Professional Documents
Culture Documents
Basic Chemistry About Acid Alkonoid and Acid Alkyl Alkonoic
Basic Chemistry About Acid Alkonoid and Acid Alkyl Alkonoic
Uploaded by
St.khalisahOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Basic Chemistry About Acid Alkonoid and Acid Alkyl Alkonoic
Basic Chemistry About Acid Alkonoid and Acid Alkyl Alkonoic
Uploaded by
St.khalisahCopyright:
Available Formats
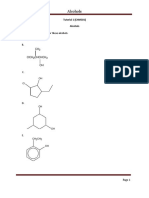
chemical
EXERCISE
1. write down the general formulas of alkonic acid and alkyl alkonat acid .
2. Write the structure of the alkonic acid (carboxylic acid).
a) methanoic acid
b) acid ethanoic
3. write the structure of the alicyl alkonoate (ester) compound.
a) methyl methanoate
b) methyl ethanoate
c) ethyl propanoate
4. Write down the isomer of C3h6o
5. write some uses of carboxylic compounds and esters (three each).
6. write the properties of alkonoic acids and alkyl konoates ( esters )
Answer :
1. general formula of alkonoic (carboxylic) acid.
R–COOH with R = H or R= CnH2n+1.
General formula alkyl alkonoat acid.
O
II
R – C - O – R or = CnH2nO
II
O
2. a). Methanoic acid
O
II
H - C – OH
b). Acid ethanoic
O
II
CH3 – C – OH
3. a). Methyl methanoate
O
II
H - C – O – CH3
b). Methyl ethanoate
O
II
CH3 - C – O – CH3
c). Ethyl propanoate
O
II
CH3 – CH2 – C – CH2 – CH3
4. Isomer CH36O
• From alkyl alkonat Acid
O
II
• CH3 – C – O - CH3 = Methyl ethanoic
• H – C – O – CH2 – CH3 = Ethyl Methanoic
• From alkonat acid ( Carboxylat)
O
II
• CH3 – CH2 - C – OH = Acid Propanoate
5. a). Alkonoic acid applications (carboxylic)
1). Formic acid is utilized in the leather tanning business as well as for the
compounding of medications (such as aspirin), insect control, and latex
coagulation.
2). Acetic acid, which is utilized as a food ingredient and preservative, has a 5%
concentration.
3). galciol acetic acid (pure vinegar) for the production of polymers, coloring agents,
and cellulose acetate.
b). Alkyl alkonoates (esters) are used
1). Alkyl alkonoates (esters) are used Amyl acetate smells like pineapple, while ethyl
acetate smells like bananas.
2). as paint/coating for automobiles and furnishings, using the formula C25H51COO -
C3OH61
3). as an ingredient for soap.
4). as a remover of nail polish
6. a). Alkonoic (carboxylic) acid characteristics
1). Weak acid .
2). More soluble in nonpolar liquids.
3). Generally nonpolar.
4). Alcohol has greater freezing and boiling points than water.
5). Water solubility of methanoic, ethanoic, propanoic, and butanoic acids.
6). Pentanoic and hexanoic acids are only a little soluble in water.
7). Water is insoluble in alkonoic acid.
b). Alkyl alkonoates' characteristics (esters)
1). Compounds of neutral carbon
2. has a fruity scent.
3). water soluble.
4). lower freezing and boiling values than alconoic acid.
