Professional Documents
Culture Documents
Jursa N: Butte0Cle
Jursa N: Butte0Cle
Uploaded by
TreisLaloun Kaiduo XoreuounCopyright:
Available Formats
You might also like
- Classroom Instruction Delivery Alignment Map General Biology 1 Grade 11 (STEM)Document6 pagesClassroom Instruction Delivery Alignment Map General Biology 1 Grade 11 (STEM)Julius Memeg PanayoNo ratings yet
- Crystallographic Analysis Transition-State Mimics Bound To Penicillopepsin: Phosphorus-Containing Peptide Analogues?Document14 pagesCrystallographic Analysis Transition-State Mimics Bound To Penicillopepsin: Phosphorus-Containing Peptide Analogues?harisankarhsNo ratings yet
- DUF89-W ?: HAT ART ThouDocument14 pagesDUF89-W ?: HAT ART ThouRamya RallabandiNo ratings yet
- Characterization of Arabidopsis Tubby Like Proteins and Redundant Function of Attlp3 and Attlp9 in Plant Response To Aba and Osmotic StressDocument13 pagesCharacterization of Arabidopsis Tubby Like Proteins and Redundant Function of Attlp3 and Attlp9 in Plant Response To Aba and Osmotic StressAhsan Jamil ChaudhryNo ratings yet
- International Journal For ParasitologyDocument10 pagesInternational Journal For Parasitologymarceloicimoto2495No ratings yet
- 1 s2.0 0014579394009325 MainDocument5 pages1 s2.0 0014579394009325 MainMuhammad Andika PerdanaNo ratings yet
- The Role of Subunit Epsilon in The Catalysis and Regulation Off F - Atp SynthaseDocument13 pagesThe Role of Subunit Epsilon in The Catalysis and Regulation Off F - Atp SynthaseBara' HammadehNo ratings yet
- Molecular Dynamics Simulations of The Unfolding of Mutant Prion Variants at Elevated TemperaturesDocument10 pagesMolecular Dynamics Simulations of The Unfolding of Mutant Prion Variants at Elevated TemperaturesLeslieTettehNo ratings yet
- RNA SilencingDocument4 pagesRNA SilencingVictor JavierNo ratings yet
- Structure of The Lysine Specific Protease KGP From Porphyromonas Gingivalis, A Target For Improved Oral HealthDocument5 pagesStructure of The Lysine Specific Protease KGP From Porphyromonas Gingivalis, A Target For Improved Oral HealthJaime Plazas RománNo ratings yet
- Nature III ArtigoDocument7 pagesNature III ArtigoNaiara CélidaNo ratings yet
- Non Pumping Function of The PumpDocument9 pagesNon Pumping Function of The PumpnkmwisNo ratings yet
- Analytical Biochemistry: Recombinant Expression of Aryl Hydrocarbon Receptor For Quantitative Ligand-Binding AnalysisDocument9 pagesAnalytical Biochemistry: Recombinant Expression of Aryl Hydrocarbon Receptor For Quantitative Ligand-Binding AnalysisJe RivasNo ratings yet
- Terpenoids and Steroids - Vol11Document260 pagesTerpenoids and Steroids - Vol11Linh Phạm100% (2)
- How To Understand An ArticleDocument10 pagesHow To Understand An ArticleAdi SetiandanaNo ratings yet
- Distinguishing The Chemical Moiety of Phosphoenolpyruvate That Contributes To Allostery in Muscle Pyruvate KinaseDocument3 pagesDistinguishing The Chemical Moiety of Phosphoenolpyruvate That Contributes To Allostery in Muscle Pyruvate KinasecpunxzatawneyNo ratings yet
- Stereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectDocument7 pagesStereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectvycttorNo ratings yet
- X-Ray Diffraction, Solution Structure, and Computational Studies On Derivatives of (3-sec-Butyl-2,3-dihydro-1H-isoquinolin-4-ylidene) Acetic Acid: Compounds With Activity As Calpain InhibitorsDocument11 pagesX-Ray Diffraction, Solution Structure, and Computational Studies On Derivatives of (3-sec-Butyl-2,3-dihydro-1H-isoquinolin-4-ylidene) Acetic Acid: Compounds With Activity As Calpain InhibitorsSilvanaMedhatNo ratings yet
- Pyruvate CarboxylaseDocument5 pagesPyruvate CarboxylasedrgerterNo ratings yet
- Cold Adaptation Proteins: Characterization, The Heat-Labile From Antarctic PsychrophileDocument6 pagesCold Adaptation Proteins: Characterization, The Heat-Labile From Antarctic PsychrophileSelfi YantiNo ratings yet
- Previews: Mitobolites: The Elixir of LifeDocument2 pagesPreviews: Mitobolites: The Elixir of LifedupuytrenNo ratings yet
- A Review Leptin Structure and Mechanism Actions"Document8 pagesA Review Leptin Structure and Mechanism Actions"elenNo ratings yet
- Twofold CH Functionalization: Palladium-Catalyzed Ortho Arylation of AnilidesDocument5 pagesTwofold CH Functionalization: Palladium-Catalyzed Ortho Arylation of Anilidesmalala000No ratings yet
