Professional Documents
Culture Documents
AAH Treatment Guidelines - V17 - 16 Aug 2021
AAH Treatment Guidelines - V17 - 16 Aug 2021
Uploaded by
Dr.Abdirahman DahirCopyright:
Available Formats
You might also like
- McCullough Baylor AlgorithmDocument1 pageMcCullough Baylor AlgorithmThe TexanNo ratings yet
- Infection Control Protocols in I.C.U.: Moderator: PresenterDocument54 pagesInfection Control Protocols in I.C.U.: Moderator: PresenterRajaNo ratings yet
- Comprehensive Psychiatric Nursing Care PlanDocument14 pagesComprehensive Psychiatric Nursing Care PlankuniquewNo ratings yet
- OKU Musculo Infection CH 04Document12 pagesOKU Musculo Infection CH 04Jose Enrique FonsecaNo ratings yet
- Covid Basics Poster v2 20211230Document2 pagesCovid Basics Poster v2 20211230Swagat MohantyNo ratings yet
- MX Protocol Book FinalDocument42 pagesMX Protocol Book FinalPawan ChoudharyNo ratings yet
- Algorithm Based On Severity: Confirmed COVID Positive Admitted As InpatientDocument2 pagesAlgorithm Based On Severity: Confirmed COVID Positive Admitted As Inpatientsefigi6746No ratings yet
- New May2020 PDFDocument7 pagesNew May2020 PDFDoctorMoodyNo ratings yet
- Suspected COVID-19 Cases Management in Triage HospitalsDocument6 pagesSuspected COVID-19 Cases Management in Triage HospitalsMuhamed RamadanNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationIulianNo ratings yet
- MoH COVID 19 Protocol - V1.8-1 PDFDocument13 pagesMoH COVID 19 Protocol - V1.8-1 PDFHCX dghhqNo ratings yet
- WB Covid Protocol Book 25.09 .20 (1)Document49 pagesWB Covid Protocol Book 25.09 .20 (1)El MirageNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument7 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationonkarratheeNo ratings yet
- NH Protocol For Covid Management FinalDocument7 pagesNH Protocol For Covid Management FinalhoneyworksNo ratings yet
- COVID Latest Guidelines-1Document48 pagesCOVID Latest Guidelines-1Digvijay ChavanNo ratings yet
- Restricted ABX PolicyDocument8 pagesRestricted ABX PolicyMario BulaciosNo ratings yet
- MoH COVID 19 Protocol - V1.1 PDFDocument6 pagesMoH COVID 19 Protocol - V1.1 PDFHCX dghhqNo ratings yet
- Marrow UpdatesDocument9 pagesMarrow UpdatesVirat KohliNo ratings yet
- VeIP GU TESDocument6 pagesVeIP GU TESMohan KumarNo ratings yet
- HandoutDocument2 pagesHandoutapi-588254706No ratings yet
- Saudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Document15 pagesSaudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19AldrinNo ratings yet
- Antiviral and Pharmacotherapy InformationDocument11 pagesAntiviral and Pharmacotherapy InformationSubahat HumaNo ratings yet
- 3.3 Medication Histories of Peptic Ulcer Disease: Table 3.3aDocument10 pages3.3 Medication Histories of Peptic Ulcer Disease: Table 3.3ab_rahman2k39603No ratings yet
- Covid Flow ChartDocument1 pageCovid Flow ChartPeeaar Green Energy SolutionsNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationAhmed EljenanNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationFarmasi RSUD Kramat JatiNo ratings yet
- COVID Latest GuidelinesDocument48 pagesCOVID Latest Guidelinesarrestedinlove123No ratings yet
- COVID-19 MoHP Protocol May 2020Document14 pagesCOVID-19 MoHP Protocol May 2020BARAKA ROKANo ratings yet
- MoH Saudi Arabia COVID 19 ProtocolDocument13 pagesMoH Saudi Arabia COVID 19 Protocoladilah fazliNo ratings yet
- Treatment Protocol Covid-19Document4 pagesTreatment Protocol Covid-19Shiv singhNo ratings yet
