Professional Documents
Culture Documents
AAS and FES Tutorial Solutions PDF
AAS and FES Tutorial Solutions PDF
Uploaded by
Arjun MaharajCopyright:
Available Formats
You might also like
- Equations of Simple Harmonic MotionDocument15 pagesEquations of Simple Harmonic MotionSansen ColipanoNo ratings yet
- Topic 4.0 Problem Solving Convective Mass TransferDocument3 pagesTopic 4.0 Problem Solving Convective Mass TransferBeatriceNo ratings yet
- ENG 9115 Assignment 3Document7 pagesENG 9115 Assignment 3latifmeijidaNo ratings yet
- Michelle Bodnar, Andrew Lohr February 5, 2018Document20 pagesMichelle Bodnar, Andrew Lohr February 5, 2018akshat sinhaNo ratings yet
- Lesson 2 pt.2Document27 pagesLesson 2 pt.2Pido, Patricia LaineNo ratings yet
- Unit 1Document64 pagesUnit 1Aditya YadavNo ratings yet
- EKC 367 Tutorial 3 AnswerDocument3 pagesEKC 367 Tutorial 3 AnswerALAMEL MANZGHAI A/P GANESONNo ratings yet
- 2016 Mmcgrade 10Document8 pages2016 Mmcgrade 10api-339611548100% (17)
- 01Document8 pages01Sadid HossainNo ratings yet
- Math Functions With Complex NumbersDocument2 pagesMath Functions With Complex NumberskekNo ratings yet
- HQ#2 SolutionDocument2 pagesHQ#2 SolutionNasser ÀlsalmiNo ratings yet
- New possible mathematical developments concerning ζ (2), ?, the Rogers- Ramanujan identity: Mathematical connections with some sectors of Particles Physics and the Black Hole physical parameters.Document197 pagesNew possible mathematical developments concerning ζ (2), ?, the Rogers- Ramanujan identity: Mathematical connections with some sectors of Particles Physics and the Black Hole physical parameters.Michele NardelliNo ratings yet
- Desarrollo:: - Hallar R - HALLAR A (H) - Remplazar en La Ecuacion Del TanqueDocument3 pagesDesarrollo:: - Hallar R - HALLAR A (H) - Remplazar en La Ecuacion Del TanqueDiego Mata PerezNo ratings yet
- Solution:: Step 1: Truss AnalysisDocument19 pagesSolution:: Step 1: Truss Analysisiyad aboissaNo ratings yet
- Objective:: (2020 Spring Semester) MESF5450 Supplementary Exercise 03Document4 pagesObjective:: (2020 Spring Semester) MESF5450 Supplementary Exercise 03Lit Pao WongNo ratings yet
- Wendy Johanna Moreno Diaz - Task 3Document17 pagesWendy Johanna Moreno Diaz - Task 3wendy jhoanna moreno diazNo ratings yet
- 1QuizExercises SolvedDocument1 page1QuizExercises SolvedclemencetrinNo ratings yet
- NM - Chap 2 Solved - ExerciesesDocument10 pagesNM - Chap 2 Solved - ExerciesesLy EsNo ratings yet
- CBSE Sample Paper Solution Class 9 Maths Set 1Document20 pagesCBSE Sample Paper Solution Class 9 Maths Set 1Gharsellaoui MuhamedNo ratings yet
- Recurrences: SE 328 Algorithms and Optimization MethodsDocument28 pagesRecurrences: SE 328 Algorithms and Optimization Methodsskj djkNo ratings yet
- Kelompok Fismod 2Document5 pagesKelompok Fismod 2WhyBoo gamingNo ratings yet
- Kelompok Fismod 2Document5 pagesKelompok Fismod 2Mahadi PratamaNo ratings yet
- HW2 SolutionDocument8 pagesHW2 SolutiontilahunNo ratings yet
- Taller Rendimiento TerminadoDocument11 pagesTaller Rendimiento Terminadosantiago hernandezNo ratings yet
- 7.1: Integration by Substitution Backward ChainDocument2 pages7.1: Integration by Substitution Backward ChainTahsina TasneemNo ratings yet
- Basic Calculus - 4th Quarter - Handouts and Worksheets - Lesson 2Document10 pagesBasic Calculus - 4th Quarter - Handouts and Worksheets - Lesson 2Louise CarolineNo ratings yet
- Mat-130 Day-1Document7 pagesMat-130 Day-1Tahsina TasneemNo ratings yet
- ProblemarioDocument15 pagesProblemarioJhoan Andre'No ratings yet
- ThermodynamicsDocument3 pagesThermodynamicsShyn GysawNo ratings yet
