Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
8 viewsBakteri Clostridium
Bakteri Clostridium
Uploaded by
EuuzThis document provides information on Clostridium, including where they live, how well they survive, and how they cause disease. Clostridia are anaerobic, spore-forming bacteria commonly found in soil and the gastrointestinal tract. They can form resistant spores and cause disease through histotoxic, enterotoxic, or neurotoxic toxins. Histotoxic clostridia like C. perfringens and C. novyi produce toxins that cause tissue necrosis. Enterotoxins from C. perfringens can cause illnesses like pulpy kidney syndrome in sheep. C. botulinum neurotoxin causes the serious disease botulism.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Project Report On RTS Juice PlantDocument7 pagesProject Report On RTS Juice PlantEIRI Board of Consultants and PublishersNo ratings yet
- Aetiology, Pathology and Management of Enterocutaneous FistulaDocument34 pagesAetiology, Pathology and Management of Enterocutaneous Fistularoselinekhadija100% (2)
- Granulosus and E. Multilocularis (Hydatid) .: Tenia Solium or T. Saginata (Teniasis)Document7 pagesGranulosus and E. Multilocularis (Hydatid) .: Tenia Solium or T. Saginata (Teniasis)moosNo ratings yet
- Lec4 Clostridium 2Document6 pagesLec4 Clostridium 2hindaoda90100% (1)
- Echinococcosis: Echinococcus GranulosusDocument3 pagesEchinococcosis: Echinococcus GranulosusJohanna FreddaNo ratings yet
- Echinococcus Granulosus E. Multilocularis E. Vogeli E. OligarthrusDocument5 pagesEchinococcus Granulosus E. Multilocularis E. Vogeli E. OligarthrusPrawesty utamiNo ratings yet
- Morphology & Identification: Clostridium SpeciesDocument14 pagesMorphology & Identification: Clostridium Specieshussain AltaherNo ratings yet
- Blood/Body Fluid Nematodes & Cestodes: Trichinella SpiralisDocument20 pagesBlood/Body Fluid Nematodes & Cestodes: Trichinella SpiralisAnthony Jay FreemanNo ratings yet
- Erinococcus GranulosusDocument9 pagesErinococcus GranulosusOomar RiderNo ratings yet
- Enterotoxemia, Anaerobic Dysentery, Bradsot, Botulism and NecrobacteriosisDocument17 pagesEnterotoxemia, Anaerobic Dysentery, Bradsot, Botulism and NecrobacteriosisNajafova SuadaNo ratings yet
- Cestodes Miriam ArellanoDocument23 pagesCestodes Miriam ArellanoAn GelNo ratings yet
- Hydatid DiseaseDocument9 pagesHydatid DiseaseAjinkya GaikwadNo ratings yet
- THE FINAL REPORT (2019-2020) : Student Name Stage Study Department Subject Semester Signature of Student Title of ReportDocument6 pagesTHE FINAL REPORT (2019-2020) : Student Name Stage Study Department Subject Semester Signature of Student Title of ReportadwNo ratings yet
- 5B - Spore Forming Gram Positive Bacteria - CLOSTRIDIUMDocument5 pages5B - Spore Forming Gram Positive Bacteria - CLOSTRIDIUMRaunaq Singh RatraNo ratings yet
- Echinococcus SP.: ClassificationDocument6 pagesEchinococcus SP.: ClassificationLucia VillacrizNo ratings yet
- CestodesDocument34 pagesCestodesمصطفي خندقاويNo ratings yet
- ParasitologyDocument29 pagesParasitologyPC NNo ratings yet
- Equine Medicine1Document208 pagesEquine Medicine1afewrk Wndimu (buxe)No ratings yet
- Tapeworms: Diphyllobothrium Latum-Fish Tapeworm Taenia Saginata - Beef Tapeworm Taenia Solium - Pork TapewormDocument21 pagesTapeworms: Diphyllobothrium Latum-Fish Tapeworm Taenia Saginata - Beef Tapeworm Taenia Solium - Pork TapewormSumit SharmaNo ratings yet
- Toxoplasma GondiiDocument40 pagesToxoplasma GondiiNeelam Joshi100% (2)
- Bacteria With Toxin-Dr AnceDocument32 pagesBacteria With Toxin-Dr AnceSuita Allemina Gloria SitepuNo ratings yet
- Penunjang Kuliah Botulisme DRDocument7 pagesPenunjang Kuliah Botulisme DRAnonymous DA8iQzNo ratings yet
- Taenia SoliumDocument8 pagesTaenia SoliumJoshua Bien FuentesNo ratings yet
- The Clostridium Specis.Document38 pagesThe Clostridium Specis.علي عبد الكريم عاصيNo ratings yet
- Int CoccidDocument43 pagesInt Coccidhanan.mahmoud272246No ratings yet
- Echinococcosis AsiyaDocument20 pagesEchinococcosis AsiyaBiswadeepan AcharyaNo ratings yet
- ToxoplasmosisDocument27 pagesToxoplasmosisKnjigeNo ratings yet
- CHAPTER 6 SporozoaDocument7 pagesCHAPTER 6 Sporozoarjamlick8228No ratings yet
- Cyclophyllidean CestodesDocument92 pagesCyclophyllidean CestodesPauline AñesNo ratings yet
- 1.4 - ClostridiumDocument45 pages1.4 - Clostridiumsajad abasNo ratings yet