- COVID-19 Protocol - MOHP 23march 2020Document4 pagesCOVID-19 Protocol - MOHP 23march 2020Sanaa SbdelghanyNo ratings yet
- Pretomanid National Drug Monograph October 2021: FDA Approval InformationDocument8 pagesPretomanid National Drug Monograph October 2021: FDA Approval InformationAndi SuhriyanaNo ratings yet
- Saudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Document12 pagesSaudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Taha ShahzadNo ratings yet
- Medical TherapyDocument6 pagesMedical Therapyvinay reddyNo ratings yet
- Increased Blood (Hyperglycemia)Document2 pagesIncreased Blood (Hyperglycemia)kharla suriagaNo ratings yet
- II-118 High Alert Medications: PurposeDocument9 pagesII-118 High Alert Medications: PurposeAhmad Al-RusasiNo ratings yet
- Beacopp Hem HL ADocument8 pagesBeacopp Hem HL AAnonymous 9dVZCnTXSNo ratings yet
- COVID-19 PKPD Treatment VietnamDocument27 pagesCOVID-19 PKPD Treatment VietnamNhanLiNo ratings yet
- Quick Reference DMARDsDocument12 pagesQuick Reference DMARDsEman MohamedNo ratings yet
- Kent and Medway Inflammatory Bowel Disease Ibd Adults High Cost Drug HCD PathwayDocument8 pagesKent and Medway Inflammatory Bowel Disease Ibd Adults High Cost Drug HCD PathwaySavan QilahsNo ratings yet
- Antiviral Management of Covid-19 How Current PerspectiveDocument29 pagesAntiviral Management of Covid-19 How Current PerspectiveBenhardiet SondaNo ratings yet
- Folfiri+cetu Gi Col PDocument12 pagesFolfiri+cetu Gi Col PJayelle2No ratings yet
- COVID 19 Anticoagulation Algorithm Version Final 1.1Document2 pagesCOVID 19 Anticoagulation Algorithm Version Final 1.1Emi PuspitasariNo ratings yet
- Early Treatment Information For Doctors Updated 11042022Document4 pagesEarly Treatment Information For Doctors Updated 11042022han tianNo ratings yet
- Daftar Obat AsoDocument2 pagesDaftar Obat AsoekarizqiamaliyahNo ratings yet
- Anticoagulant Oral GuidelinesforprescribingmonitoringandmanagementDocument29 pagesAnticoagulant Oral GuidelinesforprescribingmonitoringandmanagementJamie PalmeriNo ratings yet
- UH Treatment Protocol Edit 6 March 24 2020Document9 pagesUH Treatment Protocol Edit 6 March 24 2020zeeNo ratings yet
- COVID-19 Antiviral and Pharmacotherapy Information: PreferentialDocument6 pagesCOVID-19 Antiviral and Pharmacotherapy Information: Preferentialmrosyidn0% (1)
- صباح عبدالحق 18Document3 pagesصباح عبدالحق 18alsyany001No ratings yet
- 596 Ifosfamide Etoposide Ie TherapyDocument6 pages596 Ifosfamide Etoposide Ie Therapyravindra0504090No ratings yet
- Protocolo Covid 19 Ihss HRN PDFDocument5 pagesProtocolo Covid 19 Ihss HRN PDFJennifer CarcamoNo ratings yet
- MSHS Treatment Guidelines COVIDDocument4 pagesMSHS Treatment Guidelines COVIDFrancisco MuñozNo ratings yet
- Antibiotic Pocket GuideDocument19 pagesAntibiotic Pocket GuideNaomi Liang100% (1)
- Ortho Guidance and Septic ArthritisDocument3 pagesOrtho Guidance and Septic ArthritisKrishna RaoNo ratings yet
- Prescriber Update Vol 43 No.2 June 2022Document17 pagesPrescriber Update Vol 43 No.2 June 2022Naeman GoetzNo ratings yet
- SanfordDocument8 pagesSanfordAndreea StancuNo ratings yet
- CAP AlgorithmDocument1 pageCAP AlgorithmdamondouglasNo ratings yet
- COVID 19 TreatmentDocument8 pagesCOVID 19 TreatmentCWS ScapeNo ratings yet
- Antibiotic Hospital ManDocument1 pageAntibiotic Hospital Manarshiya.manasekiNo ratings yet
