Professional Documents
Culture Documents
Abstract SyngasproductionComplete
Abstract SyngasproductionComplete
Uploaded by
Jose Daniel Ballestero MontielCopyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Diseño de Reactores para Reacciones ComplejasDocument12 pagesDiseño de Reactores para Reacciones ComplejasJose Daniel Ballestero MontielNo ratings yet
- Lista de Articulos1Document1 pageLista de Articulos1Jose Daniel Ballestero MontielNo ratings yet
- Pirólisis de Hidrocarburos - Parte 1Document39 pagesPirólisis de Hidrocarburos - Parte 1Jose Daniel Ballestero MontielNo ratings yet
- Datasheet LGNDocument91 pagesDatasheet LGNJose Daniel Ballestero MontielNo ratings yet
- Diagrama de FlujoDocument1 pageDiagrama de FlujoJose Daniel Ballestero MontielNo ratings yet
- Datasheet LGN MergedDocument93 pagesDatasheet LGN MergedJose Daniel Ballestero MontielNo ratings yet
- MVC Y PVCDocument27 pagesMVC Y PVCJose Daniel Ballestero MontielNo ratings yet
- Unidad IIDocument55 pagesUnidad IIJose Daniel Ballestero MontielNo ratings yet
- La CarboquimicaDocument30 pagesLa CarboquimicaJose Daniel Ballestero MontielNo ratings yet
- Leotaudjesus Parte1Document90 pagesLeotaudjesus Parte1Jose Daniel Ballestero MontielNo ratings yet
- Practica 4 UV14Document8 pagesPractica 4 UV14Jose Daniel Ballestero MontielNo ratings yet
- Macur 20864528 PDFDocument2 pagesMacur 20864528 PDFJose Daniel Ballestero MontielNo ratings yet
- Proceso de Fraccionamiento de Los Liquidos Del Gas Natural (LGN)Document13 pagesProceso de Fraccionamiento de Los Liquidos Del Gas Natural (LGN)Jose Daniel Ballestero MontielNo ratings yet
- Practica 3Document8 pagesPractica 3Jose Daniel Ballestero MontielNo ratings yet
- Abstract Natural Gas ProcessingPlantDocument2 pagesAbstract Natural Gas ProcessingPlantJose Daniel Ballestero MontielNo ratings yet
- Production of Natural GasDocument1 pageProduction of Natural GasJose Daniel Ballestero MontielNo ratings yet
- UntitledDocument24 pagesUntitledJose Daniel Ballestero MontielNo ratings yet
- Tablas para El 3er ParcialDocument2 pagesTablas para El 3er ParcialJose Daniel Ballestero MontielNo ratings yet
- Formulario Temas 4 y 5Document3 pagesFormulario Temas 4 y 5Jose Daniel Ballestero MontielNo ratings yet
- Unidad IDocument57 pagesUnidad IJose Daniel Ballestero MontielNo ratings yet
- Instructivo para La Seguridad e Higiene Del Trabajo en El Lab F-Q 1ero 2012Document5 pagesInstructivo para La Seguridad e Higiene Del Trabajo en El Lab F-Q 1ero 2012Jose Daniel Ballestero MontielNo ratings yet
- Practica 7 - UVDocument8 pagesPractica 7 - UVJose Daniel Ballestero MontielNo ratings yet
- Practica 2Document7 pagesPractica 2Jose Daniel Ballestero MontielNo ratings yet
- Guia. II ParcialDocument9 pagesGuia. II ParcialJose Daniel Ballestero MontielNo ratings yet
- Reportes de La Web Del EstudianteDocument1 pageReportes de La Web Del EstudianteJose Daniel Ballestero MontielNo ratings yet
- Humidificacion 1Document15 pagesHumidificacion 1Jose Daniel Ballestero MontielNo ratings yet
- Problemas Unidad IIIDocument6 pagesProblemas Unidad IIIJose Daniel Ballestero MontielNo ratings yet
- Olefinas 1 y 2Document33 pagesOlefinas 1 y 2Jose Daniel Ballestero MontielNo ratings yet
- Clase 3er Parcial AdsorDocument35 pagesClase 3er Parcial AdsorJose Daniel Ballestero MontielNo ratings yet
- Guía I ParcialDocument5 pagesGuía I ParcialJose Daniel Ballestero MontielNo ratings yet
Abstract SyngasproductionComplete
Abstract SyngasproductionComplete
Uploaded by
Jose Daniel Ballestero MontielOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Abstract SyngasproductionComplete
Abstract SyngasproductionComplete
Uploaded by
Jose Daniel Ballestero MontielCopyright:
Available Formats
Syngas production from re-gasified liquefied natural gas and its simulation using
DWSIM
Shardul Kale
Laxminarayan Institute of Technology, R.T.M Nagpur University, Nagpur
Introduction
This aim of this project was to convert existing literature into a steady state simulation of Syngas production using liquefied natural gas (R-
LNG) as a raw material. The simulation uses the provided data of mass flow rates, temperature and pressure obtained from Fertilizers and
Chemicals Travancore Ltd (FACT), ammonia plant. The novelty of using R-LNG is that it has a lower carbon/hydrogen ratio, resulting in a
total reduction of steam: carbon ratio and subsequently reduces CO2 emissions and thus reduces the carbon footprint of the process.