- Modern Physics Test 2 - Elevate Classes - 10352883 - 2022 - 12!25!19 - 53Document6 pagesModern Physics Test 2 - Elevate Classes - 10352883 - 2022 - 12!25!19 - 53dixitratan68No ratings yet
- Answer On Question #59908 - Math - CalculusDocument2 pagesAnswer On Question #59908 - Math - CalculusAfzaal Ghazi100% (1)
- L3 Evaluation A Function Using A Matrix-1Document16 pagesL3 Evaluation A Function Using A Matrix-1Sphumelele AndiswaNo ratings yet
- Final Exam: N+ N Sin NDocument9 pagesFinal Exam: N+ N Sin NtehepiconeNo ratings yet
- Kelompok 3 - Sheet 5Document8 pagesKelompok 3 - Sheet 5Satrya Budi PratamaNo ratings yet
- Questions For 2nd YearDocument6 pagesQuestions For 2nd YearRowin Mark SabornidoNo ratings yet
- ND RDDocument5 pagesND RDSadid HossainNo ratings yet
- Tarea2 TD GaelFernandoRodriguezLopez C2G2Document8 pagesTarea2 TD GaelFernandoRodriguezLopez C2G2Rodríguez López Gael FernandoNo ratings yet
- Assignment 12 AnsDocument10 pagesAssignment 12 AnsAnuska DeyNo ratings yet
- Expt. No. 1.6: Moments, Skewness and Kurtosis - I (Ungrouped Data)Document3 pagesExpt. No. 1.6: Moments, Skewness and Kurtosis - I (Ungrouped Data)Suraj GundNo ratings yet
- Ws1 - Probability Generating FunctionssolDocument12 pagesWs1 - Probability Generating FunctionssolkelerongNo ratings yet
- Calculus (Test 1 Question)Document5 pagesCalculus (Test 1 Question)Bala GanabathyNo ratings yet
- Task 3-Eder - CastañedaDocument12 pagesTask 3-Eder - CastañedaEver Castañeda100% (1)
- Repeated QuesDocument8 pagesRepeated QuesMohamed TahmeedNo ratings yet
- Answer 59910Document3 pagesAnswer 59910Lytzkie George PaladaNo ratings yet
- Miscellaneous Practice 2 BryanDocument6 pagesMiscellaneous Practice 2 BryanBryan FuryNo ratings yet
- SPHA031-23 Online Assignment No 1 Solutions 2023Document4 pagesSPHA031-23 Online Assignment No 1 Solutions 2023Ntokozo MasemulaNo ratings yet
- M1 - L15 - Triple IntegralDocument15 pagesM1 - L15 - Triple Integralsaranshchaturvedu10No ratings yet
- Mark Scheme January 2002 GCE: Physics A Unit PA08Document5 pagesMark Scheme January 2002 GCE: Physics A Unit PA08Ali WanNo ratings yet
- Midterm SolutionDocument3 pagesMidterm SolutionJad GhorraNo ratings yet
- Toaz - Info Solution Manual Optical Fiber Communication Gerd Keiser 3rd Ed PRDocument63 pagesToaz - Info Solution Manual Optical Fiber Communication Gerd Keiser 3rd Ed PRdalvirphotonNo ratings yet
- Vector Calculus HW10Document4 pagesVector Calculus HW10ghostNo ratings yet
- 11th Phy Problem EnglishDocument52 pages11th Phy Problem EnglishwhiteshadowsgamerNo ratings yet
- JEE Main Math 24th Feb Shift 2Document9 pagesJEE Main Math 24th Feb Shift 2Abhinav KumarNo ratings yet
- Mathematical Physics Sample Exam 1Document5 pagesMathematical Physics Sample Exam 1Perry EsguerraNo ratings yet
- Exercise Model AnswersDocument19 pagesExercise Model AnswersMohamed EmamNo ratings yet
- Cap 9 Incroperaaaaaaa1298716e67372910Document2 pagesCap 9 Incroperaaaaaaa1298716e67372910Nata Serra TananNo ratings yet
- Lesson 8.3 Implicit Differentiation and Related RatesDocument37 pagesLesson 8.3 Implicit Differentiation and Related RatesAC TadtadNo ratings yet
- Trigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsFrom EverandTrigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsRating: 5 out of 5 stars5/5 (1)
- Tutorial Questions On ICP PDFDocument1 pageTutorial Questions On ICP PDFArjun MaharajNo ratings yet
- DG-Infrared (IR) SpectrosDocument68 pagesDG-Infrared (IR) SpectrosArjun MaharajNo ratings yet
- DG NMRDocument62 pagesDG NMRArjun MaharajNo ratings yet
- Electroanalysis Tutorial Solutions PDFDocument4 pagesElectroanalysis Tutorial Solutions PDFArjun MaharajNo ratings yet