- Atomic Structure of The Cystic Fibrosis Transmembrane Conductance Regulator 2016 CellDocument22 pagesAtomic Structure of The Cystic Fibrosis Transmembrane Conductance Regulator 2016 CellEd Branco VictorNo ratings yet
- Cortactin Binding To F-Actin Revealed by Electron Microscopy and 3D ReconstructionDocument8 pagesCortactin Binding To F-Actin Revealed by Electron Microscopy and 3D ReconstructionLilian Elisa HernándezNo ratings yet
- Booth 2016Document22 pagesBooth 2016Miyyada AichaouiNo ratings yet
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967From EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterNo ratings yet
- Masato Nozawa Et Al - Total Synthesis of The Hallucinogenic Neoclerodane Diterpenoid Salvinorin ADocument5 pagesMasato Nozawa Et Al - Total Synthesis of The Hallucinogenic Neoclerodane Diterpenoid Salvinorin ABic0000No ratings yet
- 1985 Biochem Molecular Structure of The b3 Adrenergic ReceptorDocument7 pages1985 Biochem Molecular Structure of The b3 Adrenergic Receptorjames mellaleievNo ratings yet
- Oxidative Aromatic C-O Bond Formation: Synthesis of 3-Functionalized Benzo (B) Furans by Fecl - Mediated Ring Closure of R-Aryl KetonesDocument4 pagesOxidative Aromatic C-O Bond Formation: Synthesis of 3-Functionalized Benzo (B) Furans by Fecl - Mediated Ring Closure of R-Aryl KetonesHao MaNo ratings yet
- Chemistry of Protein Kinases and Dephosphorylases: Advanced ArticleDocument8 pagesChemistry of Protein Kinases and Dephosphorylases: Advanced ArticleazzaassNo ratings yet
- MBSS 2011 Program With AbstractsDocument12 pagesMBSS 2011 Program With AbstractsphotopidgeNo ratings yet
- The CLPXP Protease Unfolds Substrates Using A Constant Rate of Pulling But Different GearsDocument11 pagesThe CLPXP Protease Unfolds Substrates Using A Constant Rate of Pulling But Different Gearstoshit jainNo ratings yet
- Farmol 2Document27 pagesFarmol 2Ayu DemiNo ratings yet
- Zacharias 2002 Science - Partitioning of LipidDocument4 pagesZacharias 2002 Science - Partitioning of LipidAlfun IqbalNo ratings yet
- tmp7478 TMPDocument4 pagestmp7478 TMPFrontiersNo ratings yet
- Oocyte Maturation: Effect of Elevating: Regulation of Mouse Cumulus Cell Camp On Oocyte Camp Levels'Document7 pagesOocyte Maturation: Effect of Elevating: Regulation of Mouse Cumulus Cell Camp On Oocyte Camp Levels'sikasartikaNo ratings yet
- BJ CasDocument14 pagesBJ Casanilkumar995472No ratings yet
- Bacterio Fag OsDocument41 pagesBacterio Fag OsGgb111No ratings yet
- 08 Protein-Protein Interactions As New Drug TargetsDocument506 pages08 Protein-Protein Interactions As New Drug TargetsMT LinNo ratings yet
- New Class of Compounds For The Treatment of African TrypanosomiasisDocument1 pageNew Class of Compounds For The Treatment of African TrypanosomiasisDibyajyotiSamantrayNo ratings yet
- Organic Letters (2009), 11 (6), 1301-1304Document4 pagesOrganic Letters (2009), 11 (6), 1301-1304James TianNo ratings yet
- Demo Lecture 2022Document25 pagesDemo Lecture 2022Prechiel Avanzado-BarredoNo ratings yet
- Differential Expression Profiling of The Hepatic Proteome in A Rat Model of Dioxin ResistanceDocument13 pagesDifferential Expression Profiling of The Hepatic Proteome in A Rat Model of Dioxin Resistancegiasco78No ratings yet
- Molecular Dynamics Study of Oligonucleotides Containing DifluorotolueneDocument9 pagesMolecular Dynamics Study of Oligonucleotides Containing DifluorotolueneSveti JeronimNo ratings yet
- NSMB 1288Document3 pagesNSMB 1288biologiebmNo ratings yet
- Characterization of The Taxol Structure-Activity Profile For The Locus of The A-Ring Side ChainDocument6 pagesCharacterization of The Taxol Structure-Activity Profile For The Locus of The A-Ring Side ChainNguyen V. N. TungNo ratings yet
- Qual ProtDocument11 pagesQual Prot12saoirse34No ratings yet
- In vivo demonstration that α-synuclein oligomers are toxic: a b c d e fDocument6 pagesIn vivo demonstration that α-synuclein oligomers are toxic: a b c d e fFatima Herranz TrilloNo ratings yet
- IEP-CV+research SummaryDocument3 pagesIEP-CV+research Summaryivanperez2014No ratings yet
- Acetylated - Tubulin Is Reduced in Individuals With P - 2014 - Fertility and SteDocument13 pagesAcetylated - Tubulin Is Reduced in Individuals With P - 2014 - Fertility and Stelucifierop6No ratings yet