Ammonia is produced from a gaseous mixture comprising of mainly N2 and H2 in a 1:3 ratio. Conventionally ammonia plants use naphtha as
a feedstock for syngas production serving as a source for H2 while the N2 is obtained from the air. Using naphtha as a feed generates high
amount of CO2 due to higher carbon to hydrogen ratio. FACT wishes to upgrade its plant by introducing syngas production using R-LNG.
This paper provides a simulation for the same.
Process Description
The ammonia production mainly consists of two steps:
(i) Syngas Production
(ii) Ammonia Synthesis
Part I will only be focused in this flow sheet. The unit operations included are steam reforming (primary reforming), air
reforming (secondary reforming), CO shift reaction, CO2 removal and finally methanation. In the primary reformer,
hydrocarbon reforming takes place in the presence of steam. This reaction is controlled to maintain 10.5% of hydrocarbon in
the output stream that is necessary to perform complete air reforming in secondary reformer. In secondary reformer, the
nitrogen for ammonia synthesis is produced by air reforming. Here heat is supplied by combustion. Burning gas provides heat
for the rest of the reforming process.
After the reforming section, the output stream contains H2, N2, CO, CO2 and along with minute presence of unreacted
hydrocarbons. Removal of carbon oxides is carried out using shift convertors and CO2 absorber. In the CO shift conversion,
the CO acts as reducing agent for water to yield H2 and CO2. Methanation is the simplest procedure to remove traces of
carbon oxides from a stream. So using the above mentioned method, the concentration of carbon oxides is reduced.
Syngas production or feed preparation for ammonia synthesis has been modeled using three conversion reactors and two
equilibrium reactors. In this particular work, desulphurized R-LNG acts as a hydrogen source. When this R-LNG is reacted
with steam, it gets reformed in a primary reformer which is a conversion reactor. Air is added to the second conversion
reactor (secondary reformer) to obtain the nitrogen. Shift conversion reactions take place in next two equilibrium reactors. It
is followed by CO2 absorption, for the removal of CO2 from the feed. Later a methanator unit, which is a conversion reactor,
is used to remove traces of carbon oxides from the stream.