- Atomisation Atomic Spectroscopy Lecture PDFDocument14 pagesAtomisation Atomic Spectroscopy Lecture PDFArjun MaharajNo ratings yet
- X-Ray Tutorial Solutions PDFDocument4 pagesX-Ray Tutorial Solutions PDFArjun MaharajNo ratings yet
- Gas Chromatography 1Document14 pagesGas Chromatography 1Arjun MaharajNo ratings yet
- DG-Lecture 1 - UV-VISDocument35 pagesDG-Lecture 1 - UV-VISArjun MaharajNo ratings yet
- X-Ray NotesDocument37 pagesX-Ray NotesArjun MaharajNo ratings yet
- HPLC 2009Document27 pagesHPLC 2009Arjun MaharajNo ratings yet
- CH301 Tutorial Solutions On UV Spectros PDFDocument3 pagesCH301 Tutorial Solutions On UV Spectros PDFArjun MaharajNo ratings yet
- CH301 Tutorial IRDocument2 pagesCH301 Tutorial IRArjun MaharajNo ratings yet
- CH301 Tutorial HNMR PDFDocument4 pagesCH301 Tutorial HNMR PDFArjun MaharajNo ratings yet
- CH301 Tutorial UV Spectros PDFDocument2 pagesCH301 Tutorial UV Spectros PDFArjun MaharajNo ratings yet
- Tutorial Sheet On Thermal Analysis With AnswersDocument3 pagesTutorial Sheet On Thermal Analysis With AnswersArjun MaharajNo ratings yet
- DG HPLCDocument17 pagesDG HPLCArjun MaharajNo ratings yet
- CH301 NMR Spectros PDFDocument88 pagesCH301 NMR Spectros PDFArjun MaharajNo ratings yet
- AAS Lecture PDFDocument58 pagesAAS Lecture PDFArjun MaharajNo ratings yet
- Tutorial Mass Spectrometry PDFDocument4 pagesTutorial Mass Spectrometry PDFArjun MaharajNo ratings yet
- ICP Lecture Notes PDFDocument21 pagesICP Lecture Notes PDFArjun MaharajNo ratings yet
- ThermogravimetryDocument20 pagesThermogravimetryArjun MaharajNo ratings yet
- 13C NMRDocument28 pages13C NMRArjun MaharajNo ratings yet
- Gas Chromatography 2Document35 pagesGas Chromatography 2Arjun MaharajNo ratings yet
- Lecture 1 General - Principles - of - Gas Chromatography PDFDocument30 pagesLecture 1 General - Principles - of - Gas Chromatography PDFArjun MaharajNo ratings yet
- AAS Lecture PDFDocument18 pagesAAS Lecture PDFArjun MaharajNo ratings yet
- Tutorial Sheet On Thermal Analysis-1Document2 pagesTutorial Sheet On Thermal Analysis-1Arjun MaharajNo ratings yet
- X-Ray Tutorial Solutions PDFDocument4 pagesX-Ray Tutorial Solutions PDFArjun MaharajNo ratings yet
- Lecture 3 NMR PDFDocument21 pagesLecture 3 NMR PDFArjun MaharajNo ratings yet
- Infrared Lecture 1 PDFDocument49 pagesInfrared Lecture 1 PDFArjun MaharajNo ratings yet
- NMR Structure Elucidation PDFDocument22 pagesNMR Structure Elucidation PDFArjun MaharajNo ratings yet
AAS and FES Tutorial Solutions PDF
AAS and FES Tutorial Solutions PDF
Uploaded by
Arjun MaharajOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AAS and FES Tutorial Solutions PDF
AAS and FES Tutorial Solutions PDF
Uploaded by
Arjun MaharajCopyright:
Available Formats
Flame Emission Spectroscopy
Tutorial Solution
Question 1
FES is used in the determination of alkali metals and occasionally Calcium. This is because
alkali metals are excited at relatively low temperatures of the flame to give a spectra that is
simple and remarkably free of interference from other metallic species. Alkali-metal spectra
generally consists of a few intense lines, many of which are in the visible region and are well
suited to quantitative emission measurements.
Question 2
1. The temperature of the flame
2. The presence of interfering chemicals (hydroxide etc.)
3. The type of gas used for the flame (acetylene, oxygen etc).
4. Background interference (sample matrix).
5. Ionisation potentials of sample (high/low).
Question 3
Part 3A (Approach 1)
First calculate 4s→ 3p and then 3p→ 3s, following which multiply the two values together.