- COVID-19 Therapeutic Guidance COVID-19 Therapeutic Guidance COVID-19 Therapeutic GuidanceDocument1 pageCOVID-19 Therapeutic Guidance COVID-19 Therapeutic Guidance COVID-19 Therapeutic GuidanceAbdullah KhanNo ratings yet
- Inpatient Guidance For Treatment of Covid-19 in Adults and ChildrenDocument7 pagesInpatient Guidance For Treatment of Covid-19 in Adults and ChildrenAmogh KurianNo ratings yet
- Empirical First Line AntibioticsDocument1 pageEmpirical First Line Antibioticsdiati zahrainiNo ratings yet
- Head Injury Discharge Instructions Mod 6Document1 pageHead Injury Discharge Instructions Mod 6Dr.Abdirahman DahirNo ratings yet
- Important Notes V1.0Document4 pagesImportant Notes V1.0Dr.Abdirahman DahirNo ratings yet
- Signs of Elder Maltreatment Mod 11Document1 pageSigns of Elder Maltreatment Mod 11Dr.Abdirahman DahirNo ratings yet
- Diabetes EmergenciesDocument7 pagesDiabetes EmergenciesDr.Abdirahman DahirNo ratings yet
- Signs of Child Maltreatment Mod 10Document1 pageSigns of Child Maltreatment Mod 10Dr.Abdirahman DahirNo ratings yet
- Prevalence of Intestinal Parasites Among School Children in District Upper Dir, Khyber Pakhtunkhwa PakistanDocument8 pagesPrevalence of Intestinal Parasites Among School Children in District Upper Dir, Khyber Pakhtunkhwa PakistanInternational Network For Natural SciencesNo ratings yet
- Rhinoplasty BrochureDocument4 pagesRhinoplasty BrochureSmoKingBuds HD (SKB)No ratings yet
- NCLEX Quick Fix III - Sessions 7 To 9 - Handouts PDFDocument34 pagesNCLEX Quick Fix III - Sessions 7 To 9 - Handouts PDFNina MoradaNo ratings yet
- Fat Embolism Syndrome: DescriptionDocument4 pagesFat Embolism Syndrome: DescriptionDayuKurnia DewantiNo ratings yet
- Parapharyngeal Space TumorDocument4 pagesParapharyngeal Space Tumorgauri kokaneNo ratings yet
- Optimization of Chronic Heart-Compressed PDFDocument47 pagesOptimization of Chronic Heart-Compressed PDFtaricuteNo ratings yet
- Psychological Domain - Nursing Care PlanDocument1 pagePsychological Domain - Nursing Care PlanPussykate DollNo ratings yet
- Zhang Xiang Theory HeartDocument77 pagesZhang Xiang Theory HeartAns JavidNo ratings yet
- Case Study Icu Sem 6Document30 pagesCase Study Icu Sem 6BM2-0619 Mohd Khairul Naaim Bin PenchariNo ratings yet
- MDWF 2090 - Examining The Perineum After BirthDocument7 pagesMDWF 2090 - Examining The Perineum After Birthapi-442131145No ratings yet
- Amniotic Membrane1Document4 pagesAmniotic Membrane1Joel JohnsonNo ratings yet
- Biomatric Approach For Complete Denture DesignDocument18 pagesBiomatric Approach For Complete Denture Designفواز نميرNo ratings yet
- Status EpilepticusDocument21 pagesStatus EpilepticusbushraNo ratings yet
- Spence Children's Anxiety ScaleDocument3 pagesSpence Children's Anxiety ScaleAnnie AlvaradoNo ratings yet
- Null PDFDocument48 pagesNull PDFfaniar tasha almaraNo ratings yet
- Basal Body Temperature Monitoring Contraceptive MethodDocument5 pagesBasal Body Temperature Monitoring Contraceptive Methodpriya vermaNo ratings yet
- Acute Febrile Illnesses: Solomon Bekele Sirak Melkeneh Sonia Worku Fri. May 15, 2014Document124 pagesAcute Febrile Illnesses: Solomon Bekele Sirak Melkeneh Sonia Worku Fri. May 15, 2014ashuNo ratings yet
- Contoh Soal FetomaternalDocument10 pagesContoh Soal FetomaternalBella AgustinNo ratings yet
- Diet TherapyDocument140 pagesDiet Therapyraghadalesawi51No ratings yet
- Ra 7719 Blood Bank MT-B & C 8Document7 pagesRa 7719 Blood Bank MT-B & C 8Fait Hee100% (1)
- Visual Dysfunction in Diabetes PDFDocument395 pagesVisual Dysfunction in Diabetes PDFMarcosNo ratings yet