- AnthraxDocument6 pagesAnthraxeutamène ramziNo ratings yet
- Beef Blackleg Clostridial DiseasesDocument4 pagesBeef Blackleg Clostridial DiseasesYan Lean DollisonNo ratings yet
- Entamoeba Histolytica Exists in Two Forms - Trophozoite and Cyst. The Trophozoite and Cyst Measure 20Document2 pagesEntamoeba Histolytica Exists in Two Forms - Trophozoite and Cyst. The Trophozoite and Cyst Measure 20Joan BarcenasNo ratings yet
- Part-2-ParasitologyDocument33 pagesPart-2-ParasitologyAli AhmedNo ratings yet
- Parasitology Lecture 6 - CoccidiaDocument4 pagesParasitology Lecture 6 - Coccidiamiguel cuevasNo ratings yet
- CryptosporidiosisDocument46 pagesCryptosporidiosisruthNo ratings yet
- Clostrdia: G Positive Spore Forming Anaerobic Toxin Producing RodsDocument36 pagesClostrdia: G Positive Spore Forming Anaerobic Toxin Producing Rodsjamal nasirNo ratings yet
- PARASITOLOGYDocument13 pagesPARASITOLOGYJoel CabañasNo ratings yet
- Intestinal Parasitism: ProtozoansDocument10 pagesIntestinal Parasitism: ProtozoansdtimtimanNo ratings yet
- Enterotoxaemia: SynonymsDocument5 pagesEnterotoxaemia: SynonymsMd Shamim AhasanNo ratings yet
- KKDocument27 pagesKKkalsagombaNo ratings yet
- Ronald G. Daroya, M.DDocument72 pagesRonald G. Daroya, M.DJhenestka Joy SorianoNo ratings yet
- Micro Chapter 17Document8 pagesMicro Chapter 17Ana AbuladzeNo ratings yet
- Miscellaneous Material From The USMLE Content OutlineDocument14 pagesMiscellaneous Material From The USMLE Content OutlineJohn SobieskiNo ratings yet
- Bacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeDocument67 pagesBacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeTania.dmp20No ratings yet
- L17-Taenia SpeciesDocument7 pagesL17-Taenia SpeciesMohammed RedhaNo ratings yet
- Presentation For Visceral Gout (VG) in Commercial POULTRYDocument27 pagesPresentation For Visceral Gout (VG) in Commercial POULTRYMohammad Wisal100% (2)
- Soliuminfections and Not Infection With Taniea Saginata.: Pathogenesis & EpimiologyDocument2 pagesSoliuminfections and Not Infection With Taniea Saginata.: Pathogenesis & EpimiologyraphaelNo ratings yet
- PSEUDOMONAS Bacilli ListreiaDocument12 pagesPSEUDOMONAS Bacilli ListreiaSheikh FaishalNo ratings yet
- General Properties of CestodesDocument42 pagesGeneral Properties of CestodesNicole NipasNo ratings yet
- CoccidiaDocument42 pagesCoccidiawadige4668No ratings yet
- Clostridiose RuminantsDocument12 pagesClostridiose Ruminantseutamène ramziNo ratings yet
- Gram Positive BacilliDocument6 pagesGram Positive BacilliSteve ShirmpNo ratings yet
- Intestinal Protozoa Amoebae & Ciliates: ObjectivesDocument5 pagesIntestinal Protozoa Amoebae & Ciliates: ObjectivesmicroperadeniyaNo ratings yet
- CestodesDocument39 pagesCestodesNachiket Vijay PotdarNo ratings yet
- Drowning 1Document11 pagesDrowning 1Dr.NEELKAMAL familyNo ratings yet
- UII Gram Pos Spore-FormfefefeDocument30 pagesUII Gram Pos Spore-FormfefefeDito TrunogatiNo ratings yet
- Background: View Media GalleryDocument6 pagesBackground: View Media GalleryAlmas TNo ratings yet
- Exotic Animal ParasitesDocument37 pagesExotic Animal ParasitesSUTHANNo ratings yet
- CHAPTER 2-ParasitDocument109 pagesCHAPTER 2-Parasitalery ahreallyNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- Cp1018 Boric Acid MsdsDocument6 pagesCp1018 Boric Acid MsdsPanneer SelvamNo ratings yet
- PintoDocument18 pagesPintosinagadianNo ratings yet
- Basic Symbals P&ID PDFDocument736 pagesBasic Symbals P&ID PDFPavar RavitejaNo ratings yet
- Acupressure Points GuidelinesDocument6 pagesAcupressure Points Guidelinesshahisk100% (1)
- Environmental Geotechniques: Theories of Ion ExchangeDocument21 pagesEnvironmental Geotechniques: Theories of Ion ExchangeTenkurala srujanaNo ratings yet
- 555-Timer AStable and MonostableDocument13 pages555-Timer AStable and MonostableenzuekNo ratings yet
- Department of Education: Learning Activity SheetDocument7 pagesDepartment of Education: Learning Activity SheetKaren May UrlandaNo ratings yet
- Dietary Computation For Pregnant ClientDocument12 pagesDietary Computation For Pregnant ClientLuis WashingtonNo ratings yet
- Parent Medical CoverageDocument9 pagesParent Medical CoveragecampeonNo ratings yet
- Reading ResponseDocument3 pagesReading ResponseJack SikoliaNo ratings yet
- Problem Sets For Solutions AnalysisDocument2 pagesProblem Sets For Solutions AnalysisKamil Guillergan100% (1)
