Professional Documents
Culture Documents
Bu Ac Production Abstract
Bu Ac Production Abstract
Uploaded by
qasdinayusrinaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bu Ac Production Abstract
Bu Ac Production Abstract
Uploaded by
qasdinayusrinaCopyright:
Available Formats
Production of Butyl Acetate from
Methyl Acetate and Methanol
Eduardo Pacheco e Silva
Federal University of Rio Grande do Sul
Background & Description:
Butyl acetate can be produced by the reaction of methyl acetate with butanol in a
reversible, liquid-phase, mildly exothermic reaction. Methanol is the second product. The
chemical equilibrium constant is less than unity, so the reactor effluent contains significant
amounts of the reactants, which must be recovered for recycle back to the reactor. The volatilities
are such that there are three distillation columns and two recycles.
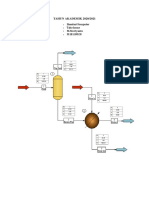
The first column C1 takes the two light components overhead (methyl acetate and
methanol) and the two heavy components out of the bottom (butanol and butyl acetate). In order
to achieve the expected bottom and distillate specifications C1 column is set to a reflux ratio (RR)
of 0.317 and a bottom mole fraction of methanol of 0.001. The C1 distillate is fed to a second
column, which produces product methanol out of the bottom and a recycle stream of the methyl
acetate/methanol azeotrope in the distillate. The C1 bottom is fed to a third column, which
produces product butyl acetate out of the bottom and a recycle stream of butanol in the distillate.
C2 and C3 specifications are as follows, RR = 1 and bottom mole fraction of methyl acetate = 0.01
, RR = 1.92 and bottom mole fraction of butanol = 0.01, respectively.
Figure 1. N - Butyl Acetate Production Flowsheet.
Results:
Table 1. Streamwise Results for the Butyl Acetate Production Flowsheet.
MeOH
Object MeAc/MeOH Feed MeAc Recycle Units
Product
Temperature 305,0 340,1 339,7 K
Pressure 15,0 15,0 1,1 atm
Mass Flow 5726,39 9000,03 3258,56 kg/h
Molar Flow 100,00 152,38 99,61 kmol/h
Molar Fraction (mixture) / 1-butanol 0,0 0,0 0,0 %
Molar Fraction (mixture) / N-butyl acetate 0,0 0,0 0,3 %
Molar Fraction (mixture) / Methyl acetate 60,0 64,2 1,0 %
Molar Fraction (mixture) / Methanol 40,0 35,8 98,7 %
Object BuOH Feed BuOH Recycle BuAc Product Units
Temperature 305,0 438,4 452,4 K
Pressure 15,0 15,0 4,0 atm
Mass Flow 4402,82 17564 6966,68 kg/h
Molar Flow 59,40 212,37 60,20 kmol/h
Molar Fraction (mixture) / 1-butanol 100,0 79,4 1,0 %
Molar Fraction (mixture) / N-butyl acetate 0,0 20,6 99,0 %
Molar Fraction (mixture) / Methyl acetate 0,0 0,0 0,0 %
Molar Fraction (mixture) / Methanol 0,0 0,0 0,0 %
Table 2. CSTR Specifications.
Object Reactor Units
Pressure Drop 10 atm
Residence Time 0,085 h
Volume 4 m³
Temperature Delta -30,1 K
Heat Load -906,4 kW
Outlet Temperature 350 K
Methyl Acetate: Conversion 37,1 %
Table 3. Columns Specifications.
Object C1 C2 C3 Units
Top Specification 0,32 1,00 1,92 RR¹
Bottom Specification MeOH = 0,1% MeAc = 1% BuOH = 1% CC²
¹ - Reflux Ratio ; ² - Component Composition.
References:
(1) Luyben, William. Design and Control of the Butyl Acetate Process. Ind. Eng. Chem.
