Professional Documents
Culture Documents
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Uploaded by
ching chingCopyright:
Available Formats
You might also like
- Katherine Byrne, Tuberculosis and The Victorian: Literary ImaginationDocument5 pagesKatherine Byrne, Tuberculosis and The Victorian: Literary ImaginationMaria Eliza GiuboruncaNo ratings yet
- Johnston (1999) Why We Feel. The Science of Human EmotionsDocument221 pagesJohnston (1999) Why We Feel. The Science of Human EmotionsNavi Setnom ArieugonNo ratings yet
- Non Deliverable ForwardDocument17 pagesNon Deliverable ForwardManish GuptaNo ratings yet
- Winters PreviewDocument265 pagesWinters PreviewcqpresscustomNo ratings yet
- Ifu40090 AuramineDocument2 pagesIfu40090 AuramineChandra Mohan RajendranNo ratings yet
- ANTIMICROBIAL SUSCEPTIBILITY TESTING and MYCOBACTERIADocument8 pagesANTIMICROBIAL SUSCEPTIBILITY TESTING and MYCOBACTERIAAlthea Jam Grezshyl GaloNo ratings yet
- Mycology Laboratory DiagnosisDocument4 pagesMycology Laboratory Diagnosisfpura210000001823No ratings yet
- BACTERIOLOGYDocument4 pagesBACTERIOLOGYjomorales7298pamNo ratings yet
- Bacte SPN Collection Table V.1Document5 pagesBacte SPN Collection Table V.1smclab eqasNo ratings yet
- Amali 1 STBP1043Document25 pagesAmali 1 STBP1043syahmimoktar2004No ratings yet
- Laboratory 9 Identification of StaphylococcusDocument2 pagesLaboratory 9 Identification of StaphylococcusRazmine RicardoNo ratings yet
- Antimicrobial Susceptibility Test: Dilution Test Method Pre-Analytical Phase (Materials)Document2 pagesAntimicrobial Susceptibility Test: Dilution Test Method Pre-Analytical Phase (Materials)ghephNo ratings yet
- BMLS 3D Practical Exam Questions - 50 PointsDocument8 pagesBMLS 3D Practical Exam Questions - 50 PointsMelody Jane PardilloNo ratings yet
- MTAP 1 Microbiology Lesson 1 Introduction Staph Strep Pt. 1Document8 pagesMTAP 1 Microbiology Lesson 1 Introduction Staph Strep Pt. 1aguilarsuzette20No ratings yet
- Encapsulation Zno Nanoparticles by Using Beeswax: Roya Namdariyan, Afshin Farahbakhsh and Hossain Alizade GolestaniDocument3 pagesEncapsulation Zno Nanoparticles by Using Beeswax: Roya Namdariyan, Afshin Farahbakhsh and Hossain Alizade GolestaniSahil GuptaNo ratings yet
- 1ST - Microbiology and Parasitology (Laboratory)Document11 pages1ST - Microbiology and Parasitology (Laboratory)Krianne Chris DimaanoNo ratings yet
- An Approach To M.TBDocument42 pagesAn Approach To M.TBKamala NathanNo ratings yet
- Para Lab Merge FileDocument72 pagesPara Lab Merge Fileehehe agikNo ratings yet
- Trans Module 3 - Microbial Control and Safety (FKHMR)Document6 pagesTrans Module 3 - Microbial Control and Safety (FKHMR)Faye Kyla Heart ResuelloNo ratings yet
- I. Basics of Identification BSNDocument13 pagesI. Basics of Identification BSNmiameyah375No ratings yet
- Microbial Biotechnolgy LabDocument19 pagesMicrobial Biotechnolgy LabChokkalingamNo ratings yet
- MICROBIOLOGY AND PARASITOLOGYChapters 1-5 BOOK-BASEDDocument6 pagesMICROBIOLOGY AND PARASITOLOGYChapters 1-5 BOOK-BASEDisabelamasiasNo ratings yet
- Fungal Cultivation and Identification (Side Notes)Document8 pagesFungal Cultivation and Identification (Side Notes)Reca Marie FRIAS100% (1)
- BacPakDocument2 pagesBacPakMinhquang NgoNo ratings yet
- Lesson5.3 LabDiaofTBDocument3 pagesLesson5.3 LabDiaofTBJAY JR. APONNo ratings yet
- Mycology Specimen CollectionDocument2 pagesMycology Specimen CollectionFuture TrekingNo ratings yet
- Microscopic Study of BacteriaDocument4 pagesMicroscopic Study of BacteriaRusselNo ratings yet
- LAB - BACTE - Bacterial Identification Methods and Strategies TABULAR - FINALS - 001Document16 pagesLAB - BACTE - Bacterial Identification Methods and Strategies TABULAR - FINALS - 001Jashmine May TadinaNo ratings yet
- Micropara Laboratory FinalDocument4 pagesMicropara Laboratory FinalIvan MaximusNo ratings yet
- Kinyoun Carbol Fuchsin Stain: Formulation Per 100 MLDocument3 pagesKinyoun Carbol Fuchsin Stain: Formulation Per 100 MLDELLNo ratings yet
- RandomDocument3 pagesRandomqdf97srrt5No ratings yet
- Lab 02 - Preparation of Smears and Gram StainingDocument10 pagesLab 02 - Preparation of Smears and Gram StainingVincent ReyesNo ratings yet
- Student Notes: Micro 1: Davao Doctors College Medical Laboratory Science DepartmentDocument5 pagesStudent Notes: Micro 1: Davao Doctors College Medical Laboratory Science DepartmentMelody Jane PardilloNo ratings yet
- Sanitakun PRDocument18 pagesSanitakun PRduaxanh71No ratings yet
