Professional Documents
Culture Documents
Brugada Syndrome
Brugada Syndrome
Uploaded by
Mohammed HarisOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Brugada Syndrome
Brugada Syndrome
Uploaded by
Mohammed HarisCopyright:
Available Formats
78 CLINICAL CASE LETTERS
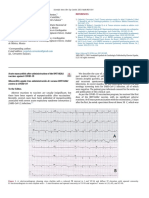
Two Faces of Brugada Syndrome nation revealed an uneven outline of the left coronary artery. In
addition, moderate mitral, pulmonary, and tricuspid valves
regurgitation were observed. An ECG recorded during an episode
of fever revealed a 2-3 mm ST-T segment elevation, such as in
Brugada syndrome is a genetically determined channelopathy, Brugada syndrome type 1 (Fig. 2). Treatment was done as per
with an incidence of 1/1,000-10,000 people. It is responsible for protocol including intravenous immunoglobulin, and later the child
4-12% of sudden cardiac deaths (SCD) with the ventricular fibril- also received intravenous steroids. In control ECG exami-nations,
lation (VF) mechanism. Brugada syndrome type 1 is charac- including the examination with the V1 and V2 electro-des placed 1
terized by a convex elevation of the ST segment ≥2 mm and nega- and 2 intercostal spaces above the conventional site, no
tive T wave. The only effective treatment reducing risk of SCD for characteristic features of Brugada syndrome were found. A gradual
a patient with Brugada syndrome is the implantation [1,2]. improvement in the clinical condition and normalization of
laboratory parameters were observed. Resting ECGs of the
Case 1: A 16-year-old boy was referred due to significant family
patient’s immediate family were normal. Patient was instructed
history (SCD with VF in father at the age of 42 year, with history
with preventive recommen-dations as in Brugada syndrome. The
of repeated episodes of syncope and wheezing at night for several
boy was directed for genetic testing and he remains under
months, and SCD of several cousins aged 24-50 in the father’s
cardiology follow-up.
family). The patient was asymptomatic, and denied symptoms
such as syncope or palpitations. The boy’s 19-year-old brother The diagnosis of Brugada syndrome can be set after recording
was diagnosed with type 1 Brugada syndrome (Fig. 1), and was the characteristic morphology in lead V1 and/or V2 (or after
managed by the implantation of a subcutaneous cardioverter- switching these electrodes to the 2nd, 3rd intercostal space -
defibrillator (s-ICD). Our patient’s resting ECG (along with nominal or high leads) during resting ECG spontaneously or after
elevated intercoastal space ECG), 72-hour Holter ECG, exercise a drug provocation test (intravenous administration of a sodium
test and echocardiographic examination showed no significant channel blocking drug) [3]. Cardiac arrest is most often preceded
deviations. Ajmaline provocation test was performed. Genetic by symptoms, such as: heart palpitations, syncope, and breath-
testing including analysis of 11 genes and 168 exons associated lessness at night. The disease is 8-times more common in males.
with Brugada syndrome showed the presence of a likely patho- Several genes are responsible for the disease, the most common
genic variant in the SCN5A c.2947_2951dupGGTCT gene, p. mutations are associated with SCN5A gene, and several pathogenic
(Leu985Valfs *162). The same mutation was also confirmed in variants are described. However, experts disagree on the
the boy’s brother. Due to the positive genetic test result, deterio- usefulness of genetic testing. In AHA/ACC/HRS 2017 guide-lines,
ration of the patient’s quality of life, another death in the family it is mentioned that ‘‘genetic testing may be useful in the diagnosis
(uncle age 58) despite the lack of clinical symptoms, the patient and care of relatives of people with Brugada syndrome.’’
was implanted a s-ICD.