- ABP3 IntoxmetalespesadosDocument18 pagesABP3 IntoxmetalespesadosFernandoLuyoNo ratings yet
- Critically Ill PaedsDocument53 pagesCritically Ill Paedsdayang rohanaNo ratings yet
- Kelompok 2 - Tugas Pengendalian Vektor - Pertemuan 2Document13 pagesKelompok 2 - Tugas Pengendalian Vektor - Pertemuan 2Irene Pramesti DiningrumNo ratings yet
- Rafaiqa-17Mar2023-Health Che PDFDocument8 pagesRafaiqa-17Mar2023-Health Che PDFmr copy xeroxNo ratings yet
- Urologi: NO Diagnosis Kode Icd 10Document9 pagesUrologi: NO Diagnosis Kode Icd 10dokter jaga rskb ddsNo ratings yet
- Figo Consensus Guidelines On Intrapartum Fetal Monitoring: Intermittent AuscultationDocument7 pagesFigo Consensus Guidelines On Intrapartum Fetal Monitoring: Intermittent AuscultationWilliam WongNo ratings yet
AAH Treatment Guidelines - V17 - 16 Aug 2021
AAH Treatment Guidelines - V17 - 16 Aug 2021
Uploaded by
Dr.Abdirahman DahirOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AAH Treatment Guidelines - V17 - 16 Aug 2021
AAH Treatment Guidelines - V17 - 16 Aug 2021
Uploaded by
Dr.Abdirahman DahirCopyright:
Available Formats
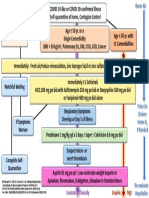
Treatment Guidelines for SARS-CoV-2 Positive Patients at Al Ain Hospital General Wards
(Version 17 – Aug 16, 2021)
SARS-CoV-2 Positive Patient Presents to ED
Supp
Basic clinical assessment, vitals, labs
Pregnant / lactating patient COVID re-screening O2 Dexamethasone 6 mg PO / IV (4)
with pneumonia, consult ID / Clinical assessment for O 2 support once daily
MDT and consider remdesivir Clinical assessment for anticoagulation
Consider sotrovimab
based on criteria
Negative HRCT / CXR Positive
Asymptomatic with abnormal
inflammatory markers or Mild
Disease
No Yes Favipiravir + camostat for 7 days
Risk Factors (1)
Moderate/Severe Disease
(2) Favipiravir + Camostat for 7 Days
Asymptomatic(2) Asymptomatic Consider remdesivir criteria
Sotrovimab criteria + OR upon MDT/ID approval
Supportive care
supportive care
Important Notes:
o For admitted patients, start enoxaparin if no contraindications.
Symptomatic(2) Symptomatic (2)
o Consider NOT starting antibiotics if procalcitonin
Sotrovimab criteria + -ve and no clinical suspicion of bacterial infection.
ED Mono Pack
ED Monopack
(Favipiravir for 5 days)
(Favipiravir for 5 days)
Remdesivir Eligibility Criteria
RED: Must | Green: Any
Total score >4: Eligible
(Consider remdesivir in patients having intolerance or
contraindications to favipiravir)
COVID-19 Medications Doses and Duration Criteria Details Score
Medication Dose Duration(Days) Moderate or severe CT lung
HRCT Changes 1
COVID-19 changes
1600 mg BID for 1 day,
Favipiravir 7 Hypoxia and O2 SPo2 < 92% or PaO2 < 70 or RR > 20 +
then 600 mg bid 1
requirement supplemental O2 > 2 L
Camostat 200 mg TID 7 Age ≥ 50, DM, CAD 1
200 mg loading dose day 1 Comorbidities
COPD or CKD 1
Remdesivir 5 (1 or ≥ 1)
then 100 mg OD Active Immunosuppression 1
Inteferon beta 1b 8M IU (250 mcg) neb bid 7
Obesity BMI > 35 1
Multivitamins 1 tab daily 5-10
LDH, CRP, ferritin, LDH > 360 or CRP > 75 or ferritin > 600
Vitamin D 50,000 weekly 14 1
urea or urea > 8
Anticoagulation in SARS-CoV-2 Positive Patients
Anticoagulation
Contraindications (CI)
Recent or active bleeding All admitted patients should be started on weight adjusted
Hemoglobin <80 g/L prophylactic dose enoxaparin except when meeting following
criteria for increasing enoxaparin dose.