Consider the formula:
N ∗ 𝑔∗ −∆𝐸⁄
= 𝑒 𝑘𝑇
𝑁0 𝑔0
Since ∆E = hc/λ [h = planks constant (6.63 x 10-34) and c = speed of light (3 x 108)], substitute
hc/λ in place of ∆E to get the final equation;
ℎ𝑐
N∗ 𝑔∗
−( )⁄
𝜆 𝐍∗ 𝒈∗ −(𝒉𝒄)⁄
= 𝑔0 𝑒 𝑘𝑇 → = 𝒆 𝝀𝒌𝑻
𝑁0 𝑵𝟎 𝒈𝟎
Step 1
4s→ 3p
We have;
λ = 1139 𝑥 10−9 , g*=1, g0 = 3, k= 1.381 x 10-23, h= 6.63x10-34, c= 3x108, T= 2100 K
and N4s/N3p = ?
−34 )(3𝑥108 ))
𝑁4𝑠 𝑔∗ −(ℎ𝑐)⁄ 1 −((6.63𝑥10 ⁄
((1139𝑥10−9 )(1.381 𝑥10−23 )(2100𝐾))
= 0 𝑒 𝜆𝑘𝑇 = 𝑒 = 𝟖. 𝟎𝟖𝟖 𝒙 𝟏𝟎−𝟒
𝑁3𝑝 𝑔 3
Step 2
3p→ 3s
We have;
λ = 589.3 𝑥 10−9 , g*=1, g0 = 3, k= 1.381 x 10-23, h= 6.63x10-34, c= 3x108, T= 2100 K
and N4s/N3p = ?
−34 )(3𝑥108 ))
𝑁4𝑠 𝑔∗ −(ℎ𝑐)⁄ 1 −((6.63𝑥10 ⁄
((589.3𝑥10−9 )(1.381 𝑥10−23 )(2100𝐾))
= 0 𝑒 𝜆𝑘𝑇 = 𝑒 = 𝟐. 𝟔𝟒𝟕 𝒙 𝟏𝟎−𝟓
𝑁3𝑝 𝑔 3
Step 3
Calculating 4s→ 3s
Consider:
𝑁4𝑠 𝑁3𝑝 𝑁4𝑠 Hence; we multiply to get; 𝑁4𝑠
𝑥 = = (8.088𝑥10−4 )(2.6471𝑥10−5 )
𝑁3𝑝 𝑁4𝑠 𝑁3𝑠 𝑁3𝑠
= 𝟐. 𝟏𝟒𝒙𝟏𝟎−𝟖
Part 3B (Approach 2)
Alternatively, the ∆E can be calculated first and then substituted in the original formula. First
calculate 4s→ 3p. Here, 4s is the excited state and the degeneracy of 4s is 1(g*) and 3p is the
ground state with a degeneracy of 3(g0). First compute the ∆E and use that to determine the
ratio of excited atoms in 4s to number of atoms in the ground state in 3p. In a similar manner,
than compute 3p→ 3s, and finally multiply the ratios to obtain the ratio of 4s→ 3s.
Note: The λ = 589.3 nm for 3p→ 3s.
Step 1
4s→ 3p
ℎ𝑐 (6.63 𝑥10−34 )(3 𝑥 108 )
∆𝐸 = = = 1.746 𝑥 10−19
𝜆 (1139 𝑥10−9 )
Now, we have;
∆E = 1.746 𝑥 10−9 , g*=1, g0 = 3, k= 1.381 x 10-23, T= 3273 K and N4s/N3p = ?
−9 )
𝑁4𝑠 𝑔∗ −∆𝐸⁄ 1 −(1.746 𝑥10 ⁄
(1.381 𝑥10−23 )(3273𝐾)
= 𝑒 𝑘𝑇 = 𝑒 = 𝟕 𝒙 𝟏𝟎−𝟑
𝑁3𝑝 𝑔0 3
Step 2
3p→ 3s
ℎ𝑐 (6.63 𝑥10−34 )(3 𝑥 108 )
∆𝐸 = = = 3.375 𝑥 10−19
𝜆 (589.3 𝑥10−9 )
Now, we have;
∆E = 3.375 𝑥 10−19 , g*=3, g0 = 1, k= 1.381 x 10-23, T= 3273 K and N3p/N3s = ?
−19 )
𝑁4𝑠 𝑔∗ −∆𝐸⁄ 3 −(3.375 𝑥10 ⁄
(1.381 𝑥10−23 )(3273𝐾)
= 0 𝑒 𝑘𝑇 = 𝑒 = 𝟏. 𝟕𝟏 𝒙 𝟏𝟎−𝟑
𝑁3𝑝 𝑔 1
Calculating 4s→ 3s
Consider:
𝑁4𝑠 𝑁3𝑝 𝑁4𝑠 Hence; we multiply to get; 𝑁4𝑠
𝑥 = = (7𝑥10−3 )(1.71𝑥10−3 ) = 𝟏. 𝟐𝒙𝟏𝟎−𝟓
𝑁3𝑝 𝑁4𝑠 𝑁3𝑠 𝑁3𝑠
Part 3C (Approach 1)
Step 1
4s→ 3p
We have;
λ = 1139 𝑥 10−9 , g*=1, g0 = 3, k= 1.381 x 10-23, h= 6.63x10-34, c= 3x108, T= 9273 K
and N4s/N3p = ?