In asymptomatic patients diagnosed with Brugada synd-
Case 2: An 11-year-old boy was referred with suspicion of rome, it is recommended to avoid drugs contraindicated in Brugada
Pediatric inflammatory multisystem syndrome temporally- syndrome, strictly prohibit the consumption of alcohol and
associated with SARS-CoV-2 infection (PIMS-TS). The patient psychoactive substances, avoid fever, avoid heavy meals, monitor
had temperatures up to of 39.5 °C along with nausea for 4 days, vital parameter (mainly at night) [2]. Several adult patients have
but no respiratory or cardiovascular complaints. Due to multi-ple been described in whom the resting ECG during the acute phase of
desaturations to SpO2 92%, he required oxygen therapy. SARS-CoV-2 disease revealed abnormalities of repolarization
Laboratory tests revealed leukocytosis with lymphopenia and suggestive of Brugada syndrome, though all had fever and ionic
thrombocytopenia. There was an increased concentration of disturbances [3-5]. In most patients, ECG changes normalized
inflammatory markers (CRP 393. 8mg/L) and borderline spontaneously after resolution of fever and did not cause serious
concentration of troponin I (0.037ng/mL). Serum IgG antibodies ventricular arrhythmias. Our second patient had several risk
against SARS-CoV-2 were positive. Echocardio-graphic exami- factors that could have led to Brugada-like changes in ECG,
Fig. 1 ECG record of patient 1 with type 1 Brugada syndrome. Fig. 2 Brugada-like changes in the ECG of patient 2 with
pediatric inflammatory multisystem syndrome (PIMS-TS).
INDIAN PEDIATRICS 342 VOLUME 59__APRIL 15, 2022
CLINICAL CASE LETTERS 79
including fever, ionic disturbances (hyponatremia, hypo- Brugada syndrome. Am Coll Cardiol. 2018;71:148-57.
phosphatemia), as well as damage to the myocardium itself in the 2. Siera J, Brugada P. The definition of Brugada syndrome. Eur
course of PIMS-TS syndrome (i.e., changes in the coronary Heart J. 2017;38:3029-34.
3. Papadakis M, Papatheodorou E, Mellor G, et al. The diagnostic
arteries) with negative family history [5-7].
yield of Brugada syndrome after sudden death with normal au-
In conclusion, Brugada syndrome is a disease, which, when topsy. J Am Coll Cardiol. 2018;71:1204-14.
detected too late, can result in SCD. However, as our two cases 4. Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017
AHA/ACC/HRS Guideline for Management of Patients With
show, its diagnosis as well as the implementation of appropriate
Ventricular Arrhythmias and the Prevention of Sudden Cardiac
preventive therapy is not always easy. Death: ExecutiveSummary. Circulation. 2018;138:210-71.
Note: Both authors contributed equally to this work. 5. Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic
manifestations of COVID-19. Am J Emerg. 2021;41: 96-103.
PIOTR KÊDZIORA,* ALEKSANDRA STASIAK 6. Vidovich MI. Transient Brugada-like electrocardiographic pat-
Department of Pediatric Cardiology and Rheumatology, tern in a patient with COVID-19. JACC: Case Reports. 2020;
Medical University of Lodz, Poland. 2:1241-49.
7. van de Poll SWE, van der Werf C. Two patients with COVID-19
*piotr.kedziora.dr@gmail.com
and a fever-induced Brugada-like electrocardiographic pattern.
REFERENCES Neth Heart J. 2020;28:431-36.
Chang D, Saleh M, Garcia-Bengo Y, et al. COVID-19 infection
1. Gonzalez Corcia MC, Sieira J, Pappaert G, et al. implantable unmasking Brugada syndrome. Heart Rhythm Case Rep. 2020;
cardioverter-defibrillators in children and adolescents with 6:237-40.
Infantile Anti-N-Methyl-D-Aspartate Initial investigations at our center revealed elevated WBC (24
×109/L, N61L30), normal CRP (1 mg/L) and procalcitonin (0.25
Receptor Encephalitis Post-SARS-CoV-2 ng/mL). SARS-CoV-2 IgG antibodies were strongly positive
Infection (index-20.7, >1.0 positive). Erythrocyte sedimentation rate (22
mm/first hour), lactate dehydrogenase (515 U/L), ferritin (19.5 ng/
mL) and echocardiography (normal) were not consistent with
MIS-C.