Res. 2011, 50, 3, 1247-1263
You might also like
- Troubleshooting Problem 5.1: Mass Balance With Recycle StreamsDocument10 pagesTroubleshooting Problem 5.1: Mass Balance With Recycle Streamsmilton ochoaNo ratings yet
- Standard Quality Assurance Plan For Ac MotorDocument6 pagesStandard Quality Assurance Plan For Ac MotorSonti Mani kumar100% (1)
- Isobutane Butane Fractionator PDFDocument7 pagesIsobutane Butane Fractionator PDFhoustonmathNo ratings yet
- HR Practices of Marks and Spencer Selfri PDFDocument46 pagesHR Practices of Marks and Spencer Selfri PDFbalach100% (1)
- Manufacturing Process of SemiconductorDocument36 pagesManufacturing Process of SemiconductorMayank Agarwal100% (1)
- Dealkylation 1 PDFDocument2 pagesDealkylation 1 PDFFamai FamaiNo ratings yet
- Dealkylation 1Document2 pagesDealkylation 1Faisal Yusuf DeshpandeNo ratings yet
- Dealkylation 1Document2 pagesDealkylation 1Yasmina ZahraNo ratings yet
- Production of Benzene Via The Hydrodealkylation of TolueneDocument2 pagesProduction of Benzene Via The Hydrodealkylation of TolueneMarco Cristian CibroNo ratings yet
- Dealkylation 1Document2 pagesDealkylation 1Yasmina ZahraNo ratings yet
- Dealkylation 1Document2 pagesDealkylation 1Naved Ahmad B235No ratings yet
- 2.2 Manual Calculation of Materials and Energy Balances 2.2.1 Mass Balance Plant Complete If The Amount That Targeted Is AchievedDocument34 pages2.2 Manual Calculation of Materials and Energy Balances 2.2.1 Mass Balance Plant Complete If The Amount That Targeted Is AchievedmmbmnbmnbNo ratings yet
- Conversion, Selectivity & YieldDocument111 pagesConversion, Selectivity & YieldVishnuboy VishnuNo ratings yet
- 1.1 Saturator 1.1.1 Process Description: H O From Distillation ColumnDocument20 pages1.1 Saturator 1.1.1 Process Description: H O From Distillation ColumnNUR AKMAL HISHAMNo ratings yet
- MTBEDocument34 pagesMTBEruben ordoñezNo ratings yet
- Energy BalanceDocument12 pagesEnergy BalanceZain Ul AbedinNo ratings yet
- CRE AssignmentDocument5 pagesCRE AssignmentKuldeepChoudharyNo ratings yet
- Chloromethan PDFDocument6 pagesChloromethan PDFMatilda Gerbi ZazoNo ratings yet
- Chapter 1Document24 pagesChapter 1Chandan Kumar YadavNo ratings yet
- Mass Balance - Guidance1Document14 pagesMass Balance - Guidance1KHORSSA AbderrahmanNo ratings yet
- Assignment 4 (7332)Document8 pagesAssignment 4 (7332)Musa KaleemNo ratings yet
- Experiment 3Document9 pagesExperiment 3Marygrace ProgellaNo ratings yet
- Table 1: Thermodynamic Properties Which Characterize The MixtureDocument2 pagesTable 1: Thermodynamic Properties Which Characterize The MixturelalelaNo ratings yet
- Conductometric Determination of TBN in Petroleum Products According To IP 400Document2 pagesConductometric Determination of TBN in Petroleum Products According To IP 400luisry1990No ratings yet
- M.Novriyanto M1B118028 - UTS SIMKOMDocument10 pagesM.Novriyanto M1B118028 - UTS SIMKOMDave N7RNo ratings yet
- Binario MEt MEtyDocument4 pagesBinario MEt MEtyHilda Piza PuentesNo ratings yet
- Book 1Document208 pagesBook 1MARTIN SUGIARTOMANURUNGNo ratings yet
- M. Novriyanto - M1B118028 - UTS - SIMKOMDocument11 pagesM. Novriyanto - M1B118028 - UTS - SIMKOMDave N7RNo ratings yet