- The Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisDocument11 pagesThe Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisUhrigNo ratings yet
- SAT5A - Flisikowski (2003)Document10 pagesSAT5A - Flisikowski (2003)Le Minh Thanh 010086No ratings yet
- Coordinate Regulation Cytochrome Alternative Pathway Respiration in Tobacco1Document6 pagesCoordinate Regulation Cytochrome Alternative Pathway Respiration in Tobacco1Srimeenakshi ShankarNo ratings yet
- Searching For Folded Proteins And: in Vitro in SilicoDocument8 pagesSearching For Folded Proteins And: in Vitro in SilicoVenkata Suryanarayana GorleNo ratings yet
- Signal Transduction:: Phosphatase 5 Function: A LINK S100 Proteins Modulate ProteinDocument13 pagesSignal Transduction:: Phosphatase 5 Function: A LINK S100 Proteins Modulate ProteinskljoleNo ratings yet
- Essential Cell Biology: Protein Structure and FunctionDocument57 pagesEssential Cell Biology: Protein Structure and Functionpetemaravich333100% (1)
- Vps41 Phosphorylation and The Rab Ypt7 Control The Targeting of The HOPS Complex To Endosome-Vacuole Fusion SitesDocument12 pagesVps41 Phosphorylation and The Rab Ypt7 Control The Targeting of The HOPS Complex To Endosome-Vacuole Fusion SitesMargarita CabreraNo ratings yet
- Amino Acid Substitutions Around The Chromophore of The Chromoprotein Rtms5 in Uence Polypeptide CleavageDocument5 pagesAmino Acid Substitutions Around The Chromophore of The Chromoprotein Rtms5 in Uence Polypeptide CleavageAndrea PilarNo ratings yet
- Organic Synthesis - 2: Plenary Lectures Presented at the Second International Symposium on Organic SynthesisFrom EverandOrganic Synthesis - 2: Plenary Lectures Presented at the Second International Symposium on Organic SynthesisS. SarelNo ratings yet
- Molecular Diseases: FEBS Federation of European Biochemical Societies: 12th Meeting, Dresden, 1978From EverandMolecular Diseases: FEBS Federation of European Biochemical Societies: 12th Meeting, Dresden, 1978G. JacobaschNo ratings yet
- Elicob 5Document7 pagesElicob 5Corrado RuffiniNo ratings yet
- Segs-Chemistry of Life: Problem SetDocument4 pagesSegs-Chemistry of Life: Problem SetMelindaSuitosColobong-GarpidaNo ratings yet
- L9-10: Genetic Engineering of Biosynthetic Pathways in Transgenic PlantsDocument72 pagesL9-10: Genetic Engineering of Biosynthetic Pathways in Transgenic PlantsTing Yan LeeNo ratings yet
- Perihal: Penawaran Harga Set Untuk Laboratorium Pemeriksaan Covid-19 Di Provinsi Banten TA 2021Document2 pagesPerihal: Penawaran Harga Set Untuk Laboratorium Pemeriksaan Covid-19 Di Provinsi Banten TA 2021M A J I D R I M B A N INo ratings yet
- Genetics Cancer - 4th EdDocument208 pagesGenetics Cancer - 4th Edjaswinireddy.potuganumaNo ratings yet
- Spring 2003 Molecular Biology Exam #3 - Final Exam: To Insert A Screen Shot Into Your Test FileDocument7 pagesSpring 2003 Molecular Biology Exam #3 - Final Exam: To Insert A Screen Shot Into Your Test File畏No ratings yet
- Lipid Maps Leipzig 2018Document72 pagesLipid Maps Leipzig 2018first name last nameNo ratings yet
- Csl-Cellas IDocument1 pageCsl-Cellas IThiết bị y tế Thịnh VượngNo ratings yet
- 1.03 Secondary HemostasisDocument8 pages1.03 Secondary HemostasisShiena ArchividoNo ratings yet
- Monte Carlo ProteinDocument4 pagesMonte Carlo ProteinMango JuiceNo ratings yet
- AP Bio 6.4-6.6 OutlineDocument5 pagesAP Bio 6.4-6.6 OutlineShivali PatelNo ratings yet
- Metabolic Biochemistry Experiment 8Document7 pagesMetabolic Biochemistry Experiment 8门门No ratings yet
- Molecular Biology of The Cell Cumulative Final Exam Study GuideDocument22 pagesMolecular Biology of The Cell Cumulative Final Exam Study Guiderazgriz1211No ratings yet
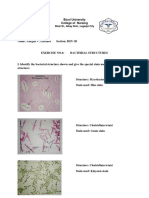
- Listanco Abegail Lab Exercise#6Document3 pagesListanco Abegail Lab Exercise#6Abegail ListancoNo ratings yet
- Good Laboratory Practise PCR Qsop38 2010Document12 pagesGood Laboratory Practise PCR Qsop38 2010Abu KanshaNo ratings yet
- LigasesDocument7 pagesLigasesSuraj PatilNo ratings yet
- Mindoro State College of Agriculture and Technology: I. ObjectivesDocument7 pagesMindoro State College of Agriculture and Technology: I. ObjectivesJunjun CaoliNo ratings yet
- Léchaudel Et Al 2018Document12 pagesLéchaudel Et Al 2018Jandira CostaNo ratings yet