The spectrum of neurological conditions associated with severe
acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is Over the next 24 hours, extrapyramidal movements worsened
evolving. Here, we describe a case of N-methyl-D-aspartate with appearance of generalized and oro-linguo-buccal dystonia
receptor encephalitis (NMDAR-E) with possible temporal with athetosis. Hence, possibility of anti-NMDA encephalitis
association with SARS-CoV-2. was considered. MRI brain was normal. CSF showed 20 cells
(95%L), sugar 65 mg/ dL (blood sugar: 102 mg/dL), protein 27 mg/
A 10-month-old typically developing boy presented with
dL. CSF-polymerase chain reaction (PCR) was negative
poor feeding and irritability for 5 days. On day 3 of illness, he
(Eschershia coli K1, Hemophlilus influenzae, Listeria mono-
developed fever and loose stools with 2 episodes of convulsions
cytogenes, Streptococcus agalactiae, Streptococcus pneumonia,
on day 5 of illness, when he was brought to our hospital. It was
Cytomegalovirus, enterovirus, HSV1, HSV2, HHV6, Human
associated with loss of pre-morbidly normal eye contact. He had
parechovirus, Varicella zoster virus and Crypto-coccus neo-
an upper respiratory tract infection (URTI) 40 days prior to
formans/gatii). CSF sample for NMDA antibodies was sent to the
illness onset. At presentation, his axillary temperature was
laboratory.
98.20 F, pulse rate was 92/minute, respiratory rate was 24/minute
and blood pressure was 84/54 mmHg. General physical and Child was started on intravenous immunoglobulin (2 g/kg)
systemic examinations were unremarkable. On neurological and pulse methylprednisolone (30 mg/kg/day for 5 days) on day
examination, baby was not interested in surroundings and had 8 of illness. CSF sample was reported strongly positive for anti-
poor interaction with caregivers. Cranial nerve examination was NMDA antibodies (indirect immunofluorescence assay). Com-
unremarkable. Motor system examination revealed normal power puted tomography (CT) of abdomen and pelvis for tumor
and tone, with brisk deep tendon reflexes. Peri-oral dyskinesias screening was negative. By day 5 of pulse steroids, there was no
and bilateral striatal toe were present. Cerebellar and meningeal improvement in extrapyramidal movements or encephalopathy.
signs were absent. In view of fever, diarrhea, seizures, acute onset Considering severe infantile form of anti-NMDAR encephalitis
encephalopathy with extrapyramidal movements, possibilities poorly responsive to first line therapy, weekly rituximab infusion
considered at admission were post-infectious immune-mediated (375 mg/m2/dose/week for 4 doses) was initiated in the second
conditions (central nervous system demyelination, autoimmune week of illness, along with addition of azathioprine for long-term
encephalitis, post-COVID multisystem inflammatory syndrome immunosuppression (2 mg/kg/day). Two weeks after last
(MIS-C)) and inherited metabolic disorder. Prior to referral, baby rituximab dose, baby remained encephalopathic. Extrapyramidal
had a normal cerebrospinal fluid (CSF) study and C-reactive movements were partially controlled with clonidine, baclofen and
protein (CRP) with elevated white cell count (WBC, 26×109/L). clonazepam. In view of refractory disease, monthly cyclophos-
INDIAN PEDIATRICS 343 VOLUME 59__APRIL 15, 2022
