocument13 pagesPowder Production Techniques: AtomizationVasantha SudasinghaNo ratings yet
- Unit 3 - Units For Expressing ConcentrationDocument24 pagesUnit 3 - Units For Expressing ConcentrationKhánh Vy NguyênNo ratings yet
- Coconut Husk Particle BoardDocument1 pageCoconut Husk Particle BoardKiran KumarNo ratings yet
- Green Concrete: By: Abhinav Srivastava 3 B. Tech. Civil Engg. February 2011Document36 pagesGreen Concrete: By: Abhinav Srivastava 3 B. Tech. Civil Engg. February 2011Abhinav Srivastava85% (13)
- Sicon MV Muffen Pi enDocument2 pagesSicon MV Muffen Pi enYip Mun FaiNo ratings yet
- Eco Friendly Cleaning ChemicalDocument12 pagesEco Friendly Cleaning ChemicalEcoChemNo ratings yet
- Unit Test Sample Paper - 1 Answer KeyDocument7 pagesUnit Test Sample Paper - 1 Answer Keymilonee lNo ratings yet
- A Presentation On Formation of Alkanes: Submitted By:-Arnab Paul (15) Dept: - Chemistry YEAR: - 1 SEM: - 2Document12 pagesA Presentation On Formation of Alkanes: Submitted By:-Arnab Paul (15) Dept: - Chemistry YEAR: - 1 SEM: - 2Arnab PaulNo ratings yet
Chemlab Project
Chemlab Project
Uploaded by
Xheena Sarabia0 ratings0% found this document useful (0 votes)
17 views2 pagesOxalic acid is an organic compound found in many plants. It has the molecular formula C2H2O4 and is an alpha,omega-dicarboxylic acid with carboxyl groups at positions 1 and 2. Oxalic acid is mainly manufactured through the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. It is a strong poison that can cause renal damage, shock, and convulsions if ingested. Acetic acid is a synthetic carboxylic acid with the molecular formula C2H4O2. It has antibacterial and antifungal properties and is commonly used as a food additive and preservative. Both acids are corros
Original Description:
Original Title
chemlab project
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentOxalic acid is an organic compound found in many plants. It has the molecular formula C2H2O4 and is an alpha,omega-dicarboxylic acid with carboxyl groups at positions 1 and 2. Oxalic acid is mainly manufactured through the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. It is a strong poison that can cause renal damage, shock, and convulsions if ingested. Acetic acid is a synthetic carboxylic acid with the molecular formula C2H4O2. It has antibacterial and antifungal properties and is commonly used as a food additive and preservative. Both acids are corros
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
17 views2 pagesChemlab Project
Chemlab Project
Uploaded by
Xheena SarabiaOxalic acid is an organic compound found in many plants. It has the molecular formula C2H2O4 and is an alpha,omega-dicarboxylic acid with carboxyl groups at positions 1 and 2. Oxalic acid is mainly manufactured through the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. It is a strong poison that can cause renal damage, shock, and convulsions if ingested. Acetic acid is a synthetic carboxylic acid with the molecular formula C2H4O2. It has antibacterial and antifungal properties and is commonly used as a food additive and preservative. Both acids are corros
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
XHEENA SARABIA Cc
“Project in AdGE 102 Laboratory for
the Final Term”
BSCRIM
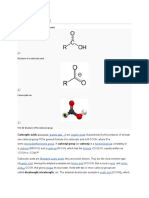
Molecular formula: C2H2O4, (COOH)2, HOOCCOOH
Oxalic acid is an alpha,omega-dicarboxylic acid that is ethane substituted by
carboxyl groups at positions 1 and 2. It has a role as a human metabolite, a plant
Other FORMULA:
metabolite and an algal metabolite. It is a conjugate acid of an oxalate(1-) and
an oxalate. Oxalic acid is an odorless white solid. Sinks and mixes with water. Oxalic
acid is an organic compound found in many plants, including leafy greens,
CC(=O)Os
vegetables, fruits, cocoa, nuts, and seeds. In plants, it's usually bound to minerals,
forming oxalate. The terms “oxalic acid” and “oxalate” are used interchangeably in
nutrition science. Oxalic acid is mainly manufactured by the oxidation
of carbohydrates or glucose using nitric acid or air in the presence of vanadium SKELETAL FORMULA:
pentoxide. A variety of precursors can be used including glycolic acid and ethylene
glycol. A newer method entails oxidative carbonylation of alcohols to give STRUCTURAL FORMULA:
the diesters of oxalic acid. 4 ROH + 4 CO + O2 → 2 (CO2R)2 + 2 H2O. The said acid is
a strong poison. The toxic symptoms include renal damage, shock, convulsions. The
toxicity arises as oxalic acid reacts with the calcium in the tissues to form calcium
oxalate, thereby upsetting the calcium potassium ratio. The deposition of oxalates in
the kidneys tubules may result in kidney damage. It is a reliable & effective cleaner
when used in appropriate applications. Oxalic Acid may be used to remove rust and
other difficult stains from areas such as buildings, boats, swimming pools, concrete
driveways, sidewalks, iron machinery, wood decks, stairs or trim.