- Docking Molekuler Senyawa Metabolit Sekunder Lantana Camara Sebagai Antiinflamasi Terhadap EnzimDocument5 pagesDocking Molekuler Senyawa Metabolit Sekunder Lantana Camara Sebagai Antiinflamasi Terhadap EnzimAsmaun AzisNo ratings yet
- Biology and Treatment of Leukemia and Bone Marrow NeoplasmsDocument200 pagesBiology and Treatment of Leukemia and Bone Marrow Neoplasmsyves2022sahaNo ratings yet
- University Institute of Pharmacy and Institute of Biosciences & Biotechnology C.S.J.M.U. KanpurDocument2 pagesUniversity Institute of Pharmacy and Institute of Biosciences & Biotechnology C.S.J.M.U. KanpurDeepak PatelNo ratings yet
- Secondary School Compiled Biology Notes PDFDocument157 pagesSecondary School Compiled Biology Notes PDFLiSiyu100% (4)
- Lecture 1 - Genes and GenomicsDocument51 pagesLecture 1 - Genes and GenomicsDeepali SinghNo ratings yet
- BS - A M: Faculty of AgricultureDocument3 pagesBS - A M: Faculty of Agriculturekingwsley njanikeNo ratings yet
- 1st Quarter Examination in ELS April DocsDocument2 pages1st Quarter Examination in ELS April DocsCindy PalenNo ratings yet
- Fatty Acid Synthesis: 28.1 Stages of FA SynthesisDocument13 pagesFatty Acid Synthesis: 28.1 Stages of FA SynthesisrJNo ratings yet
- Mechanism of Drug ActionDocument24 pagesMechanism of Drug ActionBandita Datta100% (1)
- Lipids 2Document6 pagesLipids 2cumbredinNo ratings yet
- Zymogen, Isozymes, Abzymes, RibozymesDocument9 pagesZymogen, Isozymes, Abzymes, Ribozymesr6jxkkg7nqNo ratings yet
Jursa N: Butte0Cle
Jursa N: Butte0Cle
Uploaded by
TreisLaloun Kaiduo XoreuounOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jursa N: Butte0Cle
Jursa N: Butte0Cle
Uploaded by
TreisLaloun Kaiduo XoreuounCopyright:
Available Formats
Biochem. J.
(1988) 255, 1061 (Printed in Great Britain)
JURSA Li II
BUttE0CLETTERS
n
respectively. Furthermore, ATP preparations from several other commercial sources were identical in this respect. AMP and ADP possessed IC50 values in excess of 5x 10-3M. It is not clear why these data differ from those in pituitary microsomes, or indeed if the inhibition by ATP is competitive; however, it still remains possible that the discrepancy between the effectiveness of Ins(1,4,5)P3 in releasing Ca2" and its affinity for receptor binding relates to the presence of greater-than-millimolar concentrations of ATP in many of the former systems. In this context it should be noted that a very recent report [8] has shown that, in the absence of ATP, Ins( 1,4,5)P3 can release Ca2" from intracellular stores of permeabilized rat basophilic leukaemic cells in a highly co-operative manner in the 4-40 nm concentration range.
1061
ATP and the binding of I3Hlinositol 1,4,5trisphosphate to its receptor
There are now several reports of the specific reversible binding of inositol 1,4,5-trisphosphate (InsP3) to permeabilized cells or particulate preparations of several tissues [1,2,3,4]. In some [1,2,3], though not all [4], of these preparations, equilibrium dissociation constants (KD) for InsP3 binding have been reported to be in the low nanomolar range, whereas effective concentrations to release Ca2" from intracellular stores, often from the same preparation, are one to two orders of magnitude greater. This could suggest that the binding site and sites associated with Ca2+ release are different or that the conditions of the assays substantially alter the binding affinity of InsP. In this context we have reported that ATP, which is generally present in millimolar concentrations in Ca2+release assays, inhibited the specific binding of [3H]Ins(1,4,5)P3 to a particulate site prepared from rat cerebellum [IC50 (concentration producing 50 % inhibition) 51 /tM] [5]. Since these sites have been characterized for stereoand positional specificity and possess qualitative properties in this respect very similar to those associated with Ca2' release [6], we considered that the difference in apparent affinity between the systems could relate to the presence of ATP in the Ca2' assays. Inhibitory effects of ATP on InsP3 binding have been confirmed in other systems [1, 3], but a recent letter to this journal [7] suggested that this inhibitory effect was not related to ATP itself but to some impurity in certain preparations. In particular, it was reported that ATP from Pharmacia (type 27-1006-0) is inactive on InsP3 binding to pituitary
microsomes.