You might also like
- Robbins Pathology - Chapter 5 TransDocument20 pagesRobbins Pathology - Chapter 5 Transnath nath100% (3)
- Amoeba Sisters Video Recap: ATPDocument2 pagesAmoeba Sisters Video Recap: ATPCaptain Marvel33% (3)
- Functional Matrix HypothesisDocument14 pagesFunctional Matrix Hypothesispriyab710No ratings yet
- 2021-Acute Myocarditis After Administration of The BNT162b2 Vaccine Against COVID-19Document3 pages2021-Acute Myocarditis After Administration of The BNT162b2 Vaccine Against COVID-19seguridadyambiente641No ratings yet
- A Case of Endomyocardial Biopsy-Proven Early Stage Cardiac Involvement in Heterozygous Fabry DiseaseDocument6 pagesA Case of Endomyocardial Biopsy-Proven Early Stage Cardiac Involvement in Heterozygous Fabry Diseasejinmao1010No ratings yet
- Brugada SyndromeDocument13 pagesBrugada SyndromeBelajar100% (1)
- European Heart Journal Supplements (2019) - SeptemberDocument82 pagesEuropean Heart Journal Supplements (2019) - SeptemberBeard BeardNo ratings yet
- Correspondence: Sudden Cardiac Death, RBBB, and Right Precordial ST-Segment ElevationDocument4 pagesCorrespondence: Sudden Cardiac Death, RBBB, and Right Precordial ST-Segment ElevationAhmad JauhariNo ratings yet
- Case Report DengueDocument11 pagesCase Report Denguevillanueva23749No ratings yet
- An Asymptomatic' Driver With Covid-19: Atypical Suspected Myocarditis by Sars-Cov-2Document2 pagesAn Asymptomatic' Driver With Covid-19: Atypical Suspected Myocarditis by Sars-Cov-2Rubén Darío Viñán VelascoNo ratings yet
- Brugada Syndrome: A New Mutation Found in NorwayDocument3 pagesBrugada Syndrome: A New Mutation Found in NorwayFiqi Syarifa NugraheniNo ratings yet
- Ecg GIC Oreto ConsensusDocument20 pagesEcg GIC Oreto ConsensusAndreaHistoryXNo ratings yet
- RCVS in Patients With Coronavirus DiseaseDocument10 pagesRCVS in Patients With Coronavirus DiseaseRicardo Otiniano SifuentesNo ratings yet
- Clinical and Genetic Heterogeneity of Right Bundle Branch Block and ST-Segment Elevation Syndrome Prospective in 52 FamiliesDocument7 pagesClinical and Genetic Heterogeneity of Right Bundle Branch Block and ST-Segment Elevation Syndrome Prospective in 52 FamiliesKs StylisternaNo ratings yet
- 2525-Main Article Text (Blinded Article File) - 5250-2!10!20201009Document3 pages2525-Main Article Text (Blinded Article File) - 5250-2!10!20201009FihzanNo ratings yet
- Bradyarrhythmias in Patientswith COVID-19Document6 pagesBradyarrhythmias in Patientswith COVID-19Marcela Soruco AyupeNo ratings yet
- MiokDocument7 pagesMiokDanijela AndjelkovicNo ratings yet
- 2021-A Series of Patients With Myocarditis Following SARS-CoV-2 Vaccination With mRNA-1279 and BNT162b2Document2 pages2021-A Series of Patients With Myocarditis Following SARS-CoV-2 Vaccination With mRNA-1279 and BNT162b2seguridadyambiente641No ratings yet
- NEJMc 1813469Document3 pagesNEJMc 1813469Emmanuel GarcíaNo ratings yet
- Cafaro 2023 Cardiovascular Risk in Systemic AutDocument2 pagesCafaro 2023 Cardiovascular Risk in Systemic AutSilvia PeresNo ratings yet
- Unilateral Common Carotid Artery Dissection in A PDocument3 pagesUnilateral Common Carotid Artery Dissection in A PidhebeatrickNo ratings yet
- Brugada Phenocopy or Congenital Brugada Syndrome PericarditidDocument3 pagesBrugada Phenocopy or Congenital Brugada Syndrome PericarditidDewi AmeliaNo ratings yet