5). At low temperatures, a small number of C atoms or low tribes are liquid.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- ACTIVITY 7 Milk of MagnesiaDocument4 pagesACTIVITY 7 Milk of Magnesiadaven25% (4)
- Note JJ JJDocument23 pagesNote JJ JJms9216974No ratings yet
- P24 Answers Kweyete AlbertDocument7 pagesP24 Answers Kweyete AlbertMuhammad HashirNo ratings yet
- Organic Molecules React in Predictable Ways: Functional GroupsDocument21 pagesOrganic Molecules React in Predictable Ways: Functional GroupsNia Rakhmayanti NurdinNo ratings yet
- ESTERSDocument37 pagesESTERSFirdausia Rahma PutriNo ratings yet
- Scienceclinic Smartprep GR12 Dbe Eng 2023 V4.1Document6 pagesScienceclinic Smartprep GR12 Dbe Eng 2023 V4.1Skyla GallantNo ratings yet
- Organic Chemistry 2 - Chem - f3 - v1Document66 pagesOrganic Chemistry 2 - Chem - f3 - v1Lubanga N JamesNo ratings yet
- c23 - Alcohols and Carboxylic AcidsDocument7 pagesc23 - Alcohols and Carboxylic AcidsgarethongshNo ratings yet
- CC One ShotDocument29 pagesCC One Shotbobbytext8904No ratings yet
- Carbon CompoundDocument16 pagesCarbon CompoundAidah AmirNo ratings yet
- Topic 3 Carbonyl CompoundDocument25 pagesTopic 3 Carbonyl CompoundacatcomelNo ratings yet
- 3 4 Carbonic Acids and Heterofunctional CompoundsDocument38 pages3 4 Carbonic Acids and Heterofunctional CompoundsDharmveer SharmaNo ratings yet
- EAMCET QR Chemistry SR Chem 17.organic Chemistry Carboxylic AcidsDocument5 pagesEAMCET QR Chemistry SR Chem 17.organic Chemistry Carboxylic AcidsJagadeesh Goli100% (2)
- Organic ChemistryDocument31 pagesOrganic ChemistrySameep Kumar SinghNo ratings yet
- 6carboxylic Acids PDFDocument29 pages6carboxylic Acids PDFsharmimiameerasanadyNo ratings yet
- EstersDocument18 pagesEstersArika FindlayNo ratings yet
- Cita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicDocument107 pagesCita High School, Port Harcourt Subject: Chemistry Class: Ss3 Week TopicYushau Muhammad LawalNo ratings yet
- Isomerism: (Ii) (Iii) (I) (Ii) (Ill)Document6 pagesIsomerism: (Ii) (Iii) (I) (Ii) (Ill)Ritik Kumar NayakNo ratings yet
- AVCL 9A Properties of Carboxylic Acids and EstersDocument8 pagesAVCL 9A Properties of Carboxylic Acids and EstersGiane MadrigalNo ratings yet
- 3D Chemistry Concepts & Questions 2023-24 2.0Document63 pages3D Chemistry Concepts & Questions 2023-24 2.0Anushka ChauhanNo ratings yet
- Experiment 3 - Spclpha615Document6 pagesExperiment 3 - Spclpha615POMPEYO BARROGANo ratings yet
- Carboxylic Acids Reactions: Presented By: Mizgin Q.AbdullahDocument10 pagesCarboxylic Acids Reactions: Presented By: Mizgin Q.AbdullahSafwan BakrmanyNo ratings yet
- Perfumery As Science (Steeve Herman)Document82 pagesPerfumery As Science (Steeve Herman)SpiceandWood87% (15)
- EAMCET QR Chemistry SR Chem 17.organic Chemistry Carbonyl CompoundsDocument11 pagesEAMCET QR Chemistry SR Chem 17.organic Chemistry Carbonyl CompoundsJagadeesh GoliNo ratings yet
- Organic Chemistry:: Functional GroupsDocument14 pagesOrganic Chemistry:: Functional GroupsRaquel da Silva JustinoNo ratings yet
- Isomerism in Alkanoic AcidsDocument6 pagesIsomerism in Alkanoic AcidsnelsonNo ratings yet
- Tutorial 1 - Alcohol PDFDocument5 pagesTutorial 1 - Alcohol PDFNurul Athirah JainiNo ratings yet
- Lab 10 N Acetylation - The Acetylation of A Primary Aromatic AmineDocument7 pagesLab 10 N Acetylation - The Acetylation of A Primary Aromatic AmineJuan Diego TrujilloNo ratings yet
- Chapter 1 SummaryDocument3 pagesChapter 1 SummaryGmat PrepNo ratings yet