Alan L. WILLCOCKS and Stefan R. NAHORSKI
Department of Pharmacology and Therapeutics, University of Leicester, P.O. Box 138, Medical Sciences Building, University Road, Leicester LEI 9HN, U.K.
1. Guillemette, G., Balla, T., Baukal, A. J. & Catt, K. J. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 8195-8199 2. Spat, A., Bradford, P. G., McKinney, J. S., Rubin, R. P. & Putney, J. W. (1986) Nature (London) 319, 514-516 3. Guillemette, G., Balla, T., Baukal, A. J. & Catt, K. J. (1988) J. Biol. Chem. 263, 4541-4548 4. Worley, P. F., Baraban, J. M., Colvin, J. S. & Snyder, S. H. (1987) Nature (London) 325, 159-161 5. Willcocks, A. L., Cooke, A. M., Potter, B. V. L. & Nahorski, S. R. (1987) Biochem. Biophys. Res. Commun. 146, 1071-1078 6. Strupish, J., Cooke, A. M., Potter, B. V. L., Gigg, R. & Nahorski, S. R. (1988) Biochem. J. 253, 901-905 7. Eberhardt, I., Kiesel, L. & Spat, A. (1988) Biochem. J. 250, 311 8. Meyer, T., Holowka, D. & Stryer, L. (1988) Science 240, 653-656
Received 28 July 1988
Using our cerebellar membrane preparation, which has been carefully evaluated for stereo- and positional specificity [5], we have failed to reproduce these results. For example, in our hands ATP (Sigma A2383) and ATP (Pharmacia, type 27-1006-0) produce superimposible inhibition curves against 15 nM-[3H]Ins(1,4,5)P3 with IC50 values of 7.9 ( + 0.67) x 10- M and 7.6 ( 0.80) x 10- M
Vol. 255
You might also like
- Classroom Instruction Delivery Alignment Map General Biology 1 Grade 11 (STEM)Document6 pagesClassroom Instruction Delivery Alignment Map General Biology 1 Grade 11 (STEM)Julius Memeg PanayoNo ratings yet
- Crystallographic Analysis Transition-State Mimics Bound To Penicillopepsin: Phosphorus-Containing Peptide Analogues?Document14 pagesCrystallographic Analysis Transition-State Mimics Bound To Penicillopepsin: Phosphorus-Containing Peptide Analogues?harisankarhsNo ratings yet
- DUF89-W ?: HAT ART ThouDocument14 pagesDUF89-W ?: HAT ART ThouRamya RallabandiNo ratings yet
- Characterization of Arabidopsis Tubby Like Proteins and Redundant Function of Attlp3 and Attlp9 in Plant Response To Aba and Osmotic StressDocument13 pagesCharacterization of Arabidopsis Tubby Like Proteins and Redundant Function of Attlp3 and Attlp9 in Plant Response To Aba and Osmotic StressAhsan Jamil ChaudhryNo ratings yet
- International Journal For ParasitologyDocument10 pagesInternational Journal For Parasitologymarceloicimoto2495No ratings yet
- 1 s2.0 0014579394009325 MainDocument5 pages1 s2.0 0014579394009325 MainMuhammad Andika PerdanaNo ratings yet
- The Role of Subunit Epsilon in The Catalysis and Regulation Off F - Atp SynthaseDocument13 pagesThe Role of Subunit Epsilon in The Catalysis and Regulation Off F - Atp SynthaseBara' HammadehNo ratings yet
- Molecular Dynamics Simulations of The Unfolding of Mutant Prion Variants at Elevated TemperaturesDocument10 pagesMolecular Dynamics Simulations of The Unfolding of Mutant Prion Variants at Elevated TemperaturesLeslieTettehNo ratings yet
- RNA SilencingDocument4 pagesRNA SilencingVictor JavierNo ratings yet
- Structure of The Lysine Specific Protease KGP From Porphyromonas Gingivalis, A Target For Improved Oral HealthDocument5 pagesStructure of The Lysine Specific Protease KGP From Porphyromonas Gingivalis, A Target For Improved Oral HealthJaime Plazas RománNo ratings yet
- Nature III ArtigoDocument7 pagesNature III ArtigoNaiara CélidaNo ratings yet
- Non Pumping Function of The PumpDocument9 pagesNon Pumping Function of The PumpnkmwisNo ratings yet
- Analytical Biochemistry: Recombinant Expression of Aryl Hydrocarbon Receptor For Quantitative Ligand-Binding AnalysisDocument9 pagesAnalytical Biochemistry: Recombinant Expression of Aryl Hydrocarbon Receptor For Quantitative Ligand-Binding AnalysisJe RivasNo ratings yet
- Terpenoids and Steroids - Vol11Document260 pagesTerpenoids and Steroids - Vol11Linh Phạm100% (2)