SKELETAL FORMULA:
Molecular formula: C2H4O2 or CH3COOH
Acetic Acid is a synthetic carboxylic acid with antibacterial and antifungal
properties. Although its mechanism of action is not fully known, undissociated acetic STRUCTURAL
acid may enhance lipid solubility allowing increased fatty acid accumulation on the
cell membrane or in other cell wall structures. Acetic acid, as a weak acid, can inhibit FORMULA:
carbohydrate metabolism resulting in subsequent death of the organism.
Acetic acid is a simple monocarboxylic acid containing two carbons. It has a role as a
protic solvent, a food acidity regulator, an antimicrobial food preservative and a
Daphnia magna metabolite. It is a conjugate acid of an acetate. Other FORMULA:
Acetic acid, glacial appears as a clear colorless liquid with a strong odor of vinegar.
Flash point 104 °F. Density 8.8 lb / gal. Corrosive to metals and tissue. Used to make
other chemicals, as a food additive, and in petroleum production. CC(=O)Os
Some metals such as magnesium, zinc, and iron undergo corrosion when exposed to
acetic acid. These reactions result in the formation of acetate salts. Glacial acetic acid
is obtained by dipping acetic acid solution in a “stalactite” of solid glacial acetic acid.
Acetic acid is naturally occurring and found in plants and animal organisms. It is
usually manufactured in a lab.
You might also like
- Conquering Chemistry Module 2Document88 pagesConquering Chemistry Module 2Shayan Lahijanian0% (1)
- Dodigen 2808 TDSDocument1 pageDodigen 2808 TDSRashid SaleemNo ratings yet
- EsterificationDocument3 pagesEsterificationKhris Sy-HandumonNo ratings yet
- Ethanoic Acid ChemDocument14 pagesEthanoic Acid ChemjasmynefNo ratings yet
- Literature Survey of Oxalic Acid ProductionDocument8 pagesLiterature Survey of Oxalic Acid Productionvarun singhNo ratings yet
- Oxalic Acid Expt 5 Chem 34 DraftDocument4 pagesOxalic Acid Expt 5 Chem 34 DraftVanessaOlgaJ.Dagondon100% (2)
- Exp 10Document9 pagesExp 10ChantalDanaNo ratings yet
- Carboxylic Acids: Basic Properties Project by Student Ghita AntoniaDocument7 pagesCarboxylic Acids: Basic Properties Project by Student Ghita AntoniaAntonia GhitaNo ratings yet
- Vol 4 Ascorbic AcidDocument1 pageVol 4 Ascorbic AcidregdddNo ratings yet
- Volumetric Analysis of Ascorbic Acid Present in Citrus FruitsDocument19 pagesVolumetric Analysis of Ascorbic Acid Present in Citrus FruitsSanjeev BabbarNo ratings yet
- Carboxylic Acid and Derivatives PDFDocument12 pagesCarboxylic Acid and Derivatives PDFÏt's RîçkgãrçīäNo ratings yet
- Chemistry Project ANTACIDDocument17 pagesChemistry Project ANTACIDMAX MusicNo ratings yet
- Acetic Acid: 2 PropertiesDocument12 pagesAcetic Acid: 2 PropertiesGudang MyosiNo ratings yet
- Bihari INVESTIGATORY PROJECT PDFDocument17 pagesBihari INVESTIGATORY PROJECT PDFDeepa SinghNo ratings yet
- Acids Bases and SaltsDocument68 pagesAcids Bases and SaltsDarien Gale EgdaneNo ratings yet
- 2019 Ch104 Organic AcidsDocument111 pages2019 Ch104 Organic Acidsgenesis gengerNo ratings yet
- Carboxylic Acid 1Document14 pagesCarboxylic Acid 1Junaid KhanNo ratings yet