- The History of The Big Bang TheoryDocument6 pagesThe History of The Big Bang Theorymay ann dimaanoNo ratings yet
- Social Studies jss2 First Term EXAMSDocument5 pagesSocial Studies jss2 First Term EXAMSalmightyfavouriteNo ratings yet
- Bicycle ProjectDocument4 pagesBicycle Projectgaming channelNo ratings yet
- MCN KweenDocument4 pagesMCN KweenAngelo SigueNo ratings yet
- Bence Bays Resume July 2015Document2 pagesBence Bays Resume July 2015api-292242662No ratings yet
- To, The Medical Superintendent, Services Hospital LahoreDocument1 pageTo, The Medical Superintendent, Services Hospital LahoreAfraz AliNo ratings yet
- Busbar Protect1Document9 pagesBusbar Protect1syahira87No ratings yet
- Benzene - It'S Characteristics and Safety in Handling, Storing & TransportationDocument6 pagesBenzene - It'S Characteristics and Safety in Handling, Storing & TransportationEhab SaadNo ratings yet
- Sec3 Well Spud and Guide StructuresDocument15 pagesSec3 Well Spud and Guide StructuresDonald StraubNo ratings yet
- Stilboestrol Tablets MSDSDocument6 pagesStilboestrol Tablets MSDSIsaac lauricNo ratings yet
- NT TR 459 - Guideline For The Validation of Functional Safety According To IEC 61508 - Nordtest Technical ReportDocument54 pagesNT TR 459 - Guideline For The Validation of Functional Safety According To IEC 61508 - Nordtest Technical ReportManish MehtaNo ratings yet
- Acceptance Criteria of Weld Defects As Per Different CodesDocument17 pagesAcceptance Criteria of Weld Defects As Per Different CodesMidhun K Chandrabose96% (25)
- Like Water For Chocolate QuestionsDocument2 pagesLike Water For Chocolate Questionslde918No ratings yet
- Call History 640273efa1d48Document2 pagesCall History 640273efa1d48Krishnapriya GovindNo ratings yet
- FEEDS and FEEDING - FEED FORMULATIONDocument55 pagesFEEDS and FEEDING - FEED FORMULATIONJane LabradorNo ratings yet
- Training - Cga ApplicationDocument34 pagesTraining - Cga ApplicationSubhan Muhammad100% (1)
Bakteri Clostridium
Bakteri Clostridium
Uploaded by
Euuz0 ratings0% found this document useful (0 votes)
8 views39 pagesThis document provides information on Clostridium, including where they live, how well they survive, and how they cause disease. Clostridia are anaerobic, spore-forming bacteria commonly found in soil and the gastrointestinal tract. They can form resistant spores and cause disease through histotoxic, enterotoxic, or neurotoxic toxins. Histotoxic clostridia like C. perfringens and C. novyi produce toxins that cause tissue necrosis. Enterotoxins from C. perfringens can cause illnesses like pulpy kidney syndrome in sheep. C. botulinum neurotoxin causes the serious disease botulism.
Original Description:
Ilmu bakteriologi veteriner
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides information on Clostridium, including where they live, how well they survive, and how they cause disease. Clostridia are anaerobic, spore-forming bacteria commonly found in soil and the gastrointestinal tract. They can form resistant spores and cause disease through histotoxic, enterotoxic, or neurotoxic toxins. Histotoxic clostridia like C. perfringens and C. novyi produce toxins that cause tissue necrosis. Enterotoxins from C. perfringens can cause illnesses like pulpy kidney syndrome in sheep. C. botulinum neurotoxin causes the serious disease botulism.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
8 views39 pagesBakteri Clostridium
Bakteri Clostridium
Uploaded by
EuuzThis document provides information on Clostridium, including where they live, how well they survive, and how they cause disease. Clostridia are anaerobic, spore-forming bacteria commonly found in soil and the gastrointestinal tract. They can form resistant spores and cause disease through histotoxic, enterotoxic, or neurotoxic toxins. Histotoxic clostridia like C. perfringens and C. novyi produce toxins that cause tissue necrosis. Enterotoxins from C. perfringens can cause illnesses like pulpy kidney syndrome in sheep. C. botulinum neurotoxin causes the serious disease botulism.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 39
CLOSTRIDIUM
Clostridia are gram positive, endospore forming, anaerobic
rods
They are commonly found in the soil or gastrointestinal tract
of animals
Produce ENDOSPORES
Catalase negative, oxidase negative, non-capsulated
They produce three major types of disease
Histotoxic
Enterotoxic
Neurotoxic
Where do they live?