- How To Understand An ArticleDocument10 pagesHow To Understand An ArticleAdi SetiandanaNo ratings yet
- Distinguishing The Chemical Moiety of Phosphoenolpyruvate That Contributes To Allostery in Muscle Pyruvate KinaseDocument3 pagesDistinguishing The Chemical Moiety of Phosphoenolpyruvate That Contributes To Allostery in Muscle Pyruvate KinasecpunxzatawneyNo ratings yet
- Stereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectDocument7 pagesStereoelectronic Effects On The Basicity and Nucleophilicity of Phosphites and Phosphates. Ab Initio Molecular Orbital Calculations and The A-EffectvycttorNo ratings yet
- X-Ray Diffraction, Solution Structure, and Computational Studies On Derivatives of (3-sec-Butyl-2,3-dihydro-1H-isoquinolin-4-ylidene) Acetic Acid: Compounds With Activity As Calpain InhibitorsDocument11 pagesX-Ray Diffraction, Solution Structure, and Computational Studies On Derivatives of (3-sec-Butyl-2,3-dihydro-1H-isoquinolin-4-ylidene) Acetic Acid: Compounds With Activity As Calpain InhibitorsSilvanaMedhatNo ratings yet
- Pyruvate CarboxylaseDocument5 pagesPyruvate CarboxylasedrgerterNo ratings yet
- Cold Adaptation Proteins: Characterization, The Heat-Labile From Antarctic PsychrophileDocument6 pagesCold Adaptation Proteins: Characterization, The Heat-Labile From Antarctic PsychrophileSelfi YantiNo ratings yet
- Previews: Mitobolites: The Elixir of LifeDocument2 pagesPreviews: Mitobolites: The Elixir of LifedupuytrenNo ratings yet
- A Review Leptin Structure and Mechanism Actions"Document8 pagesA Review Leptin Structure and Mechanism Actions"elenNo ratings yet
- Twofold CH Functionalization: Palladium-Catalyzed Ortho Arylation of AnilidesDocument5 pagesTwofold CH Functionalization: Palladium-Catalyzed Ortho Arylation of Anilidesmalala000No ratings yet
- Atomic Structure of The Cystic Fibrosis Transmembrane Conductance Regulator 2016 CellDocument22 pagesAtomic Structure of The Cystic Fibrosis Transmembrane Conductance Regulator 2016 CellEd Branco VictorNo ratings yet
- Cortactin Binding To F-Actin Revealed by Electron Microscopy and 3D ReconstructionDocument8 pagesCortactin Binding To F-Actin Revealed by Electron Microscopy and 3D ReconstructionLilian Elisa HernándezNo ratings yet
- Booth 2016Document22 pagesBooth 2016Miyyada AichaouiNo ratings yet
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967From EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterNo ratings yet
- Masato Nozawa Et Al - Total Synthesis of The Hallucinogenic Neoclerodane Diterpenoid Salvinorin ADocument5 pagesMasato Nozawa Et Al - Total Synthesis of The Hallucinogenic Neoclerodane Diterpenoid Salvinorin ABic0000No ratings yet
- 1985 Biochem Molecular Structure of The b3 Adrenergic ReceptorDocument7 pages1985 Biochem Molecular Structure of The b3 Adrenergic Receptorjames mellaleievNo ratings yet
- Oxidative Aromatic C-O Bond Formation: Synthesis of 3-Functionalized Benzo (B) Furans by Fecl - Mediated Ring Closure of R-Aryl KetonesDocument4 pagesOxidative Aromatic C-O Bond Formation: Synthesis of 3-Functionalized Benzo (B) Furans by Fecl - Mediated Ring Closure of R-Aryl KetonesHao MaNo ratings yet
- Chemistry of Protein Kinases and Dephosphorylases: Advanced ArticleDocument8 pagesChemistry of Protein Kinases and Dephosphorylases: Advanced ArticleazzaassNo ratings yet
- MBSS 2011 Program With AbstractsDocument12 pagesMBSS 2011 Program With AbstractsphotopidgeNo ratings yet
- The CLPXP Protease Unfolds Substrates Using A Constant Rate of Pulling But Different GearsDocument11 pagesThe CLPXP Protease Unfolds Substrates Using A Constant Rate of Pulling But Different Gearstoshit jainNo ratings yet
- Farmol 2Document27 pagesFarmol 2Ayu DemiNo ratings yet
- Zacharias 2002 Science - Partitioning of LipidDocument4 pagesZacharias 2002 Science - Partitioning of LipidAlfun IqbalNo ratings yet
- tmp7478 TMPDocument4 pagestmp7478 TMPFrontiersNo ratings yet