- Gram Staining: Risks and BenefitsDocument3 pagesGram Staining: Risks and BenefitsNiña Georgette ViñaNo ratings yet
- Simlab2 Bacte paraDocument5 pagesSimlab2 Bacte paraEDJEY TOLENTINONo ratings yet
- BacteriologyDocument1 pageBacteriologyHanna MaraeNo ratings yet
- Bacteriologic Specimen Collection V.1Document4 pagesBacteriologic Specimen Collection V.1smclab eqasNo ratings yet
- Sop Gram StainDocument6 pagesSop Gram Staindavid mchembeNo ratings yet
- E9 - Bacterial Endospores and Capsules OutlineDocument2 pagesE9 - Bacterial Endospores and Capsules OutlineJoyce AstoNo ratings yet
- CONTROLDocument7 pagesCONTROLAbDul RehManNo ratings yet
- Use of Uv-C Treatment For The Inactivation of MicroorganismsDocument6 pagesUse of Uv-C Treatment For The Inactivation of MicroorganismsGonzalo BoninoNo ratings yet
- SBL 1023 Lab 9 Exp Gram Staining AsepticDocument10 pagesSBL 1023 Lab 9 Exp Gram Staining Asepticapi-384057570No ratings yet
- Identification System of Enterobacteriaceae and Other Gram Negative, Oxidase Negative BacteriaDocument9 pagesIdentification System of Enterobacteriaceae and Other Gram Negative, Oxidase Negative BacteriaNerdyPotatoNo ratings yet
- Beeswax AbstractDocument4 pagesBeeswax Abstractsb.bavani 5No ratings yet
- Micropara ReviewerDocument24 pagesMicropara ReviewerBarangay SamputNo ratings yet
- Micro para Leclab - ReeviewerDocument22 pagesMicro para Leclab - Reeviewerzyyw.abello.uiNo ratings yet
- TDS - KING B AGAR - BM105 - ENv9Document3 pagesTDS - KING B AGAR - BM105 - ENv9saddoukNo ratings yet
- Activity 2 - Gram Staining - GR 4Document6 pagesActivity 2 - Gram Staining - GR 4leenquishayuNo ratings yet
- Bacte Comprehensive ReviewDocument116 pagesBacte Comprehensive ReviewFaith Theresa OroscoNo ratings yet
- Inoculation: Dr. Sadia IkramDocument62 pagesInoculation: Dr. Sadia IkramsummiyaNo ratings yet
- Prelims MidDocument36 pagesPrelims MidRusselNo ratings yet
- Lab 3.1 Acid Fast Staining (Exercise 9)Document1 pageLab 3.1 Acid Fast Staining (Exercise 9)Miguel CuevasNo ratings yet
- PRELIM BacteDocument8 pagesPRELIM BacteHersey MiayoNo ratings yet
- Histopath TransDocument5 pagesHistopath TransRobNo ratings yet
- JRA - 3401-I (93L) Exercise For Spore Stain and Acid Fast StainDocument3 pagesJRA - 3401-I (93L) Exercise For Spore Stain and Acid Fast StainjonathonNo ratings yet
- Acid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)Document3 pagesAcid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)62991No ratings yet
- Staining and Micros PDFDocument51 pagesStaining and Micros PDFIshaanNo ratings yet
- Microbiology A Laboratory Manual 10th Edition Cappuccino Solutions ManualDocument9 pagesMicrobiology A Laboratory Manual 10th Edition Cappuccino Solutions ManualChadHallwaiy100% (60)
- A. Conventional Method: Disk Diffusion: Kirby BauerDocument4 pagesA. Conventional Method: Disk Diffusion: Kirby Bauercayla mae carlosNo ratings yet
- Combined Science Study Pack Biology SectionDocument73 pagesCombined Science Study Pack Biology Sectionjamb2316No ratings yet
- Agar Brucella Campy PDFDocument2 pagesAgar Brucella Campy PDFMICHELLE GENESIS SOTO BURGOSNo ratings yet
- Lab Report 2 Acid Fast StainingDocument4 pagesLab Report 2 Acid Fast StainingjoeNo ratings yet
- HFLD Literature ReviewDocument5 pagesHFLD Literature ReviewMuhammad AamirNo ratings yet
- PhilosophyDocument4 pagesPhilosophyJudalineNo ratings yet
- Data Warehouse Slide3Document43 pagesData Warehouse Slide3Kai EnezhuNo ratings yet
- HP System Management Homepage 6.0 User Guide: HP-UX, Linux, and Windows Operating SystemsDocument102 pagesHP System Management Homepage 6.0 User Guide: HP-UX, Linux, and Windows Operating SystemsFábio AndréNo ratings yet
- Data Flow DiagramsDocument3 pagesData Flow DiagramsHaco Chinedu ObasiNo ratings yet
- Taylor's Scientific ManagementDocument7 pagesTaylor's Scientific Managementsipanjegiven0% (1)
- BiossDocument11 pagesBiossPriyanka SharmaNo ratings yet
- How To Use Survey MonkeyDocument122 pagesHow To Use Survey MonkeyAlfie AbiabiNo ratings yet
- Ruskin Bonds "THE KITE MAKER"Document14 pagesRuskin Bonds "THE KITE MAKER"Dhruti Galgali38% (8)
- Module 2: Most Essential Learning Competencies (Melcs)Document5 pagesModule 2: Most Essential Learning Competencies (Melcs)Teresita EspinosaNo ratings yet
- D D D D D D: Description/ordering InformationDocument13 pagesD D D D D D: Description/ordering InformationWelleyNo ratings yet