Their distribution varies with the species involved but may
be found in soil, freshwater and marine sediments.
Some appear to be common inhabitants of the intestinal
tract of man and other animals. For some species, the
intestinal canal is the main habitat and their presence in soil
is due to faecal contamination.
They persist in ecological niches with a suitably low
oxidation-reduction potential, and can survive adverse
environments as SPORES.
How well do they survive?
As vegetative cells they are no more resistant to heat than any
other vegetative organism.
In the sporing stage all members have a pronounced but variable
resistance to heat. Spores of C.botulinum, for example, withstand
boiling for 3 to 4 hours. C.novyi spores are less resistant than
C.botulinum. Boiling for less than 5 minutes destroys C.perfringens
spores. It is the presence of these resistant spores that makes
autoclaving an essential part of sterilization of instruments for
surgery.
All Clostridia are sensitive to PENICILLIN although their sensitivity
to other antimicrobial agents is NOT predictable.
How do they cause disease?
Clostridia produce a large variety of different EXOTOXINS,
which have a wide range of properties.
Exotoxin production may be encoded on genomic DNA, but
more frequently is associated with plasmids or phages.
Clostridial diseases can be divided into three broad
categories:
HISTOTOXIC –toxin in tissues (muscle, udder, liver)
ENTEROTOXIC –toxin in blood but absorbed from intestines
NEUROTOXIC –toxin in nerves
HISTOTOXIC CLOSTRIDIA
GAS GANGRENE/ MYONECROSIS/ MALIGNANT EDEMA
The name “gas gangrene” is due to the production of gas by the
Clostridia (mainly hydrogen and nitrogen), leading to the classical
“crepitus” (feels like “bubble wrap” when you touch the skin)
The organisms then multiply, produce their toxins and enzymes.
The lesion spreads by continued growth of the bacteria in
conditions created by toxin activity ie. necrosis resulting from
reduced blood supply and reduced tissue oxidation - reduction
potential which follows oedema.
The pathology of gas gangrene due to C.perfringens is probably
the result of alpha toxin but other proteases, eg. DNases,
collagenases, may be involved.
PATHOGENESIS
Spores enter wound often secondary to trauma If
conditions are right germination of spore takes place
EXOTOXINS especially alpha, cause muscle necrosis
allowing more replication of bacteria Factors such as
collagenase and gas cause further muscle damage
Exotoxins and tissue products are absorbed into circulation
causing widespread damage to endothelial and blood cells,
liver and muscle DEATH
BLACKLEG
The name 'blackleg' is specifically reserved for
emphysematous myonecrosis due to C.chauvoei, mainly
in cattle (but sometimes sheep) and frequently in the
absence of apparent penetrating wounds
INFECTIOUS NECROTIC HEPATITIS (BLACK DISEASE)
The name “Black disease” arises from the darkened
undersurface of the skin of affected animals (especially
seen after drying of the hide), due to engorgement of
subcutaneous blood vessels.
Lesions are seen in the liver of sheep (and on rare occasions
in pigs, cattle, horses, dogs, cats). It has been suggested that
the spores of C.novyi are lying dormant in the liver waiting
for the appropriate damage to be inflicted, which allows
them to germinate.
The most usual trigger in sheep is the wandering of liver
fluke larvae.
However, neoplasms and other tissue destructive lesions
may result in the multiplication of C.novyi and death
PATHOGENESIS
Organism are ingested and spores lodge in the liver
Migrating liver fluke larvae cause local necrosis Lower
redox potential allows germination and multiplication
Toxins, especially alpha, enter circulation Increased
capillary permeability, damage to muscles including heart
Congestion of subcutaneous vessels Sudden Death
Diagnosis Of Histotoxic Clostridial Disease
1. Clinical features and pathology - gas, necrosis, haemorrhage,
toxaemia and death.
2. Visualisation of organisms in tissues - Impression smears should be
made of necrotic muscle, liver, or other affected tissue. Diff Quik
(Giemsa) stains will show large, thick rods that may or may not have
spores. Gram staining will show these as being gram positive, gram
variable or gram negative. On careful inspection, a range of other
organisms may also be seen.
3. Fluorescent antibody (FAb) tests performed on impression smears for
C.chauvoei and C.septicum (which share spore antigens) used to be
available through EMAI but are no longer available.
Treatment And Control
If an animal is diagnosed with histotoxic clostridial
disease in time (ie. before it dies!), urgent and
aggressive debridement of the wound should be
implemented.
It is necessary to remove all the necrotic tissue in which
the organisms are multiplying, and to re-establish
blood supply to the affected area
Treatment has to be given early to animals and should
include PENICILLIN and ANTITOXIN. If the condition is
left even a few hours without appropriate therapy, the
animal will die before the diagnosis is confirmed.
As many of these affected animals may simply be
found dead, the only effective management of this
type of disease is preventive VACCINATION programs
2. ENTEROTOXEMIA / ENTEROPATHY
DYSENTRY / ENTERITIS
ENTEROTOXEMIA (PULPY KIDNEY)
This is a special category of disease that manifests as a
SYSTEMIC (TOXAEMIC) DISEASE - even though the multiplication
of the organisms occurs locally in the intestine it seems to produce
very little damage at that site.