- Clinical Features and Predictors of Diphtheritic Cardiomyopathy in Vietnamese ChildrenDocument8 pagesClinical Features and Predictors of Diphtheritic Cardiomyopathy in Vietnamese ChildrenanindiawNo ratings yet
- An Uncommon Stemi Masquerader: A Case of Imipramine Induced Brugada Phenocopy (BRP)Document5 pagesAn Uncommon Stemi Masquerader: A Case of Imipramine Induced Brugada Phenocopy (BRP)IJAR JOURNALNo ratings yet
- The Association Between Covid-19 and ST Elevation Myocardial Infarction: Variable Clinical Presentations On A Case Report SeriesDocument7 pagesThe Association Between Covid-19 and ST Elevation Myocardial Infarction: Variable Clinical Presentations On A Case Report SeriesLila FathiaNo ratings yet
- 10 1016@j Jelectrocard 2012 06 004Document10 pages10 1016@j Jelectrocard 2012 06 004Chris SoilworkNo ratings yet
- Jurnal PPT Sindrom BrugadaDocument64 pagesJurnal PPT Sindrom Brugadasoraya olyfiaNo ratings yet
- Arif 2009Document2 pagesArif 2009FihzanNo ratings yet
- Ajmaline TestDocument9 pagesAjmaline TestJasenko RadovicNo ratings yet
- Reading On Brugada SyndromeDocument10 pagesReading On Brugada SyndromeDonald BidenNo ratings yet
- Cardiovascular Complications of The Guillain-Barré Syndrome.Document4 pagesCardiovascular Complications of The Guillain-Barré Syndrome.Cocosul Cocosului CocosaruluiNo ratings yet
- Journal Pre-Proof: JACC Case ReportsDocument7 pagesJournal Pre-Proof: JACC Case ReportsDupekNo ratings yet
- Kosiborod Et Al.Document12 pagesKosiborod Et Al.LuluNo ratings yet
- Multiple Sclerosis and Type 1 Diabetes in SardiniaDocument1 pageMultiple Sclerosis and Type 1 Diabetes in SardinianabinshresthacyzNo ratings yet
- Texas Heart Institute Journal: Fever-Induced Brugada Syndrome in Patients With Malaria Plasmodium FalciparumDocument7 pagesTexas Heart Institute Journal: Fever-Induced Brugada Syndrome in Patients With Malaria Plasmodium FalciparumJimmy JimmyNo ratings yet
- m20 2003 PDFDocument14 pagesm20 2003 PDFTobias Ernesto Rivas GarciaNo ratings yet
- Dengue With Bradycardia: Clinical Predictors and Prognostic SignificanceDocument5 pagesDengue With Bradycardia: Clinical Predictors and Prognostic SignificanceIjsrnet EditorialNo ratings yet
- A Review On Dengue and Treatments 13 23Document3 pagesA Review On Dengue and Treatments 13 23Karina Mega WNo ratings yet
- Full Text Brugada - AsmihaDocument4 pagesFull Text Brugada - AsmihaNajla MasturaNo ratings yet
- Jurnal CovidDocument15 pagesJurnal CovidFitriani NurFadillahNo ratings yet
- StrokeDocument7 pagesStrokechristianyecyecanNo ratings yet
- Jurnal CovidDocument5 pagesJurnal CovidsafiraNo ratings yet
- Agmr 21 0030 2Document4 pagesAgmr 21 0030 2rodribeatsNo ratings yet
- DEXTROCARDIADocument4 pagesDEXTROCARDIAamalia elisene mondoñedo sanchezNo ratings yet
- Morbitz Type I Second Degree Av Block During Recov-Ery From Dengue Hemorrhagic FeverDocument4 pagesMorbitz Type I Second Degree Av Block During Recov-Ery From Dengue Hemorrhagic FeverMunawarahNo ratings yet
- Delayed Acute Myocarditis and COVID 19 Relatedmultisystem in Ammatory SyndromeDocument6 pagesDelayed Acute Myocarditis and COVID 19 Relatedmultisystem in Ammatory SyndromedrKranklNo ratings yet