- Puzzles: The Question: There Is Only One Correct Answer To This Question. Which Answer Is This? AnsDocument7 pagesPuzzles: The Question: There Is Only One Correct Answer To This Question. Which Answer Is This? AnsSk Tausif ShakeelNo ratings yet
- New Record TDocument2 pagesNew Record Tapi-309280225No ratings yet
- The Sophia Sun: in This Issue..Document32 pagesThe Sophia Sun: in This Issue..Gi3iBq7uAANo ratings yet
- A30 CismDocument14 pagesA30 CismRohan SharmaNo ratings yet
- Perils of PlagiarismDocument3 pagesPerils of Plagiarismapi-306005383No ratings yet
- Akinlo and Asaolo, 2012 PDFDocument9 pagesAkinlo and Asaolo, 2012 PDFJason KurniawanNo ratings yet
- Unit 4 HadoopDocument86 pagesUnit 4 HadoopShivanshuSinghNo ratings yet
- (1142) Grade XI Admissions 2016-17Document7 pages(1142) Grade XI Admissions 2016-17Naveen ShankarNo ratings yet
- Eclipse ManualDocument10 pagesEclipse ManualgiancarlohoneyNo ratings yet
- Khidmat - The ServiceDocument6 pagesKhidmat - The ServiceAjay Prakash VermaNo ratings yet
- Japanese TrifectaDocument9 pagesJapanese TrifectaJustin Lloyd FloroNo ratings yet
- UPI Linking SpecificationDocument42 pagesUPI Linking Specificationananya26196No ratings yet
- What Is CommunicationDocument4 pagesWhat Is CommunicationsamsimNo ratings yet
- 8 (1) .Factor Endowment TheoryDocument64 pages8 (1) .Factor Endowment Theorylilianamici59No ratings yet
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Uploaded by
ching chingOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Afb Staining: 2 SEMESTER - A.Y 2022-2023 - CLIBAC Lecturer: Ms - Junalen and Ms - Janine
Uploaded by
ching chingCopyright:
Available Formats
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
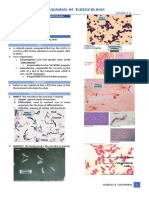
AFB STAINING
SPUTUM EXAMINATION AFB STAINS
SPUTUM CONTAINERS Ziehl-Neelsen Method
-hot method convention method
Disposable -w/steaming or heating brightfield microscope
Clean and sterile C.A.M reagents
MAIN METHODS Kinyoun Method
Unbreakable -cold method
Unleadable -w/out steaming/heating
Wide-mounted w/a screw cap or tightly fiiting cap -detergent
Auramine-Rhodamine Method
LABELING OF SPUTUM CONTAINER
-fluorescent microscope
-bacili appear yellow/orange
The label should be on the body, not on the Cooper’s Modification
lid of the cup and w/ complete label. (kung Gabbett Modification
sala ang ging himo sang patient/s, IBALIK Muller-Chermock Carbolfuchsin Tergitol Cold stain
SAILA!) Pappenheims
Baumgarten’s Method
COLLECTION TECHNIQUE Fluorochrome Stain
Prior collection NOTE: The heat/steam drives the stain into the cells.
During collection Once the organisms have taken up the carbolfuchsin,
they are not easily decolorized by acid alcohol, and
SPUTUM SMEAR EXAMINATION RESULTS
hence are termed ACID-FAST.
Doubtful
GOOD SMEAR=3cmx2cm
Negative
Positive PROPER SCANNING=Horizontal scanning and Vertical
scanning
THE SPUTUM MUST BE COLLECTED IN THE 1 EARLY ST
MORNING AND IT MUST BE COLLECTED W/IN 2 HOURS
AFTER WAKE UP TIME.
SPUTUM SPECIMEN STORAGE
Examine as soon as possible
Keep in the refrigerator if delay
keep in cool, dark place, away from the sunlight
and protected from insects or rodents
Never leave in room temp
The storage period of sputum can be considered
about same as for milk BACTERIA WHICH AFB (+)
IMPORTANT POINTS TO REMEMBER DURING SPUTUM Mycobacteria
COLLECTION Nocardia
Instruct the patient properly ACID FAST CELL WALL
Sputum collection should be done in an open air
Spot sputum collection more than 60% of the cell well is LIPID
3 sputum specimens should be sent immediately major lipid component is MYCOLIC ACID(strong
Avoid exposure to sunlight hydrophobic molecule that forms a lipid shell
around the organisms and affect its permeability)
IDEAL SPUTUM SPECIMEN FOR DIAGNOSIS
(MACROSCOPIC) PRINCIPLE OF AFB
Yellowish acid fastness is due to the high lipid content
Muco-purulent (mycolic acid) in the cell wall of bacteria which
Cheesy material resist decolorization with acid alcohol
(MICROSCOPIC) INTERPRETATION OF RESULTS (AFB)
greater than 25WBC’s(LPO) or 5WBC’s(OIO) Positive= at least one sputum smear is + for AFB
presence of alveolar macrophages or dust cells Negative=both sputum smears are – for AFB
(SPUTUM-deep lung tissue contains many dust cells and RESISTANCE
desquamated ciliated cells which is good specimen
When protected from sunlight, they remain:
from direct smear examination
-in putrefying for weeks
-in dried sputum for 6 to 8 months
Droplets of dried sputum in the air remains
infectious for 8 to 10 days.