Disease of sheep and goats found worldwide. It is particularly a
problem with feedlot lambs fed rich rations of grains
(“overeating disease”, feedlot animals) or lambs (3-10 weeks
old) suckling heavily lactating ewes grazed on lush pastures. The
major toxin elaborated by C.perfringens Type D, which is the
most common cause of pulpy kidney in Australia, is the epsilon
toxin.
PATHOGENESIS
Change in food conditions (rich diet, increases amount of undigested
starch in intestines and reduces peristalsis) Rapid proliferation of
C.perfringens Type D Epsilon prototoxin released and activated by
trypsin and chymotrypsin Large amount of active epsilon toxin
accumulates and increases intestinal permeability Epsilon toxin is
absorbed into circulation Epsilon toxin increases capillary
permeability in brain causing oedema, degeneration and necrosis of
neural tissue. Also causes damage to capillaries in Loop of Henle and to
the renal tubules resulting in glycosuria Lesions in brain stimulate
catecholamine release from adrenal medulla, causing glycogenolysis and
hyperglycaemia Death with convulsions and hypovolaemia, oedema
of lungs, pericardium and other cavities
This protoxin of course, is also normally produced in the large
intestine but there is not sufficient trypsin there to make it active.
In the small intestine there are specific receptors on the
enterocytes that facilitate absorption of the toxin into the
circulation.
Once in the circulation, the toxin causes necrosis of cells and
damage to vascular endothelial cells, resulting in the local
liquefactive necrosis of brain tissue, necrosis of renal cortex
(“pulpy kidney – this is the result of rapid post-mortem autolysis
in th toxin damaged tissue), and perivascular oedema in
meninges and brain
Diagnosis Of Pulpy Kidney
Visualisation of large numbers of the organisms in the
upper small intestine may be a useful indicator. Collect
samples quickly after death, otherwise organisms from
the large intestines will enter the small intestines and
multiply.
Identification of the epsilon toxin in the small intestinal
contents (last 1 to 1.5 m of ileum) or serum of affected
individuals is required (counterimmunoelectrophoresis or
ELISA tests used).
Treatment and Prevention
There is no specific treatment, as animals are usually
found de
Routine vaccination with a toxoid (active against the
effects of the epsilon toxin) should be carried out to
ensure that the flock is protected.
NEUROTOXIC
BOTULISM
neurological disease
As well as the neurotoxin, C.botulinum possesses a
haemagglutinating factor that has been associated with
vascular thrombus formation. Respiratory failure is the
usual cause of death.
PATHOGENESIS
Toxin is absorbed from its site of entry to the body (usually the
gastrointestinal tract, but possibly also wounds) Transported
to neurones via the blood stream Circulating toxin reaches
susceptible nerve endings but can only bind at the
neuromuscular junctions or nerve-nerve junctions (cholinergic
junctions of peripheral nerves) The toxin acts
presynaptically and blocks acetylcholine release Flaccid
paralysis, respiratory depression, possible vascular thrombus
formation
The toxin that causes botulism may be acquired in three main ways:
1. FORAGE POISONING (FOOD POISONING)
The toxin is preformed in food and is subsequently ingested. This
usually involves high protein foods (which are rich in proteases) or
foods contaminated by rotting cadavers (dead rats or cats),
vegetation or other foodstuffs.
The rotting provides the nutrients and the enzymes, if required, to
enable full activation of the toxin before it is ingested.
Animals can acquire this form of botulism from improperly stored
silage, silage or hay contaminated by the bodies of small rodents
which are a good source of protein and enzymes, or from carcasses
etc
2. WOUND BOTULISM
Rare form of intoxication
TOXICOINFECTIOUS BOTULISM
in foals less than 8 months old - usually 3 - 4 weeks.
The disease is contracted by ingestion of spores from
food such as honey
CLINICAL SIGNS
In general it is seen as an ascending flaccid paralysis.
Response to painful stimuli may persist. Ultimately
death is caused by respiratory paralysis and failure.
Signs of decreased cholinergic function may also be
observed (eg. constipation, urinary retention,
decreased salivation and lacrimation, dilated pupils)
Diagnosis
A tentative diagnosis can usually be made based on
history and clinical signs.
Confirmation of the diagnosis of botulism is based on
finding toxin in the serum, faeces, vomitus or samples of
suspected food
Treatment and Prophylaxis
Antitoxin (if available) – must be given early in disease and
must be of the correct Type eg most cases of C.botulinum in dogs
are type C therefore the equine product with antibodies to types
A,B and E is of no use.
Supportive therapy - fluids, rest, nutritional support, artificial
ventilation.
Antibiotics – while metronidazle or penicillin based antibiotics
are given in an attempt to reduce any intestinal population of
C.botulinim, this is done with the understanding that most cases of
botulism are the result of ingestion of preformed toxin.