- Project On ChemistryDocument13 pagesProject On ChemistryBadhal PaudelNo ratings yet
- Chemistry of Ascorbic AcidDocument16 pagesChemistry of Ascorbic AcidGordon YapNo ratings yet
- Inorganic Compounds With Economic, Industrial, Environmental and Social ImpactDocument4 pagesInorganic Compounds With Economic, Industrial, Environmental and Social ImpactScribdTranslationsNo ratings yet
- Part 1Document5 pagesPart 1eljon18No ratings yet
- Acetic AcidDocument11 pagesAcetic AcidPushpa KaladeviNo ratings yet
- PHARCHEM1 Lec C3Document6 pagesPHARCHEM1 Lec C3qwertyuioppoiuewqNo ratings yet
- Ascorbic Acid - The Chemistry Underlying Its Antioxidant PropertiesDocument29 pagesAscorbic Acid - The Chemistry Underlying Its Antioxidant PropertiesfgonzalezNo ratings yet
- Carboxylic Acid - SPM ChemistryDocument11 pagesCarboxylic Acid - SPM ChemistryVinnySha SelvarajahNo ratings yet
- I. Experiment Title: Aldehyde, Ketone and Carboxylic AcidDocument10 pagesI. Experiment Title: Aldehyde, Ketone and Carboxylic AcidLaily SafitriNo ratings yet
- Acid Base PH Chemical Equations 33Document10 pagesAcid Base PH Chemical Equations 33Girish JoshiNo ratings yet
- Note JJ JJDocument23 pagesNote JJ JJms9216974No ratings yet
- Carboxylic AcidsDocument15 pagesCarboxylic Acidsmadhu bonamNo ratings yet
- Uses of Laccases in The Food IndustryDocument9 pagesUses of Laccases in The Food Industrymayaalouane450No ratings yet
- Number of Atoms in Na2CO3Document3 pagesNumber of Atoms in Na2CO3Floila Jane YmasNo ratings yet
- Baqai J Health Sci 2011 14 2 33 40Document8 pagesBaqai J Health Sci 2011 14 2 33 40Cristina ElenaNo ratings yet
- Alcohols, Phenols and EthersDocument99 pagesAlcohols, Phenols and EthersLesterwin UdarbeNo ratings yet
- Acetic Acid-Properties and Its UsesDocument4 pagesAcetic Acid-Properties and Its UseskarthikeyanNo ratings yet
- Acids & Bases: Neha Kapil Scientific Officer NitraDocument33 pagesAcids & Bases: Neha Kapil Scientific Officer NitraNeha KapilNo ratings yet
- Analitik PresenDocument4 pagesAnalitik PresenMuhammad IrsyadNo ratings yet
- The Alkaline Hydrolysis ProcessDocument6 pagesThe Alkaline Hydrolysis ProcessNemanja MartinovićNo ratings yet
- Oxalic AcidDocument12 pagesOxalic AcidAnwaar KhanNo ratings yet
- Glycerol: Inorganic Chemical Compound Salt K Mno Oxidizing AgentDocument6 pagesGlycerol: Inorganic Chemical Compound Salt K Mno Oxidizing AgentfilchibuffNo ratings yet
- Carboxylic Acid byDocument38 pagesCarboxylic Acid byAuroraNo ratings yet
- Carboxylic Acid: From Wikipedia, The Free EncyclopediaDocument11 pagesCarboxylic Acid: From Wikipedia, The Free EncyclopediaDipti BagdeNo ratings yet
- Carboxylic AcidDocument13 pagesCarboxylic AcidJeremy NayagamNo ratings yet
- Agsam Shiela May R MODULE 8 Learning ActivitiesDocument3 pagesAgsam Shiela May R MODULE 8 Learning ActivitiesSecretNo ratings yet
- Lecture 3 - CarbohydratesDocument91 pagesLecture 3 - CarbohydratesAsyraf Arshat0% (1)
- AVCL 9A Properties of Carboxylic Acids and EstersDocument8 pagesAVCL 9A Properties of Carboxylic Acids and EstersGiane MadrigalNo ratings yet
- Chem Project Class 12 With Investigatory Project On ' Antacids'Document15 pagesChem Project Class 12 With Investigatory Project On ' Antacids'SHAHBAN55550% (1)
- Carboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeDocument9 pagesCarboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeFighter_ace_97No ratings yet
- Text 10.1Document6 pagesText 10.1Ashley MessamNo ratings yet
- Calcium OxalateDocument1 pageCalcium Oxalatekishe50No ratings yet
- 65 Ess16016 Dikonversi DikonversiDocument6 pages65 Ess16016 Dikonversi DikonversiMuhammad Ilham TaufiqNo ratings yet
- A. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentDocument27 pagesA. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentKeyvir AulinzNo ratings yet
- LayoutDocument55 pagesLayoutHenok Moges KassahunNo ratings yet
- Practical BiochemistryDocument108 pagesPractical BiochemistryCindy Nona100% (1)
- Names: Bryle Kristiann CamaroteDocument18 pagesNames: Bryle Kristiann CamaroteKateNo ratings yet
- Chem Project Class 12 With Investigatory Project OnDocument23 pagesChem Project Class 12 With Investigatory Project OnshashankNo ratings yet
- Chapter No.02 Carbohydrates IIDocument56 pagesChapter No.02 Carbohydrates IIsolutionsexpert70059165No ratings yet
- Jawahar Navodaya Vidhyalaya Angul: Analytical Project Chemistr YDocument16 pagesJawahar Navodaya Vidhyalaya Angul: Analytical Project Chemistr YCY CYMONNo ratings yet
- CHEMISTRY Project For Class 12Document19 pagesCHEMISTRY Project For Class 12Naveen Doss100% (1)
- Biochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingFrom EverandBiochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingRating: 4 out of 5 stars4/5 (1)
- Activity 2Document8 pagesActivity 2Xheena SarabiaNo ratings yet
- Answer Sheet For Activity 1 Lab Group 3Document5 pagesAnswer Sheet For Activity 1 Lab Group 3Xheena SarabiaNo ratings yet
- Act 3 J Gen ChemDocument5 pagesAct 3 J Gen ChemXheena SarabiaNo ratings yet
- Genchem Lec Act1Document5 pagesGenchem Lec Act1Xheena SarabiaNo ratings yet
- Genchem LecDocument2 pagesGenchem LecXheena SarabiaNo ratings yet
- GEN CHEM ACT 2, SerevoDocument12 pagesGEN CHEM ACT 2, SerevoXheena SarabiaNo ratings yet
- ReflectionLABPaperOnGenChem - XheenaSarabia IIIDDocument5 pagesReflectionLABPaperOnGenChem - XheenaSarabia IIIDXheena SarabiaNo ratings yet
- GenChem Activity 3Document3 pagesGenChem Activity 3Xheena SarabiaNo ratings yet
- Batomalaki, JelynT Activity1Document8 pagesBatomalaki, JelynT Activity1Xheena SarabiaNo ratings yet
- Dahdahchemlab ProjDocument3 pagesDahdahchemlab ProjXheena SarabiaNo ratings yet
- AbibibbibibibibibDocument1 pageAbibibbibibibibibXheena SarabiaNo ratings yet
- Abibi Chemlab ProjDocument2 pagesAbibi Chemlab ProjXheena SarabiaNo ratings yet
- Technicalwriting ReqsDocument6 pagesTechnicalwriting ReqsXheena SarabiaNo ratings yet
- SARABIA, XHEENA - Lesson 15 Properties of GasesDocument3 pagesSARABIA, XHEENA - Lesson 15 Properties of GasesXheena SarabiaNo ratings yet
- Part3 DRUGSDocument3 pagesPart3 DRUGSXheena SarabiaNo ratings yet
- Excuse LetterDocument1 pageExcuse LetterXheena SarabiaNo ratings yet
- Dictionary of Terms in CriminologyDocument27 pagesDictionary of Terms in CriminologyXheena Sarabia100% (1)