−34 )(3𝑥108 ))
𝑁4𝑠 𝑔∗ −(ℎ𝑐)⁄ 1 −((6.63𝑥10 ⁄
((1139𝑥10−9 )(1.381 𝑥10−23 )(9273𝐾))
= 0 𝑒 𝜆𝑘𝑇 = 𝑒 = 𝟖. 𝟓𝟐𝟒 𝒙 𝟏𝟎−𝟐
𝑁3𝑝 𝑔 3
Step 2
3p→ 3s
We have;
λ = 589.3 𝑥 10−9 , g*=1, g0 = 3, k= 1.381 x 10-23, h= 6.63x10-34, c= 3x108, T= 9273 K
and N4s/N3p = ?
−34 )(3𝑥108 ))
𝑁4𝑠 𝑔∗ −(ℎ𝑐)⁄ 1 −((6.63𝑥10 ⁄
((589.3𝑥10−9 )(1.381 𝑥10−23 )(9273𝐾))
= 0 𝑒 𝜆𝑘𝑇 = 𝑒 = 𝟎. 𝟐𝟏𝟓
𝑁3𝑝 𝑔 3
Step 3
Calculating 4s→ 3s
Consider:
𝑁4𝑠 𝑁3𝑝 𝑁4𝑠 Hence; we multiply to get; 𝑁4𝑠
𝑥 = = (8.524𝑥10−2 )(0.215)
𝑁3𝑝 𝑁4𝑠 𝑁3𝑠 𝑁3𝑠
= 𝟏. 𝟖𝟑𝒙𝟏𝟎−𝟐 𝑜𝑟 𝟎. 𝟎𝟏𝟖𝟑
Question 4
First calculate the population of excited Na atoms at 2600K and then at 2610K. Following
this, find the percentage difference between the two values.
Step 1; (at 2600 K)
We have;
∆E = 3.371 𝑥 10−19 , g*=2, g0 = 1, k= 1.381 x 10-23, T= 2600 K and N*/N0 = ?
−19 )
𝑁4𝑠 𝑔∗ −∆𝐸⁄ 2 −(3.371 𝑥10 ⁄
(1.381 𝑥10−23 )(2600𝐾)
= 0 𝑒 𝑘𝑇 = 𝑒 = 𝟏. 𝟔𝟕 𝒙 𝟏𝟎−𝟒
𝑁3𝑝 𝑔 1
Step 2; (at 2610 K)
We have;
∆E = 3.371 𝑥 10−19 , g*=2, g0 = 1, k= 1.381 x 10-23, T= 2610 K and N*/N0 = ?
−19 )
𝑁4𝑠 𝑔∗ −∆𝐸⁄ 2 −(3.371 𝑥10 ⁄
(1.381 𝑥10−23 )(2610𝐾)
= 𝑒 𝑘𝑇 = 𝑒 = 𝟏. 𝟕𝟒 𝒙 𝟏𝟎−𝟒
𝑁3𝑝 𝑔0 1
Step 3; (Percentage difference)
1.74 𝑥10−4 − 1.67 𝑥10−4
𝑥 100 = 𝟒. 𝟎𝟐%
1.74 𝑥10−4
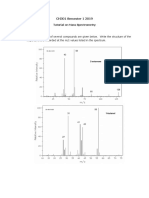
Question 5
Consider the relation and formula below;
𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑎𝑛𝑎𝑙𝑦𝑡𝑒 𝑖𝑛 𝑖𝑛𝑖𝑡𝑖𝑎𝑙 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑆𝑖𝑔𝑛𝑎𝑙 𝑓𝑟𝑜𝑚 𝑖𝑛𝑖𝑡𝑖𝑎𝑙
=
𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑎𝑛𝑎𝑙𝑦𝑡𝑒 𝑝𝑙𝑢𝑠 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑖𝑛 𝑓𝑖𝑛𝑎𝑙 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 𝑆𝑖𝑔𝑛𝑎𝑙 𝑓𝑟𝑜𝑚 𝑓𝑖𝑛𝑎𝑙
[𝑋]𝑖 𝐼𝑋
=
[𝑆]𝑓 + [𝑋]𝑓 𝐼𝑆+𝑋
Step 1
Compute [𝑆]𝑓 𝑎𝑛𝑑 [𝑋]𝑓 and make [𝑋]𝑖 the subject of the formula.