Organisms from culture are killed within 2
hours when exposed to sunlight; but in
sputum, they require 20 to 30 hours exposure
before they are killed.
They are generally more resistant to chemical
disinfection than other vegetative organisms
(requires 24 hours of exposure in 5% phenol).
Easily killed by moist heat (boiling for
10mins), pasteurization or steam under
pressure (autoclave).
ABELLAR, LACONSE | M24
1
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
known as Tubercle bacilli
Mycobacterium Microincinerator or Bunsen burner
Mycolic acid-layer on its cell wall
tuberculosis Inoculating loop
Aerobic, rod shaped, thin and slightly curve staining tray
(0.2-0.6um in diameter. 1-4um length) Glass slide
Slow grower in Lowenstein Jensen medium (5- Lens paper
10% carbon dioxide) microscope.
ph 6.5-6.8
no capsules, spores, flagella PROCEDURE OF N.S
describe by Robert Koch in 1882
Place a small drop of nigrosin close to one end of a
causative agent of tuberculosis(oldest
clean slide.
documented communicable disease
Place a slide against the drop of suspended
Non-motile
organisms at a 45° angle and allow the drop to
Colonies are nonpigmented and classically
spread along the edge of the applied slide.
described as being buff-colored
Using aseptic technique, place a loopful of
It transmits through INHALATION
inoculum from the bacterial
DIAGNOSIS culture in the drop of nigrosin and mix.
Push the slide away from the drop of suspended
Direct sputum smear microscopy organisms to form a thin
Culture (most accurate test) smear. Air-dry.
Tuberculin skin test Note: Do not heat fix the slide.
DOTS(Directly Observed Treatment Short Examine the slides under oil immersion.
Course)-internationally recommended
strategy for TB control by the WHO and STAINS OF N.S
IUATLD and recognized as highly efficient
India Ink
and cost-effective strategy
Nigrosin
Eosin
NEGATIVE STAINING
study of morphological shape, size, and
arrangement of the bacteria cells that is
difficult to stain
it does not stain the BACTERIAL
CELLS(due to repulsion between the
negative charges of the stains and the
negatively charged bacterial wall, the dye
stains the background) directly, instead it
strains the background and strains the
actual glass slide and it also uses a
negatively charged dye
ADVANTAGES OF N.S
use of only 1 stain and absence of heat fixation of
the sample.
NS employs the use of an acidic stain and due to
repulsion between the (-) charges of the stain and
bacterial surface and the dye will not penetrate
the cell
useful for determining cell size and arrangement.
also, can be used to stain cells that are too
delicate to be heat fixed.
PRINCIPE OF N.S
requires an acidic dye such as INDIA INK OR
NIGROSIN (means that the stain readily gives up a
hydrogen ion (proton) and the chromophore of the
dye becomes negatively charged)
the surface of most bacterial cells is negatively
charged, the cell surface repels the stain. The
glass of the slide will stain, but the bacterial cells
will not. The bacteria will show up as clear spots
against a dark background.
REQUIREMENT OF N.S
Nigrosin
ABELLAR, LACONSE | M24
2
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
CONTROL OF MICROORGANISM
Sterilization-destruction of all forms of life.
including bacterial spores
-Physical Method
-Chemical Method
Disinfection-process that eliminates a defined
scope of microorganisms including some
spores
-Physical M
-Chemical M (Disinfectants)(Antiseptic)
-Disinfectant-chemical agent applied on
inanimate objects or surfaces
-Antispetic-chemical agent applied to
skin/tissue(animate) for the purpose of
reducing the number of bacteria
FACTORS THAT INFLUENCE THE DEGREE OF KILLING
A. Types of Organisms
-Vary deeply in their ability to withstand
treatment
-Depends on their physical and chemical
components and how they protect themselves
DIFFERENT TYPES OF MICROBES
-Prions (most resistant)
naked pieces of protein similar to virus but
without nucleic acid
transmitted to humans via contaminated
medical
products, therapeutic devices, body fluids ,
and food products
-Bacterial spores
-Mycobacteria
ABELLAR, LACONSE | M24
3
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
-Non-lipid viruses
-Fungi
-Bacteria
-Lipid viruses
B. Number of Organisms
-Microbial load
aka “bioburden”
total number of microorganisms
Note: In general, higher numbers of organisms require
longer exposure time to the killing agents
C. Concentration of disinfecting agent
-amount of disinfectant needed to destroy PHYSICAL METHODS
microbes varies with different agents
-Proper concentrations ensure: HEAT FILTRATION RADIATION
1.the inactivation of target organisms Moist heat Air-HEPA filter Ionizing
2.promote safe and cost-effective practices Dry heat Liquid-thin Non-ionizing
D. Presence of organic material membrane filters
-Organic materials, such as blood, mucus, and Pasteurization
pus, affects killing activity by: Boiling
inactivating the disinfecting agent HEAT
preventing full contact between
object and agent -Moist heat
E. Nature of surface to be disinfected
heat under steam pressure
-made up of biomaterials that exclude the use
Agent used in autoclaves
of certain disinfection or sterilization methods
Steam under 15 psi (or 1 ATM pressure)
because of possible damage to the instruments
Temperature and time required: 121 °C for 15
F. Contact time
mins
-Too little contact time does not allow the
Application: method of choice for heat stable
agent work properly
objects
-Example: Betadine requires 1-2 minutes
contact time -DRY HEAT
G. Temperature