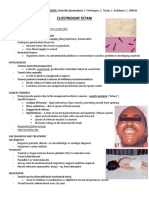
TETANUS
Spores of C.tetani are found worldwide in the soil
(especially heavily manured soils) and in the intestine of
man and other animals,
PATHOGENESIS
Spores are introduced into the animal via deep penetrating wound with
minimal trauma Germination of the spore occurs, leading to production of
small numbers of Clostridium tetani vegetative bacteria which produce LARGE
volumes of tetanus toxin at the deep wound Tetanus toxin is transported
within the axons of the peripheral motor and sensory fibres (intra-axonal
retrograde transport) to the CENTRAL NERVOUS SYSTEM Tetanus toxin has a
high affinity for di and tri- sialogangliosides in cells from all parts of the nervous
system but preferentially the spinal cord. Tetanus toxin acts centrally rather than
locally The tetanus neurotoxin acts specifically on the inhibitory synapses,
preventing the release of glycine and GABA from the inhibitory interneurones in
the brain and spinal cord. The lack of inhibition leads to overexcitation of
motor neurones that manifests as increased muscle tone, rigidity and spasm
(SPASTIC PARALYSIS)
Diagnosis
Usually made on clinical grounds alone. Isolation of
organisms is usually extremely difficult or impossible
even if the location of the wound is known (little
multiplication).
May follow castration, shearing, docking, mulesing,
injections etc. Docking by use of elastic band ligatures
is especially hazardous.
Clinical signs
Incubation period usually 3–10 days but could be as
long as several months
advanced disease is characterised by convulsive
contractions of voluntary muscles
Treatment
If a wound that may be contaminated with tetanus
spores is noticed soon after it has occurred, tetanus can
be prevented by ensuring that an anaerobic
environment does not develop. Debridement of all
necrotic tissue, exposure to oxygen and use of
chemoprophylaxis is important. In practice, the use of
penicillin (both locally within the wound, and
systemically) and injection of antitoxin should be
practiced in any animal
Antitoxin cannot neutralise the toxin already fixed to
the nervous tissue but is able to neutralize toxin that is
still in the muscle tissue.
Be mindful that medications may not get to intended
sites when blood supply and tissue penetration is poor
as in areas of necrosis, which is why debridement is
needed if the site of origin can be identified.
Muscle relaxation may be achieved by use of
diazepam, acetyl promazine or one of the longer
acting barbiturates
Supportive treatment such as i/v fluids administered if
the animal is unable to eat or drink
Prevention
At risk animals can be vaccinated with a TOXOID
Toxoid vaccines : A vaccine made from a toxin
(poison) that has been made harmless but that elicits an
immune response against the toxin. are based on the
toxin produced by certain bacteria (e.g. tetanus or
diphtheria
You might also like
- Project Report On RTS Juice PlantDocument7 pagesProject Report On RTS Juice PlantEIRI Board of Consultants and PublishersNo ratings yet
- Aetiology, Pathology and Management of Enterocutaneous FistulaDocument34 pagesAetiology, Pathology and Management of Enterocutaneous Fistularoselinekhadija100% (2)
- Granulosus and E. Multilocularis (Hydatid) .: Tenia Solium or T. Saginata (Teniasis)Document7 pagesGranulosus and E. Multilocularis (Hydatid) .: Tenia Solium or T. Saginata (Teniasis)moosNo ratings yet
- Lec4 Clostridium 2Document6 pagesLec4 Clostridium 2hindaoda90100% (1)
- Echinococcosis: Echinococcus GranulosusDocument3 pagesEchinococcosis: Echinococcus GranulosusJohanna FreddaNo ratings yet
- Echinococcus Granulosus E. Multilocularis E. Vogeli E. OligarthrusDocument5 pagesEchinococcus Granulosus E. Multilocularis E. Vogeli E. OligarthrusPrawesty utamiNo ratings yet
- Morphology & Identification: Clostridium SpeciesDocument14 pagesMorphology & Identification: Clostridium Specieshussain AltaherNo ratings yet
- Blood/Body Fluid Nematodes & Cestodes: Trichinella SpiralisDocument20 pagesBlood/Body Fluid Nematodes & Cestodes: Trichinella SpiralisAnthony Jay FreemanNo ratings yet
- Erinococcus GranulosusDocument9 pagesErinococcus GranulosusOomar RiderNo ratings yet
- Enterotoxemia, Anaerobic Dysentery, Bradsot, Botulism and NecrobacteriosisDocument17 pagesEnterotoxemia, Anaerobic Dysentery, Bradsot, Botulism and NecrobacteriosisNajafova SuadaNo ratings yet