𝑉𝑠 5
[𝑆]𝑓 = [𝑆]𝑖 ∗ ( ) = [2.08 𝑀] ∗ ( ) = 𝟎. 𝟏𝟎𝟒
𝑉 100
𝑉0 95
[𝑋]𝑓 = [𝑋]𝑖 ∗ ( ) = [𝑋]𝑖 ∗ ( ) = 𝟎. 𝟗𝟓 [𝑿]𝒊
𝑉 100
Step 2
Substitute the variables in the original equation and solve for [𝑿]𝒊 .
[𝑋]𝑖 𝐼𝑋
=
[𝑆]𝑓 + [𝑋]𝑓 𝐼𝑆+𝑋
[𝑋]𝑖 4.27 𝑚𝑉
=
0.104 + 0.95 [𝑋]𝑖 6.50 𝑚𝑉
[𝑋]𝑖
= 0.65
0.104 + 0.95 [𝑋]𝑖
[𝑋]𝑖 = 0.068 + 0.62[𝑋]𝑖
0.38[𝑋]𝑖 = 0.068
[𝑿]𝒊 = 𝟎. 𝟏𝟖
Alternatively, use the formula:
Cs x Vs
[X]i = [R(Vs+Vo) − Vo]
Question 6
Explanation – Iron forms a compound with low volatility with the sulfate ions resulting in a
decrease in signal. (Iron sulfate has a much greater thermal stability)
3 Possible Methods:
1. Add a releasing agent like Sr which would react with the sulfate, leaving Iron free for
atomisation.
2. Add a protective agent like EDTA
3. Increase the flame temperature until the compound breaks down.
You might also like
- Equations of Simple Harmonic MotionDocument15 pagesEquations of Simple Harmonic MotionSansen ColipanoNo ratings yet
- Topic 4.0 Problem Solving Convective Mass TransferDocument3 pagesTopic 4.0 Problem Solving Convective Mass TransferBeatriceNo ratings yet
- ENG 9115 Assignment 3Document7 pagesENG 9115 Assignment 3latifmeijidaNo ratings yet
- Michelle Bodnar, Andrew Lohr February 5, 2018Document20 pagesMichelle Bodnar, Andrew Lohr February 5, 2018akshat sinhaNo ratings yet
- Lesson 2 pt.2Document27 pagesLesson 2 pt.2Pido, Patricia LaineNo ratings yet
- Unit 1Document64 pagesUnit 1Aditya YadavNo ratings yet
- EKC 367 Tutorial 3 AnswerDocument3 pagesEKC 367 Tutorial 3 AnswerALAMEL MANZGHAI A/P GANESONNo ratings yet
- 2016 Mmcgrade 10Document8 pages2016 Mmcgrade 10api-339611548100% (17)
- 01Document8 pages01Sadid HossainNo ratings yet
- Math Functions With Complex NumbersDocument2 pagesMath Functions With Complex NumberskekNo ratings yet
- HQ#2 SolutionDocument2 pagesHQ#2 SolutionNasser ÀlsalmiNo ratings yet
- New possible mathematical developments concerning ζ (2), ?, the Rogers- Ramanujan identity: Mathematical connections with some sectors of Particles Physics and the Black Hole physical parameters.Document197 pagesNew possible mathematical developments concerning ζ (2), ?, the Rogers- Ramanujan identity: Mathematical connections with some sectors of Particles Physics and the Black Hole physical parameters.Michele NardelliNo ratings yet
- Desarrollo:: - Hallar R - HALLAR A (H) - Remplazar en La Ecuacion Del TanqueDocument3 pagesDesarrollo:: - Hallar R - HALLAR A (H) - Remplazar en La Ecuacion Del TanqueDiego Mata PerezNo ratings yet
- Solution:: Step 1: Truss AnalysisDocument19 pagesSolution:: Step 1: Truss Analysisiyad aboissaNo ratings yet
- Objective:: (2020 Spring Semester) MESF5450 Supplementary Exercise 03Document4 pagesObjective:: (2020 Spring Semester) MESF5450 Supplementary Exercise 03Lit Pao WongNo ratings yet
- Wendy Johanna Moreno Diaz - Task 3Document17 pagesWendy Johanna Moreno Diaz - Task 3wendy jhoanna moreno diazNo ratings yet
- 1QuizExercises SolvedDocument1 page1QuizExercises SolvedclemencetrinNo ratings yet
- NM - Chap 2 Solved - ExerciesesDocument10 pagesNM - Chap 2 Solved - ExerciesesLy EsNo ratings yet
- CBSE Sample Paper Solution Class 9 Maths Set 1Document20 pagesCBSE Sample Paper Solution Class 9 Maths Set 1Gharsellaoui MuhamedNo ratings yet
- Recurrences: SE 328 Algorithms and Optimization MethodsDocument28 pagesRecurrences: SE 328 Algorithms and Optimization Methodsskj djkNo ratings yet
- Kelompok Fismod 2Document5 pagesKelompok Fismod 2WhyBoo gamingNo ratings yet