- Carboxylic Acids DerivativesDocument18 pagesCarboxylic Acids DerivativesshyroneruttoNo ratings yet
- cARBOXYLIC ACID DerivativesDocument171 pagescARBOXYLIC ACID DerivativesRaymond OforiNo ratings yet
- Chemistry 10Document14 pagesChemistry 10BehruzNo ratings yet
- Aldehydes and KetonesDocument16 pagesAldehydes and Ketonesmonika.rani.fasvNo ratings yet
- 4.6 Organic Chemistry 2Document30 pages4.6 Organic Chemistry 2Lenah MoiNo ratings yet
- DGT Alcohols Phenols and EthersDocument69 pagesDGT Alcohols Phenols and Ethersjayashree krishnaNo ratings yet
- 4.6 Organic Chemistry 2Document30 pages4.6 Organic Chemistry 2PeterNo ratings yet
- Previous HSE Questions and Answers For The Chapter "Alcohols, Phenols and Ethers"Document10 pagesPrevious HSE Questions and Answers For The Chapter "Alcohols, Phenols and Ethers"Adithya K SanjeevNo ratings yet
- Ch.22 Alkanols - TDocument16 pagesCh.22 Alkanols - Tlk2021202100% (1)
- CH 11 Upl Hints15887759321589447013Document4 pagesCH 11 Upl Hints15887759321589447013Zayed EqbalNo ratings yet
- CCMR Educational Programs: Title: Esterfication Date Created: Author(s) : Appropriate Level: AbstractDocument10 pagesCCMR Educational Programs: Title: Esterfication Date Created: Author(s) : Appropriate Level: AbstractShenique ClarkeNo ratings yet
- (L-1) DPP Chapter 11 Chemistry Class 12th - 14117630 - 2023 - 01 - 03 - 17 - 01Document4 pages(L-1) DPP Chapter 11 Chemistry Class 12th - 14117630 - 2023 - 01 - 03 - 17 - 01OB CREATIONNo ratings yet
- Esterification: Preparation of Benzyl Acetate Microscale Experiment IvDocument9 pagesEsterification: Preparation of Benzyl Acetate Microscale Experiment IvNimzNo ratings yet
- Lecture 7Document19 pagesLecture 7Mustafa SaßerNo ratings yet
- Carboxylic AcidDocument21 pagesCarboxylic AcidShalsabila NHNo ratings yet
- Carboxylic AcidDocument21 pagesCarboxylic AcidShalsabila NHNo ratings yet
- 4.6 Organic Chemistry 2Document30 pages4.6 Organic Chemistry 2caroprinters01No ratings yet
- 1st Final Examination of OcDocument2 pages1st Final Examination of OcRahmanida SusianaNo ratings yet
- Carboxylic Acids ModuleDocument6 pagesCarboxylic Acids ModuleimanNo ratings yet
- Carboxylic Acids: Frederick A. Bettelheim William H. Brown Mary K. Campbell Shawn O. FarrellDocument33 pagesCarboxylic Acids: Frederick A. Bettelheim William H. Brown Mary K. Campbell Shawn O. FarrellShereen Al-ObinayNo ratings yet
- Alcohols - ROHDocument11 pagesAlcohols - ROHappleNo ratings yet
- Alcohol Ether PhenolDocument80 pagesAlcohol Ether PhenolMAJR 104100% (1)
- Chemistry of Natural ProductsDocument22 pagesChemistry of Natural ProductsKhalid LoveNo ratings yet
- Lecture # 6 (Carboxylic Acids and Macromolecules)Document64 pagesLecture # 6 (Carboxylic Acids and Macromolecules)hamzaali227004No ratings yet
- Chemistry Notes PT 2Document37 pagesChemistry Notes PT 2Leng RyanNo ratings yet
- 487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .Document4 pages487 - Concept, Sources, Nomenclature and Isomerism in Alkanoate (Esters) .emmanuelirem805No ratings yet
- EthersDocument11 pagesEthersbomzanaakritiNo ratings yet
- Differentiation of Chiral Compounds Using NMR SpectroscopyFrom EverandDifferentiation of Chiral Compounds Using NMR SpectroscopyNo ratings yet
- Beton Bertulang 2Document2 pagesBeton Bertulang 2St.khalisahNo ratings yet
- Matematika 3Document4 pagesMatematika 3St.khalisahNo ratings yet
- How To Make Essay About Talking MyselftDocument1 pageHow To Make Essay About Talking MyselftSt.khalisahNo ratings yet