Reactions
The following reactions take place in their respective reactors:
Primary reforming
(a) CH4 + H2O →CO + 3H2 (Conversion reaction)
(b) CH4 + 2H2O →CO2 + 4H2 (Conversion reaction)
(c) CO + H2O →CO2 + H2 (Equilibrium)
Secondary reforming
(a) CH4 + 2O2→CO2 + 2H2O (Conversion reaction)
(b) CH4 + 1.5O2→CO + 2H2O (Conversion reaction)
Shift conversion
(a) CO + H2O →CO2 + H2 (Equilibrium reaction)
Methanation
(a) CO + 3H2→CH4 + H2O (Conversion reaction)
(b) CO2 + 3H2→CH4 + 2H2O (Conversion reaction)
Flowsheet
Fig 1. Syngas production from re-gasified liquefied natural gas in DWSIM
Results
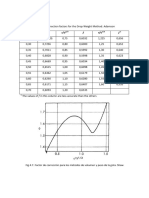
Stream wise analysis of major material streams is given below.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Diseño de Reactores para Reacciones ComplejasDocument12 pagesDiseño de Reactores para Reacciones ComplejasJose Daniel Ballestero MontielNo ratings yet
- Lista de Articulos1Document1 pageLista de Articulos1Jose Daniel Ballestero MontielNo ratings yet
- Pirólisis de Hidrocarburos - Parte 1Document39 pagesPirólisis de Hidrocarburos - Parte 1Jose Daniel Ballestero MontielNo ratings yet
- Datasheet LGNDocument91 pagesDatasheet LGNJose Daniel Ballestero MontielNo ratings yet
- Diagrama de FlujoDocument1 pageDiagrama de FlujoJose Daniel Ballestero MontielNo ratings yet
- Datasheet LGN MergedDocument93 pagesDatasheet LGN MergedJose Daniel Ballestero MontielNo ratings yet
- MVC Y PVCDocument27 pagesMVC Y PVCJose Daniel Ballestero MontielNo ratings yet
- Unidad IIDocument55 pagesUnidad IIJose Daniel Ballestero MontielNo ratings yet
- La CarboquimicaDocument30 pagesLa CarboquimicaJose Daniel Ballestero MontielNo ratings yet
- Leotaudjesus Parte1Document90 pagesLeotaudjesus Parte1Jose Daniel Ballestero MontielNo ratings yet
- Practica 4 UV14Document8 pagesPractica 4 UV14Jose Daniel Ballestero MontielNo ratings yet
- Macur 20864528 PDFDocument2 pagesMacur 20864528 PDFJose Daniel Ballestero MontielNo ratings yet
- Proceso de Fraccionamiento de Los Liquidos Del Gas Natural (LGN)Document13 pagesProceso de Fraccionamiento de Los Liquidos Del Gas Natural (LGN)Jose Daniel Ballestero MontielNo ratings yet
- Practica 3Document8 pagesPractica 3Jose Daniel Ballestero MontielNo ratings yet
- Abstract Natural Gas ProcessingPlantDocument2 pagesAbstract Natural Gas ProcessingPlantJose Daniel Ballestero MontielNo ratings yet
- Production of Natural GasDocument1 pageProduction of Natural GasJose Daniel Ballestero MontielNo ratings yet
- UntitledDocument24 pagesUntitledJose Daniel Ballestero MontielNo ratings yet
- Tablas para El 3er ParcialDocument2 pagesTablas para El 3er ParcialJose Daniel Ballestero MontielNo ratings yet
- Formulario Temas 4 y 5Document3 pagesFormulario Temas 4 y 5Jose Daniel Ballestero MontielNo ratings yet
- Unidad IDocument57 pagesUnidad IJose Daniel Ballestero MontielNo ratings yet
- Instructivo para La Seguridad e Higiene Del Trabajo en El Lab F-Q 1ero 2012Document5 pagesInstructivo para La Seguridad e Higiene Del Trabajo en El Lab F-Q 1ero 2012Jose Daniel Ballestero MontielNo ratings yet
- Practica 7 - UVDocument8 pagesPractica 7 - UVJose Daniel Ballestero MontielNo ratings yet
- Practica 2Document7 pagesPractica 2Jose Daniel Ballestero MontielNo ratings yet
- Guia. II ParcialDocument9 pagesGuia. II ParcialJose Daniel Ballestero MontielNo ratings yet
- Reportes de La Web Del EstudianteDocument1 pageReportes de La Web Del EstudianteJose Daniel Ballestero MontielNo ratings yet
- Humidificacion 1Document15 pagesHumidificacion 1Jose Daniel Ballestero MontielNo ratings yet
- Problemas Unidad IIIDocument6 pagesProblemas Unidad IIIJose Daniel Ballestero MontielNo ratings yet
- Olefinas 1 y 2Document33 pagesOlefinas 1 y 2Jose Daniel Ballestero MontielNo ratings yet
- Clase 3er Parcial AdsorDocument35 pagesClase 3er Parcial AdsorJose Daniel Ballestero MontielNo ratings yet
- Guía I ParcialDocument5 pagesGuía I ParcialJose Daniel Ballestero MontielNo ratings yet