- Kelompok Fismod 2Document5 pagesKelompok Fismod 2Mahadi PratamaNo ratings yet
- HW2 SolutionDocument8 pagesHW2 SolutiontilahunNo ratings yet
- Taller Rendimiento TerminadoDocument11 pagesTaller Rendimiento Terminadosantiago hernandezNo ratings yet
- 7.1: Integration by Substitution Backward ChainDocument2 pages7.1: Integration by Substitution Backward ChainTahsina TasneemNo ratings yet
- Basic Calculus - 4th Quarter - Handouts and Worksheets - Lesson 2Document10 pagesBasic Calculus - 4th Quarter - Handouts and Worksheets - Lesson 2Louise CarolineNo ratings yet
- Mat-130 Day-1Document7 pagesMat-130 Day-1Tahsina TasneemNo ratings yet
- ProblemarioDocument15 pagesProblemarioJhoan Andre'No ratings yet
- ThermodynamicsDocument3 pagesThermodynamicsShyn GysawNo ratings yet
- Modern Physics Test 2 - Elevate Classes - 10352883 - 2022 - 12!25!19 - 53Document6 pagesModern Physics Test 2 - Elevate Classes - 10352883 - 2022 - 12!25!19 - 53dixitratan68No ratings yet
- Answer On Question #59908 - Math - CalculusDocument2 pagesAnswer On Question #59908 - Math - CalculusAfzaal Ghazi100% (1)
- L3 Evaluation A Function Using A Matrix-1Document16 pagesL3 Evaluation A Function Using A Matrix-1Sphumelele AndiswaNo ratings yet
- Final Exam: N+ N Sin NDocument9 pagesFinal Exam: N+ N Sin NtehepiconeNo ratings yet
- Kelompok 3 - Sheet 5Document8 pagesKelompok 3 - Sheet 5Satrya Budi PratamaNo ratings yet
- Questions For 2nd YearDocument6 pagesQuestions For 2nd YearRowin Mark SabornidoNo ratings yet
- ND RDDocument5 pagesND RDSadid HossainNo ratings yet
- Tarea2 TD GaelFernandoRodriguezLopez C2G2Document8 pagesTarea2 TD GaelFernandoRodriguezLopez C2G2Rodríguez López Gael FernandoNo ratings yet
- Assignment 12 AnsDocument10 pagesAssignment 12 AnsAnuska DeyNo ratings yet
- Expt. No. 1.6: Moments, Skewness and Kurtosis - I (Ungrouped Data)Document3 pagesExpt. No. 1.6: Moments, Skewness and Kurtosis - I (Ungrouped Data)Suraj GundNo ratings yet
- Ws1 - Probability Generating FunctionssolDocument12 pagesWs1 - Probability Generating FunctionssolkelerongNo ratings yet
- Calculus (Test 1 Question)Document5 pagesCalculus (Test 1 Question)Bala GanabathyNo ratings yet
- Task 3-Eder - CastañedaDocument12 pagesTask 3-Eder - CastañedaEver Castañeda100% (1)
- Repeated QuesDocument8 pagesRepeated QuesMohamed TahmeedNo ratings yet
- Answer 59910Document3 pagesAnswer 59910Lytzkie George PaladaNo ratings yet
- Miscellaneous Practice 2 BryanDocument6 pagesMiscellaneous Practice 2 BryanBryan FuryNo ratings yet
- SPHA031-23 Online Assignment No 1 Solutions 2023Document4 pagesSPHA031-23 Online Assignment No 1 Solutions 2023Ntokozo MasemulaNo ratings yet
- M1 - L15 - Triple IntegralDocument15 pagesM1 - L15 - Triple Integralsaranshchaturvedu10No ratings yet
- Mark Scheme January 2002 GCE: Physics A Unit PA08Document5 pagesMark Scheme January 2002 GCE: Physics A Unit PA08Ali WanNo ratings yet
- Midterm SolutionDocument3 pagesMidterm SolutionJad GhorraNo ratings yet
- Toaz - Info Solution Manual Optical Fiber Communication Gerd Keiser 3rd Ed PRDocument63 pagesToaz - Info Solution Manual Optical Fiber Communication Gerd Keiser 3rd Ed PRdalvirphotonNo ratings yet
- Vector Calculus HW10Document4 pagesVector Calculus HW10ghostNo ratings yet
- 11th Phy Problem EnglishDocument52 pages11th Phy Problem EnglishwhiteshadowsgamerNo ratings yet
- JEE Main Math 24th Feb Shift 2Document9 pagesJEE Main Math 24th Feb Shift 2Abhinav KumarNo ratings yet