- Political Science - The Influence of Scientific and Technological Advances in A CountryDocument1 pagePolitical Science - The Influence of Scientific and Technological Advances in A CountrySt.khalisahNo ratings yet
- (O) Registered Pesticide October 2005-September 2010Document295 pages(O) Registered Pesticide October 2005-September 2010sk8terboyNo ratings yet
- Codan Chemical Resistance 2015Document4 pagesCodan Chemical Resistance 2015Gokul VenugopalNo ratings yet
- ECSA Chemicals Flavours and Fragrances Catalogue en 16 03.cleanedDocument28 pagesECSA Chemicals Flavours and Fragrances Catalogue en 16 03.cleanedVarun MaheshwariNo ratings yet
- Results Lipidomics Claudia Grewe 12 - 2023Document3 pagesResults Lipidomics Claudia Grewe 12 - 2023Gonçalo BastosNo ratings yet
- AMRI API ProductCatalog WebDocument16 pagesAMRI API ProductCatalog Webমোঃ এমদাদুল হকNo ratings yet
- Chemical Resistance List2Document8 pagesChemical Resistance List2Jean Paul Pamo ValdiviaNo ratings yet
- BFSA (Food Additives)Document48 pagesBFSA (Food Additives)shahriar.codexNo ratings yet
- Lime (Material) : Lime Is A Calcium-Containing Inorganic Mineral Composed Primarily of Oxides, andDocument6 pagesLime (Material) : Lime Is A Calcium-Containing Inorganic Mineral Composed Primarily of Oxides, andShahid BhatNo ratings yet
- 2014 Isocyanate and Phosgene Free Routes PDFDocument17 pages2014 Isocyanate and Phosgene Free Routes PDFPilar MayaNo ratings yet
- Alcohol, PhenolDocument3 pagesAlcohol, PhenolFact GamerNo ratings yet
- Infion 21 Mei 2018Document4 pagesInfion 21 Mei 2018megaNo ratings yet
- All Organic Reagent PDFDocument6 pagesAll Organic Reagent PDFreaper1371293No ratings yet
- Student Safety Sheets: Sodium Sulfites, Thiosulfate & PersulfateDocument1 pageStudent Safety Sheets: Sodium Sulfites, Thiosulfate & PersulfateOlaNo ratings yet
- Essential Chemistry SPM Question Bank Chapter 8 2010Document10 pagesEssential Chemistry SPM Question Bank Chapter 8 2010Thilagavathy MuruganNo ratings yet
- Aldehyde and Ketone ReactionsDocument21 pagesAldehyde and Ketone ReactionsChelsea MartinezNo ratings yet
- Brand Name - Generic NameDocument6 pagesBrand Name - Generic Namejonette carataoNo ratings yet
- Formularium Obat 1Document14 pagesFormularium Obat 1Winda NingsihNo ratings yet
- Carbonyl Compounds - XII PDFDocument39 pagesCarbonyl Compounds - XII PDFMathoholic OjaNo ratings yet
- Carbonyl Compounds: SEM-3, CC-7 Problems: Assignment 1Document7 pagesCarbonyl Compounds: SEM-3, CC-7 Problems: Assignment 1Pedro SilvaNo ratings yet
- List of Copper Salts - WikipediaDocument6 pagesList of Copper Salts - WikipediababuNo ratings yet
- Formularium KF 58 - EviDocument8 pagesFormularium KF 58 - EviNurul Evi kurniatiNo ratings yet
- Complete List of Inorganic AcidsDocument2 pagesComplete List of Inorganic AcidsNormina AboNo ratings yet
- Sodium Acid Pyrophosphate PowderDocument2 pagesSodium Acid Pyrophosphate PowderOmar SaaedNo ratings yet
- Amity Institute of Applied Sciences: B Tech Engineering Chemistry CHEM136Document10 pagesAmity Institute of Applied Sciences: B Tech Engineering Chemistry CHEM136gaurav toppoNo ratings yet
- Cations and AnionsDocument22 pagesCations and AnionsDoe BlackNo ratings yet
- Practice Organic Compounds TestDocument4 pagesPractice Organic Compounds TesthelloblargNo ratings yet
- Chemical Weekly Mar07 PDFDocument246 pagesChemical Weekly Mar07 PDFGanshNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidSsNo ratings yet
- Standards CompiledDocument686 pagesStandards CompiledSalazar OmeprazoleNo ratings yet