- Oocyte Maturation: Effect of Elevating: Regulation of Mouse Cumulus Cell Camp On Oocyte Camp Levels'Document7 pagesOocyte Maturation: Effect of Elevating: Regulation of Mouse Cumulus Cell Camp On Oocyte Camp Levels'sikasartikaNo ratings yet
- BJ CasDocument14 pagesBJ Casanilkumar995472No ratings yet
- Bacterio Fag OsDocument41 pagesBacterio Fag OsGgb111No ratings yet
- 08 Protein-Protein Interactions As New Drug TargetsDocument506 pages08 Protein-Protein Interactions As New Drug TargetsMT LinNo ratings yet
- New Class of Compounds For The Treatment of African TrypanosomiasisDocument1 pageNew Class of Compounds For The Treatment of African TrypanosomiasisDibyajyotiSamantrayNo ratings yet
- Organic Letters (2009), 11 (6), 1301-1304Document4 pagesOrganic Letters (2009), 11 (6), 1301-1304James TianNo ratings yet
- Demo Lecture 2022Document25 pagesDemo Lecture 2022Prechiel Avanzado-BarredoNo ratings yet
- Differential Expression Profiling of The Hepatic Proteome in A Rat Model of Dioxin ResistanceDocument13 pagesDifferential Expression Profiling of The Hepatic Proteome in A Rat Model of Dioxin Resistancegiasco78No ratings yet
- Molecular Dynamics Study of Oligonucleotides Containing DifluorotolueneDocument9 pagesMolecular Dynamics Study of Oligonucleotides Containing DifluorotolueneSveti JeronimNo ratings yet
- NSMB 1288Document3 pagesNSMB 1288biologiebmNo ratings yet
- Characterization of The Taxol Structure-Activity Profile For The Locus of The A-Ring Side ChainDocument6 pagesCharacterization of The Taxol Structure-Activity Profile For The Locus of The A-Ring Side ChainNguyen V. N. TungNo ratings yet
- Qual ProtDocument11 pagesQual Prot12saoirse34No ratings yet
- In vivo demonstration that α-synuclein oligomers are toxic: a b c d e fDocument6 pagesIn vivo demonstration that α-synuclein oligomers are toxic: a b c d e fFatima Herranz TrilloNo ratings yet
- IEP-CV+research SummaryDocument3 pagesIEP-CV+research Summaryivanperez2014No ratings yet
- Acetylated - Tubulin Is Reduced in Individuals With P - 2014 - Fertility and SteDocument13 pagesAcetylated - Tubulin Is Reduced in Individuals With P - 2014 - Fertility and Stelucifierop6No ratings yet
- The Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisDocument11 pagesThe Plant Journal - 2010 - J Rgens - A Role For ABIL3 in Plant Cell MorphogenesisUhrigNo ratings yet
- SAT5A - Flisikowski (2003)Document10 pagesSAT5A - Flisikowski (2003)Le Minh Thanh 010086No ratings yet
- Coordinate Regulation Cytochrome Alternative Pathway Respiration in Tobacco1Document6 pagesCoordinate Regulation Cytochrome Alternative Pathway Respiration in Tobacco1Srimeenakshi ShankarNo ratings yet
- Searching For Folded Proteins And: in Vitro in SilicoDocument8 pagesSearching For Folded Proteins And: in Vitro in SilicoVenkata Suryanarayana GorleNo ratings yet
- Signal Transduction:: Phosphatase 5 Function: A LINK S100 Proteins Modulate ProteinDocument13 pagesSignal Transduction:: Phosphatase 5 Function: A LINK S100 Proteins Modulate ProteinskljoleNo ratings yet
- Essential Cell Biology: Protein Structure and FunctionDocument57 pagesEssential Cell Biology: Protein Structure and Functionpetemaravich333100% (1)
- Vps41 Phosphorylation and The Rab Ypt7 Control The Targeting of The HOPS Complex To Endosome-Vacuole Fusion SitesDocument12 pagesVps41 Phosphorylation and The Rab Ypt7 Control The Targeting of The HOPS Complex To Endosome-Vacuole Fusion SitesMargarita CabreraNo ratings yet
- Amino Acid Substitutions Around The Chromophore of The Chromoprotein Rtms5 in Uence Polypeptide CleavageDocument5 pagesAmino Acid Substitutions Around The Chromophore of The Chromoprotein Rtms5 in Uence Polypeptide CleavageAndrea PilarNo ratings yet
- Organic Synthesis - 2: Plenary Lectures Presented at the Second International Symposium on Organic SynthesisFrom EverandOrganic Synthesis - 2: Plenary Lectures Presented at the Second International Symposium on Organic SynthesisS. SarelNo ratings yet