- Bibliografi A: Carta Cientı Fica / Rev Esp Cardiol. 2020 73 (8) :665-687Document4 pagesBibliografi A: Carta Cientı Fica / Rev Esp Cardiol. 2020 73 (8) :665-687DANIA LUCERO SANCHEZ SOTONo ratings yet
- A Case of Vaccine-Associated Myocarditis Following Pneumococcal Immunization Leading To Acute Mitral RegurgitationDocument8 pagesA Case of Vaccine-Associated Myocarditis Following Pneumococcal Immunization Leading To Acute Mitral RegurgitationAlina Elisabeta Tiniuc căs LehaciNo ratings yet
- SGLT2i CV Outcomes Various Populations 2023Document11 pagesSGLT2i CV Outcomes Various Populations 2023vladbvs16No ratings yet
- Dengue Myocarditis: Case ReportDocument3 pagesDengue Myocarditis: Case Reportallfanz_krenNo ratings yet
- Revisi A Suspected COVID - 2Document11 pagesRevisi A Suspected COVID - 2Nicholas PranandaNo ratings yet
- dm2 SubtiposDocument13 pagesdm2 SubtiposCesar GuilhermeNo ratings yet
- 1 s2.0 S0022073620304969 MainDocument5 pages1 s2.0 S0022073620304969 MainSivaRaman JayaramanNo ratings yet
- Infective EndocarditisDocument5 pagesInfective Endocarditismustafa malvi100% (1)
- Chyu Shah 2022 Electrocardiograms in Critical Care CardiologyDocument5 pagesChyu Shah 2022 Electrocardiograms in Critical Care Cardiologytegar ksatriaNo ratings yet
- Bern Jak 2021Document6 pagesBern Jak 2021Meirina KhairatNo ratings yet
- Brugada Syndrome: Progress in Diagnosis and Management: Clinical ArrhythmiasDocument6 pagesBrugada Syndrome: Progress in Diagnosis and Management: Clinical ArrhythmiasAndi Tiara S. AdamNo ratings yet
- Cerebellar Stroke in A COVID-19 Infected Patient. A Case ReportDocument6 pagesCerebellar Stroke in A COVID-19 Infected Patient. A Case ReportMuhammad Reza FirdausNo ratings yet
- 10 0000@Www Jstage JST Go jp@Article@Jat@23@11@2335436@ArticleDocument9 pages10 0000@Www Jstage JST Go jp@Article@Jat@23@11@2335436@ArticleLanna HarumiyaNo ratings yet
- PDF - Vol 98-12-N02Document2 pagesPDF - Vol 98-12-N02Carla CarvalhoNo ratings yet
- Acute Myocarditis Mimicking ST-elevation Myocardial Infarction: A Diagnostic Challenge For Frontline CliniciansDocument3 pagesAcute Myocarditis Mimicking ST-elevation Myocardial Infarction: A Diagnostic Challenge For Frontline CliniciansZazaNo ratings yet
- Cardiac Sarcoidosis: Key Concepts in Pathogenesis, Disease Management, and Interesting CasesFrom EverandCardiac Sarcoidosis: Key Concepts in Pathogenesis, Disease Management, and Interesting CasesNo ratings yet
- Biological Macromolecules PDFDocument31 pagesBiological Macromolecules PDFAngeline CortezNo ratings yet
- Embryogenesis 1Document4 pagesEmbryogenesis 1kopipaym8No ratings yet
- Class 12 Biology Chapter 2 Revision NotesDocument25 pagesClass 12 Biology Chapter 2 Revision NotesSrushti BhagitNo ratings yet
- Plasma ProteinsDocument28 pagesPlasma ProteinsAyesha AzamNo ratings yet
- Chapter 2 Nucleic AcidDocument26 pagesChapter 2 Nucleic Acidapi-19916399No ratings yet
- Alma Angélica Del Villar-Martínez (Editor), Juan Arturo Ragazzo-Sánchez (Editor), Pablo Emilio Vanegas-Espinoza (Editor), Octavio Paredes-López (Editor) - Advances in Plant Biotechnology_ in Vitro ProDocument279 pagesAlma Angélica Del Villar-Martínez (Editor), Juan Arturo Ragazzo-Sánchez (Editor), Pablo Emilio Vanegas-Espinoza (Editor), Octavio Paredes-López (Editor) - Advances in Plant Biotechnology_ in Vitro ProCarlosNo ratings yet
- MergeDocument370 pagesMergeVaibhav JindalNo ratings yet