- Cestodes Miriam ArellanoDocument23 pagesCestodes Miriam ArellanoAn GelNo ratings yet
- Hydatid DiseaseDocument9 pagesHydatid DiseaseAjinkya GaikwadNo ratings yet
- THE FINAL REPORT (2019-2020) : Student Name Stage Study Department Subject Semester Signature of Student Title of ReportDocument6 pagesTHE FINAL REPORT (2019-2020) : Student Name Stage Study Department Subject Semester Signature of Student Title of ReportadwNo ratings yet
- 5B - Spore Forming Gram Positive Bacteria - CLOSTRIDIUMDocument5 pages5B - Spore Forming Gram Positive Bacteria - CLOSTRIDIUMRaunaq Singh RatraNo ratings yet
- Echinococcus SP.: ClassificationDocument6 pagesEchinococcus SP.: ClassificationLucia VillacrizNo ratings yet
- CestodesDocument34 pagesCestodesمصطفي خندقاويNo ratings yet
- ParasitologyDocument29 pagesParasitologyPC NNo ratings yet
- Equine Medicine1Document208 pagesEquine Medicine1afewrk Wndimu (buxe)No ratings yet
- Tapeworms: Diphyllobothrium Latum-Fish Tapeworm Taenia Saginata - Beef Tapeworm Taenia Solium - Pork TapewormDocument21 pagesTapeworms: Diphyllobothrium Latum-Fish Tapeworm Taenia Saginata - Beef Tapeworm Taenia Solium - Pork TapewormSumit SharmaNo ratings yet
- Toxoplasma GondiiDocument40 pagesToxoplasma GondiiNeelam Joshi100% (2)
- Bacteria With Toxin-Dr AnceDocument32 pagesBacteria With Toxin-Dr AnceSuita Allemina Gloria SitepuNo ratings yet
- Penunjang Kuliah Botulisme DRDocument7 pagesPenunjang Kuliah Botulisme DRAnonymous DA8iQzNo ratings yet
- Taenia SoliumDocument8 pagesTaenia SoliumJoshua Bien FuentesNo ratings yet
- The Clostridium Specis.Document38 pagesThe Clostridium Specis.علي عبد الكريم عاصيNo ratings yet
- Int CoccidDocument43 pagesInt Coccidhanan.mahmoud272246No ratings yet
- Echinococcosis AsiyaDocument20 pagesEchinococcosis AsiyaBiswadeepan AcharyaNo ratings yet
- ToxoplasmosisDocument27 pagesToxoplasmosisKnjigeNo ratings yet
- CHAPTER 6 SporozoaDocument7 pagesCHAPTER 6 Sporozoarjamlick8228No ratings yet
- Cyclophyllidean CestodesDocument92 pagesCyclophyllidean CestodesPauline AñesNo ratings yet
- 1.4 - ClostridiumDocument45 pages1.4 - Clostridiumsajad abasNo ratings yet
- AnthraxDocument6 pagesAnthraxeutamène ramziNo ratings yet
- Beef Blackleg Clostridial DiseasesDocument4 pagesBeef Blackleg Clostridial DiseasesYan Lean DollisonNo ratings yet
- Entamoeba Histolytica Exists in Two Forms - Trophozoite and Cyst. The Trophozoite and Cyst Measure 20Document2 pagesEntamoeba Histolytica Exists in Two Forms - Trophozoite and Cyst. The Trophozoite and Cyst Measure 20Joan BarcenasNo ratings yet
- Part-2-ParasitologyDocument33 pagesPart-2-ParasitologyAli AhmedNo ratings yet
- Parasitology Lecture 6 - CoccidiaDocument4 pagesParasitology Lecture 6 - Coccidiamiguel cuevasNo ratings yet
- CryptosporidiosisDocument46 pagesCryptosporidiosisruthNo ratings yet
- Clostrdia: G Positive Spore Forming Anaerobic Toxin Producing RodsDocument36 pagesClostrdia: G Positive Spore Forming Anaerobic Toxin Producing Rodsjamal nasirNo ratings yet
- PARASITOLOGYDocument13 pagesPARASITOLOGYJoel CabañasNo ratings yet
- Intestinal Parasitism: ProtozoansDocument10 pagesIntestinal Parasitism: ProtozoansdtimtimanNo ratings yet
- Enterotoxaemia: SynonymsDocument5 pagesEnterotoxaemia: SynonymsMd Shamim AhasanNo ratings yet
- KKDocument27 pagesKKkalsagombaNo ratings yet
- Ronald G. Daroya, M.DDocument72 pagesRonald G. Daroya, M.DJhenestka Joy SorianoNo ratings yet
- Micro Chapter 17Document8 pagesMicro Chapter 17Ana AbuladzeNo ratings yet
- Miscellaneous Material From The USMLE Content OutlineDocument14 pagesMiscellaneous Material From The USMLE Content OutlineJohn SobieskiNo ratings yet
- Bacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeDocument67 pagesBacillus and Biological Warfare: Dr. Samah Binte Latif M - Phil (Part-1), Microbiology Dhaka Medical CollegeTania.dmp20No ratings yet
- L17-Taenia SpeciesDocument7 pagesL17-Taenia SpeciesMohammed RedhaNo ratings yet
- Presentation For Visceral Gout (VG) in Commercial POULTRYDocument27 pagesPresentation For Visceral Gout (VG) in Commercial POULTRYMohammad Wisal100% (2)
- Soliuminfections and Not Infection With Taniea Saginata.: Pathogenesis & EpimiologyDocument2 pagesSoliuminfections and Not Infection With Taniea Saginata.: Pathogenesis & EpimiologyraphaelNo ratings yet