Platelet count <50 x 109/L
INR >1.8 Criteria for Intermediate Dose Enoxaparin
Coagulopathy On supp O2 >5 L
Dual antiplatelet therapy (relative CI – eligible if moderate or severe (CT finding)
one of the antiplatelets can be safely stopped)
Pregnancy (relative CI – consult OBGYN) No contraindications to anticoagulation
Enoxaparin Prophylactic Weight-based Dosing Enoxaparin Intermediate Dose
Cr.Cl>30ml/min 0.5 mg/kg of actual body weight twice daily
Weight Enoxaparin dose Enoxaparin Therapeutic Dose
<50 kg 20 mg SC daily Cr.Cl>30ml/min
50 - 100 kg 40 mg SC daily
Weight Enoxaparin dose
100 - 150 kg 40 mg BID
> 150 kg 60 mg SC BID <100 kg 1 mg / kg / dose bid
Enoxaparin Prophylactic Renal Function based-Dosing Please consult MDT and
> 100 kg
Creatinine Clearance Enoxaparin dose clinical pharmacist
CrCl > 30 ml/min Weight-based dosing as above. Enoxaparin Renal Function Based Therapeutic Dose
Weight >50 kg, SC 30 mg daily Creatinine Clearance Enoxaparin dose
CrCl < 30 ml/min
Weight <50 kg , SC 20 mg daily CrCl > 30 ml/min Weight-based dosing as above.
Dialysis Consider UFH SC 5000 units BID CrCl < 30 ml/min 1 mg / kg / dose daily
General Information and Foot Notes
Notes:
Tocilizumab Eligibility Criteria (1) Comorbidities and risk factors: CVD, diabetes, hypertension, chronic
(Should be approved by MDT / ID) lung disease, cancer, chronic kidney disease, immunocompromised,
Extensive or bilateral lung disease and severely ill patients obesity BMI > 30 or weight > 100 kg, age > 60 years, smoking.
(2) Symptomatic: Sore throat, myalgia, runny nose, headache, dry cough.
Worsening of respiratory exchanges such as require non-invasive (3) Remdesivir: Patients with mild CT lung COVID19 changes do not
(> 5 L) or invasive ventilation. qualify. Consult ID for remdesivir consideration if patient is worsening on
Laboratory parameters supportive of cytokine syndrome dual therapy. If ALT raised > 10 times the ULN, consider remdesivir
discontinuation.
Serum IL-6 ≥ 5 X upper normal limit (4) Dexamethasone: Monitor for hyperglycemia (both in diabetic and
Ferritin ≥ 1000 non-diabetic), hypokalemia and psychiatric manifestations. Please add
LDH > 300 U/L POCT glucose monitoring for all patients started on dexamethasone.
(5) Camostat can be used as an alternative agent
CRP >75 mg/dL
Significant Adverse Drug Reactions:
Favipiravir: Increased LFTs, neutropenia, increased uric acid, increased
q Dose: triglycerides, hypokalemia.
>90kg: 800mg Remdesivir: Increased LFTs, Infusion related reaction, nausea,
>65kg & ≤90kg: 600mg vomiting, hypotension, diaphoresis, shivering.