- Parvus Therapeutics and Ardena Enter Agreement For Tech Transfer, Scale-Up and GMP Manufacturing of Navacim NanoparticlesDocument3 pagesParvus Therapeutics and Ardena Enter Agreement For Tech Transfer, Scale-Up and GMP Manufacturing of Navacim NanoparticlesPR.comNo ratings yet
- Definition AnthropologyDocument8 pagesDefinition AnthropologyMYREN ARELLANONo ratings yet
- Chapter 13 Section 1-2 Study StationsDocument9 pagesChapter 13 Section 1-2 Study StationsBao HoangNo ratings yet
- Huseynov Et Al 2016 Developmental Evidence For Obstetric Adaptation of The Human Female PelvisDocument6 pagesHuseynov Et Al 2016 Developmental Evidence For Obstetric Adaptation of The Human Female PelvisTaakeNo ratings yet
- Dealing With Listeria: A Guide For Food ManufacturersDocument8 pagesDealing With Listeria: A Guide For Food ManufacturersNicolas BenavidezNo ratings yet
- Aptamers in HIV Research Diagnosis and TherapyDocument11 pagesAptamers in HIV Research Diagnosis and TherapymikiNo ratings yet
- Class 12 Biology Zoology EM Chapter 2. Human Reproduction Question Bank by Rana Sakthi Durga, Puducherry.Document2 pagesClass 12 Biology Zoology EM Chapter 2. Human Reproduction Question Bank by Rana Sakthi Durga, Puducherry.joshan jayapaul pandiyanNo ratings yet
- National Geographic 2011-02Document188 pagesNational Geographic 2011-02Wade Web100% (6)
- Als Et Al - 2023 - Depression Pathophysiology, Risk Prediction of Recurrence and ComorbidDocument39 pagesAls Et Al - 2023 - Depression Pathophysiology, Risk Prediction of Recurrence and Comorbidkeon.arbabi.altNo ratings yet
- Ichthyosis: A Road Model For Skin Research: Review ArticleDocument10 pagesIchthyosis: A Road Model For Skin Research: Review ArticlebaihaqiNo ratings yet
- Rticle: Structural and Functional Analysis of Cyclin D1 Reveals p27 and Substrate Inhibitor Binding RequirementsDocument14 pagesRticle: Structural and Functional Analysis of Cyclin D1 Reveals p27 and Substrate Inhibitor Binding RequirementsSamyabrata BhaduriNo ratings yet
- 21-22 S2 Week 18 Biology Warm Up Responses May 9 - May 13Document2 pages21-22 S2 Week 18 Biology Warm Up Responses May 9 - May 13Samuel BinkleyNo ratings yet
- Iponatic®: Portable Molecule Workstation (4 Modules)Document2 pagesIponatic®: Portable Molecule Workstation (4 Modules)abhinaya baskaranNo ratings yet
- Biomedicines: Multi-Modal Biological Destruction by Cold Atmospheric Plasma: Capability and MechanismDocument21 pagesBiomedicines: Multi-Modal Biological Destruction by Cold Atmospheric Plasma: Capability and MechanismAndrei VasileNo ratings yet
- PONCE - Module 1 - BSN-2 - A18Document7 pagesPONCE - Module 1 - BSN-2 - A18Ponce Kristel Mae ONo ratings yet
- Tiologia Class 3Document24 pagesTiologia Class 3Joyce GalvezNo ratings yet
- Psychology Mcqs Class 12 NewDocument5 pagesPsychology Mcqs Class 12 NewAkshita BatraNo ratings yet
- 4 Genetics Lecture - Karyotyping of Normal Male, Female and Different Chromosomal Anomalies.Document43 pages4 Genetics Lecture - Karyotyping of Normal Male, Female and Different Chromosomal Anomalies.AMIRA HELAYELNo ratings yet
- General Bio I - Final Exam - 2013Document7 pagesGeneral Bio I - Final Exam - 2013Yonas Isa'acNo ratings yet
- GPB 321 Crop Improvement Manual FinalDocument71 pagesGPB 321 Crop Improvement Manual FinalSultan AhmadNo ratings yet