- Molecular Diseases: FEBS Federation of European Biochemical Societies: 12th Meeting, Dresden, 1978From EverandMolecular Diseases: FEBS Federation of European Biochemical Societies: 12th Meeting, Dresden, 1978G. JacobaschNo ratings yet
- Elicob 5Document7 pagesElicob 5Corrado RuffiniNo ratings yet
- Segs-Chemistry of Life: Problem SetDocument4 pagesSegs-Chemistry of Life: Problem SetMelindaSuitosColobong-GarpidaNo ratings yet
- L9-10: Genetic Engineering of Biosynthetic Pathways in Transgenic PlantsDocument72 pagesL9-10: Genetic Engineering of Biosynthetic Pathways in Transgenic PlantsTing Yan LeeNo ratings yet
- Perihal: Penawaran Harga Set Untuk Laboratorium Pemeriksaan Covid-19 Di Provinsi Banten TA 2021Document2 pagesPerihal: Penawaran Harga Set Untuk Laboratorium Pemeriksaan Covid-19 Di Provinsi Banten TA 2021M A J I D R I M B A N INo ratings yet
- Genetics Cancer - 4th EdDocument208 pagesGenetics Cancer - 4th Edjaswinireddy.potuganumaNo ratings yet
- Spring 2003 Molecular Biology Exam #3 - Final Exam: To Insert A Screen Shot Into Your Test FileDocument7 pagesSpring 2003 Molecular Biology Exam #3 - Final Exam: To Insert A Screen Shot Into Your Test File畏No ratings yet
- Lipid Maps Leipzig 2018Document72 pagesLipid Maps Leipzig 2018first name last nameNo ratings yet
- Csl-Cellas IDocument1 pageCsl-Cellas IThiết bị y tế Thịnh VượngNo ratings yet
- 1.03 Secondary HemostasisDocument8 pages1.03 Secondary HemostasisShiena ArchividoNo ratings yet
- Monte Carlo ProteinDocument4 pagesMonte Carlo ProteinMango JuiceNo ratings yet
- AP Bio 6.4-6.6 OutlineDocument5 pagesAP Bio 6.4-6.6 OutlineShivali PatelNo ratings yet
- Metabolic Biochemistry Experiment 8Document7 pagesMetabolic Biochemistry Experiment 8门门No ratings yet
- Molecular Biology of The Cell Cumulative Final Exam Study GuideDocument22 pagesMolecular Biology of The Cell Cumulative Final Exam Study Guiderazgriz1211No ratings yet
- Listanco Abegail Lab Exercise#6Document3 pagesListanco Abegail Lab Exercise#6Abegail ListancoNo ratings yet
- Good Laboratory Practise PCR Qsop38 2010Document12 pagesGood Laboratory Practise PCR Qsop38 2010Abu KanshaNo ratings yet
- LigasesDocument7 pagesLigasesSuraj PatilNo ratings yet
- Mindoro State College of Agriculture and Technology: I. ObjectivesDocument7 pagesMindoro State College of Agriculture and Technology: I. ObjectivesJunjun CaoliNo ratings yet
- Léchaudel Et Al 2018Document12 pagesLéchaudel Et Al 2018Jandira CostaNo ratings yet
- Docking Molekuler Senyawa Metabolit Sekunder Lantana Camara Sebagai Antiinflamasi Terhadap EnzimDocument5 pagesDocking Molekuler Senyawa Metabolit Sekunder Lantana Camara Sebagai Antiinflamasi Terhadap EnzimAsmaun AzisNo ratings yet
- Biology and Treatment of Leukemia and Bone Marrow NeoplasmsDocument200 pagesBiology and Treatment of Leukemia and Bone Marrow Neoplasmsyves2022sahaNo ratings yet
- University Institute of Pharmacy and Institute of Biosciences & Biotechnology C.S.J.M.U. KanpurDocument2 pagesUniversity Institute of Pharmacy and Institute of Biosciences & Biotechnology C.S.J.M.U. KanpurDeepak PatelNo ratings yet
- Secondary School Compiled Biology Notes PDFDocument157 pagesSecondary School Compiled Biology Notes PDFLiSiyu100% (4)
- Lecture 1 - Genes and GenomicsDocument51 pagesLecture 1 - Genes and GenomicsDeepali SinghNo ratings yet
- BS - A M: Faculty of AgricultureDocument3 pagesBS - A M: Faculty of Agriculturekingwsley njanikeNo ratings yet
- 1st Quarter Examination in ELS April DocsDocument2 pages1st Quarter Examination in ELS April DocsCindy PalenNo ratings yet
- Fatty Acid Synthesis: 28.1 Stages of FA SynthesisDocument13 pagesFatty Acid Synthesis: 28.1 Stages of FA SynthesisrJNo ratings yet
- Mechanism of Drug ActionDocument24 pagesMechanism of Drug ActionBandita Datta100% (1)
- Lipids 2Document6 pagesLipids 2cumbredinNo ratings yet
- Zymogen, Isozymes, Abzymes, RibozymesDocument9 pagesZymogen, Isozymes, Abzymes, Ribozymesr6jxkkg7nqNo ratings yet