- PSEUDOMONAS Bacilli ListreiaDocument12 pagesPSEUDOMONAS Bacilli ListreiaSheikh FaishalNo ratings yet
- General Properties of CestodesDocument42 pagesGeneral Properties of CestodesNicole NipasNo ratings yet
- CoccidiaDocument42 pagesCoccidiawadige4668No ratings yet
- Clostridiose RuminantsDocument12 pagesClostridiose Ruminantseutamène ramziNo ratings yet
- Gram Positive BacilliDocument6 pagesGram Positive BacilliSteve ShirmpNo ratings yet
- Intestinal Protozoa Amoebae & Ciliates: ObjectivesDocument5 pagesIntestinal Protozoa Amoebae & Ciliates: ObjectivesmicroperadeniyaNo ratings yet
- CestodesDocument39 pagesCestodesNachiket Vijay PotdarNo ratings yet
- Drowning 1Document11 pagesDrowning 1Dr.NEELKAMAL familyNo ratings yet
- UII Gram Pos Spore-FormfefefeDocument30 pagesUII Gram Pos Spore-FormfefefeDito TrunogatiNo ratings yet
- Background: View Media GalleryDocument6 pagesBackground: View Media GalleryAlmas TNo ratings yet
- Exotic Animal ParasitesDocument37 pagesExotic Animal ParasitesSUTHANNo ratings yet
- CHAPTER 2-ParasitDocument109 pagesCHAPTER 2-Parasitalery ahreallyNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- Cp1018 Boric Acid MsdsDocument6 pagesCp1018 Boric Acid MsdsPanneer SelvamNo ratings yet
- PintoDocument18 pagesPintosinagadianNo ratings yet
- Basic Symbals P&ID PDFDocument736 pagesBasic Symbals P&ID PDFPavar RavitejaNo ratings yet
- Acupressure Points GuidelinesDocument6 pagesAcupressure Points Guidelinesshahisk100% (1)
- Environmental Geotechniques: Theories of Ion ExchangeDocument21 pagesEnvironmental Geotechniques: Theories of Ion ExchangeTenkurala srujanaNo ratings yet
- 555-Timer AStable and MonostableDocument13 pages555-Timer AStable and MonostableenzuekNo ratings yet
- Department of Education: Learning Activity SheetDocument7 pagesDepartment of Education: Learning Activity SheetKaren May UrlandaNo ratings yet
- Dietary Computation For Pregnant ClientDocument12 pagesDietary Computation For Pregnant ClientLuis WashingtonNo ratings yet
- Parent Medical CoverageDocument9 pagesParent Medical CoveragecampeonNo ratings yet
- Reading ResponseDocument3 pagesReading ResponseJack SikoliaNo ratings yet
- Problem Sets For Solutions AnalysisDocument2 pagesProblem Sets For Solutions AnalysisKamil Guillergan100% (1)
- The History of The Big Bang TheoryDocument6 pagesThe History of The Big Bang Theorymay ann dimaanoNo ratings yet
- Social Studies jss2 First Term EXAMSDocument5 pagesSocial Studies jss2 First Term EXAMSalmightyfavouriteNo ratings yet
- Bicycle ProjectDocument4 pagesBicycle Projectgaming channelNo ratings yet
- MCN KweenDocument4 pagesMCN KweenAngelo SigueNo ratings yet
- Bence Bays Resume July 2015Document2 pagesBence Bays Resume July 2015api-292242662No ratings yet
- To, The Medical Superintendent, Services Hospital LahoreDocument1 pageTo, The Medical Superintendent, Services Hospital LahoreAfraz AliNo ratings yet
- Busbar Protect1Document9 pagesBusbar Protect1syahira87No ratings yet
- Benzene - It'S Characteristics and Safety in Handling, Storing & TransportationDocument6 pagesBenzene - It'S Characteristics and Safety in Handling, Storing & TransportationEhab SaadNo ratings yet
- Sec3 Well Spud and Guide StructuresDocument15 pagesSec3 Well Spud and Guide StructuresDonald StraubNo ratings yet
- Stilboestrol Tablets MSDSDocument6 pagesStilboestrol Tablets MSDSIsaac lauricNo ratings yet
- NT TR 459 - Guideline For The Validation of Functional Safety According To IEC 61508 - Nordtest Technical ReportDocument54 pagesNT TR 459 - Guideline For The Validation of Functional Safety According To IEC 61508 - Nordtest Technical ReportManish MehtaNo ratings yet
- Acceptance Criteria of Weld Defects As Per Different CodesDocument17 pagesAcceptance Criteria of Weld Defects As Per Different CodesMidhun K Chandrabose96% (25)
- Like Water For Chocolate QuestionsDocument2 pagesLike Water For Chocolate Questionslde918No ratings yet
- Call History 640273efa1d48Document2 pagesCall History 640273efa1d48Krishnapriya GovindNo ratings yet
- FEEDS and FEEDING - FEED FORMULATIONDocument55 pagesFEEDS and FEEDING - FEED FORMULATIONJane LabradorNo ratings yet
- Training - Cga ApplicationDocument34 pagesTraining - Cga ApplicationSubhan Muhammad100% (1)