>40kg & ≤65kg: 400mg Camostat: Hyperkalemia, Increased LFTs,, increased serum creatinine,
≤40kg: 8mg/kg thrombocytopenia, leukopenia, eosinophilia, xerostomia
Approved by clinical guide team, Al Ain Hospital
You might also like
- McCullough Baylor AlgorithmDocument1 pageMcCullough Baylor AlgorithmThe TexanNo ratings yet
- Infection Control Protocols in I.C.U.: Moderator: PresenterDocument54 pagesInfection Control Protocols in I.C.U.: Moderator: PresenterRajaNo ratings yet
- Comprehensive Psychiatric Nursing Care PlanDocument14 pagesComprehensive Psychiatric Nursing Care PlankuniquewNo ratings yet
- OKU Musculo Infection CH 04Document12 pagesOKU Musculo Infection CH 04Jose Enrique FonsecaNo ratings yet
- Covid Basics Poster v2 20211230Document2 pagesCovid Basics Poster v2 20211230Swagat MohantyNo ratings yet
- MX Protocol Book FinalDocument42 pagesMX Protocol Book FinalPawan ChoudharyNo ratings yet
- Algorithm Based On Severity: Confirmed COVID Positive Admitted As InpatientDocument2 pagesAlgorithm Based On Severity: Confirmed COVID Positive Admitted As Inpatientsefigi6746No ratings yet
- New May2020 PDFDocument7 pagesNew May2020 PDFDoctorMoodyNo ratings yet
- Suspected COVID-19 Cases Management in Triage HospitalsDocument6 pagesSuspected COVID-19 Cases Management in Triage HospitalsMuhamed RamadanNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationIulianNo ratings yet
- MoH COVID 19 Protocol - V1.8-1 PDFDocument13 pagesMoH COVID 19 Protocol - V1.8-1 PDFHCX dghhqNo ratings yet
- WB Covid Protocol Book 25.09 .20 (1)Document49 pagesWB Covid Protocol Book 25.09 .20 (1)El MirageNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument7 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationonkarratheeNo ratings yet
- NH Protocol For Covid Management FinalDocument7 pagesNH Protocol For Covid Management FinalhoneyworksNo ratings yet
- COVID Latest Guidelines-1Document48 pagesCOVID Latest Guidelines-1Digvijay ChavanNo ratings yet
- Restricted ABX PolicyDocument8 pagesRestricted ABX PolicyMario BulaciosNo ratings yet
- MoH COVID 19 Protocol - V1.1 PDFDocument6 pagesMoH COVID 19 Protocol - V1.1 PDFHCX dghhqNo ratings yet
- Marrow UpdatesDocument9 pagesMarrow UpdatesVirat KohliNo ratings yet
- VeIP GU TESDocument6 pagesVeIP GU TESMohan KumarNo ratings yet
- HandoutDocument2 pagesHandoutapi-588254706No ratings yet
- Saudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Document15 pagesSaudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19AldrinNo ratings yet
- Antiviral and Pharmacotherapy InformationDocument11 pagesAntiviral and Pharmacotherapy InformationSubahat HumaNo ratings yet
- 3.3 Medication Histories of Peptic Ulcer Disease: Table 3.3aDocument10 pages3.3 Medication Histories of Peptic Ulcer Disease: Table 3.3ab_rahman2k39603No ratings yet
- Covid Flow ChartDocument1 pageCovid Flow ChartPeeaar Green Energy SolutionsNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationAhmed EljenanNo ratings yet
- Guidance For Treatment of Covid-19 in Adults and Children: Patient PopulationDocument10 pagesGuidance For Treatment of Covid-19 in Adults and Children: Patient PopulationFarmasi RSUD Kramat JatiNo ratings yet
- COVID Latest GuidelinesDocument48 pagesCOVID Latest Guidelinesarrestedinlove123No ratings yet
- COVID-19 MoHP Protocol May 2020Document14 pagesCOVID-19 MoHP Protocol May 2020BARAKA ROKANo ratings yet
- MoH Saudi Arabia COVID 19 ProtocolDocument13 pagesMoH Saudi Arabia COVID 19 Protocoladilah fazliNo ratings yet
- Treatment Protocol Covid-19Document4 pagesTreatment Protocol Covid-19Shiv singhNo ratings yet
- COVID-19 Protocol - MOHP 23march 2020Document4 pagesCOVID-19 Protocol - MOHP 23march 2020Sanaa SbdelghanyNo ratings yet