- DisputeRes XSDocument2 pagesDisputeRes XSXheena SarabiaNo ratings yet
- ANSI-IsO Turning Inserts 1Document5 pagesANSI-IsO Turning Inserts 1Parameshwaran PraveenNo ratings yet
- Pricelist Wef. 01112022 TRADEDocument5 pagesPricelist Wef. 01112022 TRADEGarena Free fireNo ratings yet
- Ds Chopped Strand Mat PBDocument2 pagesDs Chopped Strand Mat PBbasanth babuNo ratings yet
- Cost - Effective HousingDocument17 pagesCost - Effective HousingpriyankaNo ratings yet
- ChemistryTestfinal - LASTDocument30 pagesChemistryTestfinal - LASTpurpleasma64No ratings yet
- Silk Manufacturing ProcessDocument2 pagesSilk Manufacturing ProcessAjay Krishna0% (1)
- Product Data Sheet LT 80P: Siegling - Total Belting SolutionsDocument2 pagesProduct Data Sheet LT 80P: Siegling - Total Belting SolutionsGuilherme AugustoNo ratings yet
- Steel SlagDocument15 pagesSteel SlagNaveen B R 4AI19CV037No ratings yet
- Technical SpecificationDocument211 pagesTechnical SpecificationVitali MihalachiNo ratings yet
- Ovoid Pipe Profiles-SimonaDocument12 pagesOvoid Pipe Profiles-SimonaSankar CdmNo ratings yet
- Application Notes Welding (English)Document8 pagesApplication Notes Welding (English)metallurgist100% (6)
- Thurmalox 8200 Painting SpecificationDocument2 pagesThurmalox 8200 Painting SpecificationFreddy Carl FredricksenNo ratings yet
- Omv Comp VDL S 100Document2 pagesOmv Comp VDL S 100Robert IsacNo ratings yet
- The Headband PatternDocument4 pagesThe Headband PatternrarrinNo ratings yet
- Waterwall - Fireside CorrosionDocument8 pagesWaterwall - Fireside CorrosionwahonodNo ratings yet
- Penguard Tie Coat 100Document3 pagesPenguard Tie Coat 100Mohamed FarhanNo ratings yet
- List of Boiler ManufacturerDocument28 pagesList of Boiler ManufacturerGp MishraNo ratings yet
- F 3204 - 16Document4 pagesF 3204 - 16jose floresNo ratings yet
- 6.chemical Precipitation (07.04.2020)Document70 pages6.chemical Precipitation (07.04.2020)HARI PRASATHNo ratings yet
- Hydropalat WE 3650Document28 pagesHydropalat WE 3650APEX SONNo ratings yet
- Powder Production Techniques: AtomizationDocument13 pagesPowder Production Techniques: AtomizationVasantha SudasinghaNo ratings yet
- Unit 3 - Units For Expressing ConcentrationDocument24 pagesUnit 3 - Units For Expressing ConcentrationKhánh Vy NguyênNo ratings yet
- Coconut Husk Particle BoardDocument1 pageCoconut Husk Particle BoardKiran KumarNo ratings yet
- Green Concrete: By: Abhinav Srivastava 3 B. Tech. Civil Engg. February 2011Document36 pagesGreen Concrete: By: Abhinav Srivastava 3 B. Tech. Civil Engg. February 2011Abhinav Srivastava85% (13)
- Sicon MV Muffen Pi enDocument2 pagesSicon MV Muffen Pi enYip Mun FaiNo ratings yet
- Eco Friendly Cleaning ChemicalDocument12 pagesEco Friendly Cleaning ChemicalEcoChemNo ratings yet
- Unit Test Sample Paper - 1 Answer KeyDocument7 pagesUnit Test Sample Paper - 1 Answer Keymilonee lNo ratings yet
- A Presentation On Formation of Alkanes: Submitted By:-Arnab Paul (15) Dept: - Chemistry YEAR: - 1 SEM: - 2Document12 pagesA Presentation On Formation of Alkanes: Submitted By:-Arnab Paul (15) Dept: - Chemistry YEAR: - 1 SEM: - 2Arnab PaulNo ratings yet