-Disinfectants are generally used at room Requires longer exposure times and increased
temperature (20-22°C) temperature than for moist heat
-↑ activity = ↑ temperature Temperature and time required: above 160°C
-↓ activity = ↓ temperature for 3 hours
Applications: commonly used to sterilize glass
H. pH wares and heat-stable substances (such as
-It is critical to make sure: oils)
at what pH the agent is active
and the pH of the material to be
exposed to the agent
I. Biofilms
-Considered as a community of bacteria or -PASTEURIZATION
other microorganisms
-To disinfect materials that may have a biofilm Method that achieves disinfection but not
present: sterilization
Concentration of the disinfectant
Contact time Temperature and time required:
J. Compatibility of disinfectants –Batch : 63 °C for 30 mins
-common mistake is to believe that the use of
two or more disinfectants are better than one –Flash : 72 °C for 15 secs
•But one may inhibit/inactivate the action of
another or work against each other Application: used mostly in the food
•Example: bleach and quaternary ammonium industry - eliminates food-borne
pathogens and organisms responsible for
METHODS OF STERILIZATION food spoilage
-Physical Methods -Boiling
-Chemical Methods Method that achieves disinfection but not
sterilization
Temperature and time required: 100 °C
for 10 to 15 minutes
FILTRATION
RADIATION
ABELLAR, LACONSE | M24
4
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
Removing physical dirt
Before and after routine patient contact
After contact with infected patients or their
surroundings
In high risk units such as ICU, and burn units
CHEMICAL METHODS Upon entering isolation units and leaving source
isolation units
Just as physical methods are used mainly to Before antiseptic procedures
achieve sterilization, chemical agents are used as
disinfectants Routine Handwashing Technique:
Some chemical agents may be used to sterilize
1.Treat the hands with antiseptic products.
devices that comes in contact with patients – these
are known as chemosterilizers 2.Wash the lower third of the forearm.
Actions of C.M 3.Rinse with tap water.
-Reaction of components of the cytoplasmic membrane 4.Dry w/ disposable or sterile towel.
results in cell death
-Denaturation of cellular proteins (CHON) – disrupts
the metabolism of the cells
-Reacts to thiol (-sh) group of enzymes – disrupts the
amino acid
-Damage of RNA and DNA - inhibits replication
MEDIA PREP
ANTISEPTIC
Germ Theory of Disease-one of the most
PRINCIPLES OF BACTERIAL CULTURE
important contributions by microbiologists to
the general welfare of the worldwide To grow and isolate all bacteria in a
population To determine which of the bacteria
medical community gradually grew aware of that grow are most likely causing
the problem of nosocomial infections and the infection and which are likely
need to practice asepsis to prevent the contaminants or colonizers.
contamination of wounds, dressings, and To obtain sufficient growth of
surgical instruments. clinically relevant bacteria to allow
germ theory of disease also contributed to the identification and characterization
development of antimicrobial
chemotherapeutics. CULTIVATION-process of growing microorganisms in culture
by taking bacteria from the infection site by some means of
specimen collection and growing them in the artificial
environment of the laboratory CULTURE MEDIA
Pioneers for
CULTURE MEDIA-Nutrient preparations that are used for
Ignatz Semmelweis(1816-1865)- ‘Father of culturing microorganisms
Handwashing” “Father of Hospital epidemiology”
-he demonstrated that routine handwashing can -Serve as artificial environment providing the same
prevent the spread of disease elements found in bacteria’s natural habitat
Joseph Lister (1827-1912)- “Father of antiseptic CLASSIFICATIONS OF CULTURE MEDIA
surgery”
-introduced handwashing and the use of phenols 1.According to physical state
for surgical wound disinfection
2.According to functional type
GOALS OF HYGIENIC HANDWASHING
3.According to the method of dispensing
To eliminate transient flora (Contracted from the
environment or from other people) According to physical state
To protect the skin with its resident flora
- Classification of bacterial culture media on the
Routine handwashing in health care settings is performed basis of consistency
in the following situations: -has a 3 physical state (ara sa table)
ABELLAR, LACONSE | M24
5
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
no agar
contains
solidifying
agent (agar)
A.LIQUID MEDIA/BROTH C.SOLID MEDIA
Applications: Contains 5 to 10% agar
initially used to propagate large numbers of Applications
microbes
Surface growth to observe colony
for fermentation studies
appearance
biochemical studies
Pure culture isolation
Disadvantage: Storage of culture
Biochemical reactions
not recommended for motility study
Examples
Examples
-NA -BAP -MacConkey -EMB -CHOC -LIA -TSI
-TSB
ACCORDING TO FUNCTIONAL TYPE
-BHI
Classification of bacterial culture media on their
-Thioglycolate ability to support bacterial growth
-Urea broth Four (4) Functional Types:
Positive Growth 1.Nonselective media- Support the growth of most non-
fastidious microbes
-Turbidity – cloudiness of the medium
Examples: -Nutrient agar -Nutrient broth
2.Selective media- Support the growth of one type or
group of microbes but not another
- may contain inhibitory substances such as antimicrobials,
dyes, or alcohol
Examples:
Mac-Conkey agar is selective for enteric gram (-)
bacilli and inhibits the growth of most gram (+)
bacteria
Lowenstein Jensen medium – for isolation of M.