- Pretomanid National Drug Monograph October 2021: FDA Approval InformationDocument8 pagesPretomanid National Drug Monograph October 2021: FDA Approval InformationAndi SuhriyanaNo ratings yet
- Saudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Document12 pagesSaudi Moh Protocol For Patients Suspected Of/Confirmed With Covid-19Taha ShahzadNo ratings yet
- Medical TherapyDocument6 pagesMedical Therapyvinay reddyNo ratings yet
- Increased Blood (Hyperglycemia)Document2 pagesIncreased Blood (Hyperglycemia)kharla suriagaNo ratings yet
- II-118 High Alert Medications: PurposeDocument9 pagesII-118 High Alert Medications: PurposeAhmad Al-RusasiNo ratings yet
- Beacopp Hem HL ADocument8 pagesBeacopp Hem HL AAnonymous 9dVZCnTXSNo ratings yet
- COVID-19 PKPD Treatment VietnamDocument27 pagesCOVID-19 PKPD Treatment VietnamNhanLiNo ratings yet
- Quick Reference DMARDsDocument12 pagesQuick Reference DMARDsEman MohamedNo ratings yet
- Kent and Medway Inflammatory Bowel Disease Ibd Adults High Cost Drug HCD PathwayDocument8 pagesKent and Medway Inflammatory Bowel Disease Ibd Adults High Cost Drug HCD PathwaySavan QilahsNo ratings yet
- Antiviral Management of Covid-19 How Current PerspectiveDocument29 pagesAntiviral Management of Covid-19 How Current PerspectiveBenhardiet SondaNo ratings yet
- Folfiri+cetu Gi Col PDocument12 pagesFolfiri+cetu Gi Col PJayelle2No ratings yet
- COVID 19 Anticoagulation Algorithm Version Final 1.1Document2 pagesCOVID 19 Anticoagulation Algorithm Version Final 1.1Emi PuspitasariNo ratings yet
- Early Treatment Information For Doctors Updated 11042022Document4 pagesEarly Treatment Information For Doctors Updated 11042022han tianNo ratings yet
- Daftar Obat AsoDocument2 pagesDaftar Obat AsoekarizqiamaliyahNo ratings yet
- Anticoagulant Oral GuidelinesforprescribingmonitoringandmanagementDocument29 pagesAnticoagulant Oral GuidelinesforprescribingmonitoringandmanagementJamie PalmeriNo ratings yet
- UH Treatment Protocol Edit 6 March 24 2020Document9 pagesUH Treatment Protocol Edit 6 March 24 2020zeeNo ratings yet
- COVID-19 Antiviral and Pharmacotherapy Information: PreferentialDocument6 pagesCOVID-19 Antiviral and Pharmacotherapy Information: Preferentialmrosyidn0% (1)
- صباح عبدالحق 18Document3 pagesصباح عبدالحق 18alsyany001No ratings yet
- 596 Ifosfamide Etoposide Ie TherapyDocument6 pages596 Ifosfamide Etoposide Ie Therapyravindra0504090No ratings yet
- Protocolo Covid 19 Ihss HRN PDFDocument5 pagesProtocolo Covid 19 Ihss HRN PDFJennifer CarcamoNo ratings yet
- MSHS Treatment Guidelines COVIDDocument4 pagesMSHS Treatment Guidelines COVIDFrancisco MuñozNo ratings yet
- Antibiotic Pocket GuideDocument19 pagesAntibiotic Pocket GuideNaomi Liang100% (1)
- Ortho Guidance and Septic ArthritisDocument3 pagesOrtho Guidance and Septic ArthritisKrishna RaoNo ratings yet
- Prescriber Update Vol 43 No.2 June 2022Document17 pagesPrescriber Update Vol 43 No.2 June 2022Naeman GoetzNo ratings yet
- SanfordDocument8 pagesSanfordAndreea StancuNo ratings yet
- CAP AlgorithmDocument1 pageCAP AlgorithmdamondouglasNo ratings yet
- COVID 19 TreatmentDocument8 pagesCOVID 19 TreatmentCWS ScapeNo ratings yet
- Antibiotic Hospital ManDocument1 pageAntibiotic Hospital Manarshiya.manasekiNo ratings yet
- COVID-19 Therapeutic Guidance COVID-19 Therapeutic Guidance COVID-19 Therapeutic GuidanceDocument1 pageCOVID-19 Therapeutic Guidance COVID-19 Therapeutic Guidance COVID-19 Therapeutic GuidanceAbdullah KhanNo ratings yet
- Inpatient Guidance For Treatment of Covid-19 in Adults and ChildrenDocument7 pagesInpatient Guidance For Treatment of Covid-19 in Adults and ChildrenAmogh KurianNo ratings yet
- Empirical First Line AntibioticsDocument1 pageEmpirical First Line Antibioticsdiati zahrainiNo ratings yet
- Head Injury Discharge Instructions Mod 6Document1 pageHead Injury Discharge Instructions Mod 6Dr.Abdirahman DahirNo ratings yet