- Mathematical Physics Sample Exam 1Document5 pagesMathematical Physics Sample Exam 1Perry EsguerraNo ratings yet
- Exercise Model AnswersDocument19 pagesExercise Model AnswersMohamed EmamNo ratings yet
- Cap 9 Incroperaaaaaaa1298716e67372910Document2 pagesCap 9 Incroperaaaaaaa1298716e67372910Nata Serra TananNo ratings yet
- Lesson 8.3 Implicit Differentiation and Related RatesDocument37 pagesLesson 8.3 Implicit Differentiation and Related RatesAC TadtadNo ratings yet
- Trigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsFrom EverandTrigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsRating: 5 out of 5 stars5/5 (1)
- Tutorial Questions On ICP PDFDocument1 pageTutorial Questions On ICP PDFArjun MaharajNo ratings yet
- DG-Infrared (IR) SpectrosDocument68 pagesDG-Infrared (IR) SpectrosArjun MaharajNo ratings yet
- DG NMRDocument62 pagesDG NMRArjun MaharajNo ratings yet
- Electroanalysis Tutorial Solutions PDFDocument4 pagesElectroanalysis Tutorial Solutions PDFArjun MaharajNo ratings yet
- Atomisation Atomic Spectroscopy Lecture PDFDocument14 pagesAtomisation Atomic Spectroscopy Lecture PDFArjun MaharajNo ratings yet
- X-Ray Tutorial Solutions PDFDocument4 pagesX-Ray Tutorial Solutions PDFArjun MaharajNo ratings yet
- Gas Chromatography 1Document14 pagesGas Chromatography 1Arjun MaharajNo ratings yet
- DG-Lecture 1 - UV-VISDocument35 pagesDG-Lecture 1 - UV-VISArjun MaharajNo ratings yet
- X-Ray NotesDocument37 pagesX-Ray NotesArjun MaharajNo ratings yet
- HPLC 2009Document27 pagesHPLC 2009Arjun MaharajNo ratings yet
- CH301 Tutorial Solutions On UV Spectros PDFDocument3 pagesCH301 Tutorial Solutions On UV Spectros PDFArjun MaharajNo ratings yet
- CH301 Tutorial IRDocument2 pagesCH301 Tutorial IRArjun MaharajNo ratings yet
- CH301 Tutorial HNMR PDFDocument4 pagesCH301 Tutorial HNMR PDFArjun MaharajNo ratings yet
- CH301 Tutorial UV Spectros PDFDocument2 pagesCH301 Tutorial UV Spectros PDFArjun MaharajNo ratings yet
- Tutorial Sheet On Thermal Analysis With AnswersDocument3 pagesTutorial Sheet On Thermal Analysis With AnswersArjun MaharajNo ratings yet
- DG HPLCDocument17 pagesDG HPLCArjun MaharajNo ratings yet
- CH301 NMR Spectros PDFDocument88 pagesCH301 NMR Spectros PDFArjun MaharajNo ratings yet
- AAS Lecture PDFDocument58 pagesAAS Lecture PDFArjun MaharajNo ratings yet
- Tutorial Mass Spectrometry PDFDocument4 pagesTutorial Mass Spectrometry PDFArjun MaharajNo ratings yet
- ICP Lecture Notes PDFDocument21 pagesICP Lecture Notes PDFArjun MaharajNo ratings yet
- ThermogravimetryDocument20 pagesThermogravimetryArjun MaharajNo ratings yet
- 13C NMRDocument28 pages13C NMRArjun MaharajNo ratings yet
- Gas Chromatography 2Document35 pagesGas Chromatography 2Arjun MaharajNo ratings yet
- Lecture 1 General - Principles - of - Gas Chromatography PDFDocument30 pagesLecture 1 General - Principles - of - Gas Chromatography PDFArjun MaharajNo ratings yet
- AAS Lecture PDFDocument18 pagesAAS Lecture PDFArjun MaharajNo ratings yet
- Tutorial Sheet On Thermal Analysis-1Document2 pagesTutorial Sheet On Thermal Analysis-1Arjun MaharajNo ratings yet
- X-Ray Tutorial Solutions PDFDocument4 pagesX-Ray Tutorial Solutions PDFArjun MaharajNo ratings yet
- Lecture 3 NMR PDFDocument21 pagesLecture 3 NMR PDFArjun MaharajNo ratings yet
- Infrared Lecture 1 PDFDocument49 pagesInfrared Lecture 1 PDFArjun MaharajNo ratings yet
- NMR Structure Elucidation PDFDocument22 pagesNMR Structure Elucidation PDFArjun MaharajNo ratings yet