tuberculosis
B. SEMI-SOLID
MEDIA 3.Differential media- Allow grouping of microbes based on
different characteristics demonstrated on the medium
Contains 0.5 to 1.5% agar
Contains less concentration of “agar” to allow Examples: -Blood agar -MacConkey
for the free spread of microorganisms
Media can be differential and selective
MacConkey agar inhibits gram-positive organisms
Applications and differentiates gram-negative bacilli on the
basis of lactose fermentation
Fermentation studies
Sheep blood agar is nonselective but
Bacterial motility
differentiates organisms on the basis of
Promotes anaerobic growth
hemolysis
Positive Growth
4.Enriched media-Contain growth enhancers that
Motile organisms extend from the are added to nonselective agar to allow fastidious
stab line and produce turbidity or organisms to flourish.
cloudiness throughout the medium
Example: -Chocolate agar is an enriched medium
Example
ACCORDING TO THE METHODS OF DISPENSING
SIM (Sulfide Indole Motility
While in the liquefied state, solid media can be
poured into either a test tube or petri plates
Agar melts 100 °C and solidifies at 45 °C
A. Agar butt (deep)
ABELLAR, LACONSE | M24
6
CLINICAL BACTERIOLOGY | LAB
2ND SEMESTER | A.Y 2022-2023 | CLIBAC
LECTURER: MS.JUNALEN AND MS.JANINE
-hardened in an upright position STORAGEOFMEDIA
B. Agar slant 1. refrigerator to avoid dehydration
-hardened in a slanted position 2.agar plates are air tight
C. Agar butt-slant 3.tube media with fitted capped is store at room temp
-hardened in a semi-upright/slanted position 4. agar/plate should be warmed before use
D. Agar plate or plated medium HOWTOPREPARECULTUREMEDIUM
-poured into a petri dish 1. wear your mask,labgown and gloves
CULTURE MEDIA PREPARATION 2.disinfect the laboratory table top
During preparation of culture media, it is 3.prepare the plates,flaskand tubes (sterile)
important to take note if the medium being
prepared is a tube or plated medium. 4. prepare the media powder, distilled water etc.
In preparing tube medium, it is necessary to
5. follow aseptic technique from preparation to dispensing.
dispense first the medium before sterilizing while
for the preparation of plated medium, it is 6.autoclave the media and store properly
important to sterilize first before dispensing in
Petri dish
Important points to remember (tube media)
Before autoclaving, cover each tube with
cotton
Place tubes in large beaker and cover with
foil.
STERILIZATION AND DISINFECTION METHOD
Moist heat (Autoclave)
Advantages:
-almost all media and anything that withstand 121
°C can be sterile in this way
-rapid and dependable
Disadvantages:
-tends to etch glass wares and leaves it damp
Dry heat
Advantage:
-commonly used to sterilize glasswares
Disadvantages:
-not as effective as moist heat
-may cause charring of cotton and papers
Bacteriologic filter
Advantage:
-some media cannot withstand heating, can be
sterilized by passing through a filter
Disadvantage:
-there will be a high chance that the filtrate will
not be sterile
ABELLAR, LACONSE | M24
7
You might also like
- Katherine Byrne, Tuberculosis and The Victorian: Literary ImaginationDocument5 pagesKatherine Byrne, Tuberculosis and The Victorian: Literary ImaginationMaria Eliza GiuboruncaNo ratings yet
- Johnston (1999) Why We Feel. The Science of Human EmotionsDocument221 pagesJohnston (1999) Why We Feel. The Science of Human EmotionsNavi Setnom ArieugonNo ratings yet
- Non Deliverable ForwardDocument17 pagesNon Deliverable ForwardManish GuptaNo ratings yet
- Winters PreviewDocument265 pagesWinters PreviewcqpresscustomNo ratings yet
- Ifu40090 AuramineDocument2 pagesIfu40090 AuramineChandra Mohan RajendranNo ratings yet
- ANTIMICROBIAL SUSCEPTIBILITY TESTING and MYCOBACTERIADocument8 pagesANTIMICROBIAL SUSCEPTIBILITY TESTING and MYCOBACTERIAAlthea Jam Grezshyl GaloNo ratings yet
- Mycology Laboratory DiagnosisDocument4 pagesMycology Laboratory Diagnosisfpura210000001823No ratings yet
- BACTERIOLOGYDocument4 pagesBACTERIOLOGYjomorales7298pamNo ratings yet
- Bacte SPN Collection Table V.1Document5 pagesBacte SPN Collection Table V.1smclab eqasNo ratings yet
- Amali 1 STBP1043Document25 pagesAmali 1 STBP1043syahmimoktar2004No ratings yet
- Laboratory 9 Identification of StaphylococcusDocument2 pagesLaboratory 9 Identification of StaphylococcusRazmine RicardoNo ratings yet