- Important Notes V1.0Document4 pagesImportant Notes V1.0Dr.Abdirahman DahirNo ratings yet
- Signs of Elder Maltreatment Mod 11Document1 pageSigns of Elder Maltreatment Mod 11Dr.Abdirahman DahirNo ratings yet
- Diabetes EmergenciesDocument7 pagesDiabetes EmergenciesDr.Abdirahman DahirNo ratings yet
- Signs of Child Maltreatment Mod 10Document1 pageSigns of Child Maltreatment Mod 10Dr.Abdirahman DahirNo ratings yet
- Prevalence of Intestinal Parasites Among School Children in District Upper Dir, Khyber Pakhtunkhwa PakistanDocument8 pagesPrevalence of Intestinal Parasites Among School Children in District Upper Dir, Khyber Pakhtunkhwa PakistanInternational Network For Natural SciencesNo ratings yet
- Rhinoplasty BrochureDocument4 pagesRhinoplasty BrochureSmoKingBuds HD (SKB)No ratings yet
- NCLEX Quick Fix III - Sessions 7 To 9 - Handouts PDFDocument34 pagesNCLEX Quick Fix III - Sessions 7 To 9 - Handouts PDFNina MoradaNo ratings yet
- Fat Embolism Syndrome: DescriptionDocument4 pagesFat Embolism Syndrome: DescriptionDayuKurnia DewantiNo ratings yet
- Parapharyngeal Space TumorDocument4 pagesParapharyngeal Space Tumorgauri kokaneNo ratings yet
- Optimization of Chronic Heart-Compressed PDFDocument47 pagesOptimization of Chronic Heart-Compressed PDFtaricuteNo ratings yet
- Psychological Domain - Nursing Care PlanDocument1 pagePsychological Domain - Nursing Care PlanPussykate DollNo ratings yet
- Zhang Xiang Theory HeartDocument77 pagesZhang Xiang Theory HeartAns JavidNo ratings yet
- Case Study Icu Sem 6Document30 pagesCase Study Icu Sem 6BM2-0619 Mohd Khairul Naaim Bin PenchariNo ratings yet
- MDWF 2090 - Examining The Perineum After BirthDocument7 pagesMDWF 2090 - Examining The Perineum After Birthapi-442131145No ratings yet
- Amniotic Membrane1Document4 pagesAmniotic Membrane1Joel JohnsonNo ratings yet
- Biomatric Approach For Complete Denture DesignDocument18 pagesBiomatric Approach For Complete Denture Designفواز نميرNo ratings yet
- Status EpilepticusDocument21 pagesStatus EpilepticusbushraNo ratings yet
- Spence Children's Anxiety ScaleDocument3 pagesSpence Children's Anxiety ScaleAnnie AlvaradoNo ratings yet
- Null PDFDocument48 pagesNull PDFfaniar tasha almaraNo ratings yet
- Basal Body Temperature Monitoring Contraceptive MethodDocument5 pagesBasal Body Temperature Monitoring Contraceptive Methodpriya vermaNo ratings yet
- Acute Febrile Illnesses: Solomon Bekele Sirak Melkeneh Sonia Worku Fri. May 15, 2014Document124 pagesAcute Febrile Illnesses: Solomon Bekele Sirak Melkeneh Sonia Worku Fri. May 15, 2014ashuNo ratings yet
- Contoh Soal FetomaternalDocument10 pagesContoh Soal FetomaternalBella AgustinNo ratings yet
- Diet TherapyDocument140 pagesDiet Therapyraghadalesawi51No ratings yet
- Ra 7719 Blood Bank MT-B & C 8Document7 pagesRa 7719 Blood Bank MT-B & C 8Fait Hee100% (1)
- Visual Dysfunction in Diabetes PDFDocument395 pagesVisual Dysfunction in Diabetes PDFMarcosNo ratings yet
- ABP3 IntoxmetalespesadosDocument18 pagesABP3 IntoxmetalespesadosFernandoLuyoNo ratings yet
- Critically Ill PaedsDocument53 pagesCritically Ill Paedsdayang rohanaNo ratings yet
- Kelompok 2 - Tugas Pengendalian Vektor - Pertemuan 2Document13 pagesKelompok 2 - Tugas Pengendalian Vektor - Pertemuan 2Irene Pramesti DiningrumNo ratings yet
- Rafaiqa-17Mar2023-Health Che PDFDocument8 pagesRafaiqa-17Mar2023-Health Che PDFmr copy xeroxNo ratings yet
- Urologi: NO Diagnosis Kode Icd 10Document9 pagesUrologi: NO Diagnosis Kode Icd 10dokter jaga rskb ddsNo ratings yet
- Figo Consensus Guidelines On Intrapartum Fetal Monitoring: Intermittent AuscultationDocument7 pagesFigo Consensus Guidelines On Intrapartum Fetal Monitoring: Intermittent AuscultationWilliam WongNo ratings yet