- Antimicrobial Susceptibility Test: Dilution Test Method Pre-Analytical Phase (Materials)Document2 pagesAntimicrobial Susceptibility Test: Dilution Test Method Pre-Analytical Phase (Materials)ghephNo ratings yet
- BMLS 3D Practical Exam Questions - 50 PointsDocument8 pagesBMLS 3D Practical Exam Questions - 50 PointsMelody Jane PardilloNo ratings yet
- MTAP 1 Microbiology Lesson 1 Introduction Staph Strep Pt. 1Document8 pagesMTAP 1 Microbiology Lesson 1 Introduction Staph Strep Pt. 1aguilarsuzette20No ratings yet
- Encapsulation Zno Nanoparticles by Using Beeswax: Roya Namdariyan, Afshin Farahbakhsh and Hossain Alizade GolestaniDocument3 pagesEncapsulation Zno Nanoparticles by Using Beeswax: Roya Namdariyan, Afshin Farahbakhsh and Hossain Alizade GolestaniSahil GuptaNo ratings yet
- 1ST - Microbiology and Parasitology (Laboratory)Document11 pages1ST - Microbiology and Parasitology (Laboratory)Krianne Chris DimaanoNo ratings yet
- An Approach To M.TBDocument42 pagesAn Approach To M.TBKamala NathanNo ratings yet
- Para Lab Merge FileDocument72 pagesPara Lab Merge Fileehehe agikNo ratings yet
- Trans Module 3 - Microbial Control and Safety (FKHMR)Document6 pagesTrans Module 3 - Microbial Control and Safety (FKHMR)Faye Kyla Heart ResuelloNo ratings yet
- I. Basics of Identification BSNDocument13 pagesI. Basics of Identification BSNmiameyah375No ratings yet
- Microbial Biotechnolgy LabDocument19 pagesMicrobial Biotechnolgy LabChokkalingamNo ratings yet
- MICROBIOLOGY AND PARASITOLOGYChapters 1-5 BOOK-BASEDDocument6 pagesMICROBIOLOGY AND PARASITOLOGYChapters 1-5 BOOK-BASEDisabelamasiasNo ratings yet
- Fungal Cultivation and Identification (Side Notes)Document8 pagesFungal Cultivation and Identification (Side Notes)Reca Marie FRIAS100% (1)
- BacPakDocument2 pagesBacPakMinhquang NgoNo ratings yet
- Lesson5.3 LabDiaofTBDocument3 pagesLesson5.3 LabDiaofTBJAY JR. APONNo ratings yet
- Mycology Specimen CollectionDocument2 pagesMycology Specimen CollectionFuture TrekingNo ratings yet
- Microscopic Study of BacteriaDocument4 pagesMicroscopic Study of BacteriaRusselNo ratings yet
- LAB - BACTE - Bacterial Identification Methods and Strategies TABULAR - FINALS - 001Document16 pagesLAB - BACTE - Bacterial Identification Methods and Strategies TABULAR - FINALS - 001Jashmine May TadinaNo ratings yet
- Micropara Laboratory FinalDocument4 pagesMicropara Laboratory FinalIvan MaximusNo ratings yet
- Kinyoun Carbol Fuchsin Stain: Formulation Per 100 MLDocument3 pagesKinyoun Carbol Fuchsin Stain: Formulation Per 100 MLDELLNo ratings yet
- RandomDocument3 pagesRandomqdf97srrt5No ratings yet
- Lab 02 - Preparation of Smears and Gram StainingDocument10 pagesLab 02 - Preparation of Smears and Gram StainingVincent ReyesNo ratings yet
- Student Notes: Micro 1: Davao Doctors College Medical Laboratory Science DepartmentDocument5 pagesStudent Notes: Micro 1: Davao Doctors College Medical Laboratory Science DepartmentMelody Jane PardilloNo ratings yet
- Sanitakun PRDocument18 pagesSanitakun PRduaxanh71No ratings yet
- Gram Staining: Risks and BenefitsDocument3 pagesGram Staining: Risks and BenefitsNiña Georgette ViñaNo ratings yet
- Simlab2 Bacte paraDocument5 pagesSimlab2 Bacte paraEDJEY TOLENTINONo ratings yet
- BacteriologyDocument1 pageBacteriologyHanna MaraeNo ratings yet
- Bacteriologic Specimen Collection V.1Document4 pagesBacteriologic Specimen Collection V.1smclab eqasNo ratings yet
- Sop Gram StainDocument6 pagesSop Gram Staindavid mchembeNo ratings yet
- E9 - Bacterial Endospores and Capsules OutlineDocument2 pagesE9 - Bacterial Endospores and Capsules OutlineJoyce AstoNo ratings yet
- CONTROLDocument7 pagesCONTROLAbDul RehManNo ratings yet
- Use of Uv-C Treatment For The Inactivation of MicroorganismsDocument6 pagesUse of Uv-C Treatment For The Inactivation of MicroorganismsGonzalo BoninoNo ratings yet
- SBL 1023 Lab 9 Exp Gram Staining AsepticDocument10 pagesSBL 1023 Lab 9 Exp Gram Staining Asepticapi-384057570No ratings yet
- Identification System of Enterobacteriaceae and Other Gram Negative, Oxidase Negative BacteriaDocument9 pagesIdentification System of Enterobacteriaceae and Other Gram Negative, Oxidase Negative BacteriaNerdyPotatoNo ratings yet
- Beeswax AbstractDocument4 pagesBeeswax Abstractsb.bavani 5No ratings yet
- Micropara ReviewerDocument24 pagesMicropara ReviewerBarangay SamputNo ratings yet
- Micro para Leclab - ReeviewerDocument22 pagesMicro para Leclab - Reeviewerzyyw.abello.uiNo ratings yet
- TDS - KING B AGAR - BM105 - ENv9Document3 pagesTDS - KING B AGAR - BM105 - ENv9saddoukNo ratings yet
- Activity 2 - Gram Staining - GR 4Document6 pagesActivity 2 - Gram Staining - GR 4leenquishayuNo ratings yet
- Bacte Comprehensive ReviewDocument116 pagesBacte Comprehensive ReviewFaith Theresa OroscoNo ratings yet
- Inoculation: Dr. Sadia IkramDocument62 pagesInoculation: Dr. Sadia IkramsummiyaNo ratings yet
- Prelims MidDocument36 pagesPrelims MidRusselNo ratings yet
- Lab 3.1 Acid Fast Staining (Exercise 9)Document1 pageLab 3.1 Acid Fast Staining (Exercise 9)Miguel CuevasNo ratings yet
- PRELIM BacteDocument8 pagesPRELIM BacteHersey MiayoNo ratings yet
- Histopath TransDocument5 pagesHistopath TransRobNo ratings yet
- JRA - 3401-I (93L) Exercise For Spore Stain and Acid Fast StainDocument3 pagesJRA - 3401-I (93L) Exercise For Spore Stain and Acid Fast StainjonathonNo ratings yet
- Acid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)Document3 pagesAcid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)62991No ratings yet
- Staining and Micros PDFDocument51 pagesStaining and Micros PDFIshaanNo ratings yet
- Microbiology A Laboratory Manual 10th Edition Cappuccino Solutions ManualDocument9 pagesMicrobiology A Laboratory Manual 10th Edition Cappuccino Solutions ManualChadHallwaiy100% (60)
- A. Conventional Method: Disk Diffusion: Kirby BauerDocument4 pagesA. Conventional Method: Disk Diffusion: Kirby Bauercayla mae carlosNo ratings yet
- Combined Science Study Pack Biology SectionDocument73 pagesCombined Science Study Pack Biology Sectionjamb2316No ratings yet
- Agar Brucella Campy PDFDocument2 pagesAgar Brucella Campy PDFMICHELLE GENESIS SOTO BURGOSNo ratings yet
- Lab Report 2 Acid Fast StainingDocument4 pagesLab Report 2 Acid Fast StainingjoeNo ratings yet
- HFLD Literature ReviewDocument5 pagesHFLD Literature ReviewMuhammad AamirNo ratings yet
- PhilosophyDocument4 pagesPhilosophyJudalineNo ratings yet
- Data Warehouse Slide3Document43 pagesData Warehouse Slide3Kai EnezhuNo ratings yet
- HP System Management Homepage 6.0 User Guide: HP-UX, Linux, and Windows Operating SystemsDocument102 pagesHP System Management Homepage 6.0 User Guide: HP-UX, Linux, and Windows Operating SystemsFábio AndréNo ratings yet
- Data Flow DiagramsDocument3 pagesData Flow DiagramsHaco Chinedu ObasiNo ratings yet
- Taylor's Scientific ManagementDocument7 pagesTaylor's Scientific Managementsipanjegiven0% (1)
- BiossDocument11 pagesBiossPriyanka SharmaNo ratings yet
- How To Use Survey MonkeyDocument122 pagesHow To Use Survey MonkeyAlfie AbiabiNo ratings yet
- Ruskin Bonds "THE KITE MAKER"Document14 pagesRuskin Bonds "THE KITE MAKER"Dhruti Galgali38% (8)
- Module 2: Most Essential Learning Competencies (Melcs)Document5 pagesModule 2: Most Essential Learning Competencies (Melcs)Teresita EspinosaNo ratings yet
- D D D D D D: Description/ordering InformationDocument13 pagesD D D D D D: Description/ordering InformationWelleyNo ratings yet
- Puzzles: The Question: There Is Only One Correct Answer To This Question. Which Answer Is This? AnsDocument7 pagesPuzzles: The Question: There Is Only One Correct Answer To This Question. Which Answer Is This? AnsSk Tausif ShakeelNo ratings yet
- New Record TDocument2 pagesNew Record Tapi-309280225No ratings yet
- The Sophia Sun: in This Issue..Document32 pagesThe Sophia Sun: in This Issue..Gi3iBq7uAANo ratings yet
- A30 CismDocument14 pagesA30 CismRohan SharmaNo ratings yet
- Perils of PlagiarismDocument3 pagesPerils of Plagiarismapi-306005383No ratings yet
- Akinlo and Asaolo, 2012 PDFDocument9 pagesAkinlo and Asaolo, 2012 PDFJason KurniawanNo ratings yet
- Unit 4 HadoopDocument86 pagesUnit 4 HadoopShivanshuSinghNo ratings yet
- (1142) Grade XI Admissions 2016-17Document7 pages(1142) Grade XI Admissions 2016-17Naveen ShankarNo ratings yet
- Eclipse ManualDocument10 pagesEclipse ManualgiancarlohoneyNo ratings yet
- Khidmat - The ServiceDocument6 pagesKhidmat - The ServiceAjay Prakash VermaNo ratings yet
- Japanese TrifectaDocument9 pagesJapanese TrifectaJustin Lloyd FloroNo ratings yet
- UPI Linking SpecificationDocument42 pagesUPI Linking Specificationananya26196No ratings yet
- What Is CommunicationDocument4 pagesWhat Is CommunicationsamsimNo ratings yet
- 8 (1) .Factor Endowment TheoryDocument64 pages8 (1) .Factor Endowment Theorylilianamici59No ratings yet