- WOrd TADocument61 pagesWOrd TAFaizal AdityaNo ratings yet
- Excel - Distillation Column Design Lab ReportDocument37 pagesExcel - Distillation Column Design Lab ReportGracylla Rose0% (1)
- Neraca MassaDocument6 pagesNeraca MassaUum LukmanNo ratings yet
- Calculation Sheet V1Document14 pagesCalculation Sheet V1Muhammad Umer RanaNo ratings yet
- CombinepdfDocument17 pagesCombinepdfNaresh GanisonNo ratings yet
- Fuel Consumption EstimateDocument28 pagesFuel Consumption EstimateAnonymous oVRvsdWzfBNo ratings yet
- Enthalpy of Combustion of AlcoholsDocument8 pagesEnthalpy of Combustion of AlcoholsKian Paolo ManolesNo ratings yet
- Material and Energy Balance LatestDocument3 pagesMaterial and Energy Balance LatestWinnieNo ratings yet
- ChE 132 Assignment 2Document2 pagesChE 132 Assignment 2Leo BesaNo ratings yet
- CO2 Capture ReportDocument15 pagesCO2 Capture ReportMuchammad AdriyanNo ratings yet
- 04 - AbsorbersDocument11 pages04 - AbsorbersAhmad TaufiqNo ratings yet
- Toluen PDFDocument31 pagesToluen PDFAnonymous NxpnI6jCNo ratings yet
- Gasoline: US Gallon 115,000 Btu 121 MJ 32 MJ/liter (LHV) - HHV 125,000 Btu/gallon 132 MJ/gallon 35 MJ/literDocument12 pagesGasoline: US Gallon 115,000 Btu 121 MJ 32 MJ/liter (LHV) - HHV 125,000 Btu/gallon 132 MJ/gallon 35 MJ/literHarryBouterNo ratings yet
- Problem No.01 Part A: Equilibrium ConversionDocument4 pagesProblem No.01 Part A: Equilibrium ConversionsadiaNo ratings yet
- Material Balance & Energy Balance - Reactor-2Document32 pagesMaterial Balance & Energy Balance - Reactor-2Xy karNo ratings yet
- Ethanol 12Document19 pagesEthanol 12Andrea LeonNo ratings yet
- Assignment 5: Optimization: (PO3, CO2)Document3 pagesAssignment 5: Optimization: (PO3, CO2)MohamedFittri0% (1)
- Efisiensi Boiler Metode Tak LangsungDocument64 pagesEfisiensi Boiler Metode Tak LangsungDicki PangestuNo ratings yet
- Chapter 3 - Equipment Design Part 6 (T-102)Document53 pagesChapter 3 - Equipment Design Part 6 (T-102)aimanrslnNo ratings yet
- Mass Balance (Final)Document26 pagesMass Balance (Final)Adeel AhmedNo ratings yet
- Catalytic Reforming and Isomerization Tutorial SheetDocument2 pagesCatalytic Reforming and Isomerization Tutorial SheetRohit Sahu100% (1)
- Cuptor 600kgDocument84 pagesCuptor 600kgIon CristinaNo ratings yet
- Product Description and Handling Guide-Ethyl AcetateDocument7 pagesProduct Description and Handling Guide-Ethyl Acetateemad hayekNo ratings yet
- Mass&Energy Balance2Document41 pagesMass&Energy Balance2Muhammad Umer RanaNo ratings yet
- RDP Exp 4 Rigorous DistillationDocument66 pagesRDP Exp 4 Rigorous Distillation22 shantanu kapadnisNo ratings yet
- Excel Work Book For Heat ExchangersDocument88 pagesExcel Work Book For Heat Exchangersanup232423No ratings yet
- Workshop 2.6 - Calibration Data For Petroleum Refinery Distillation ExampleDocument4 pagesWorkshop 2.6 - Calibration Data For Petroleum Refinery Distillation Example李天No ratings yet
- M.Novriyanto M1B118028 - UTS SIMKOMDocument11 pagesM.Novriyanto M1B118028 - UTS SIMKOMDave N7RNo ratings yet
- Assignment Reaction EngineeringDocument6 pagesAssignment Reaction Engineeringnur hidayatiNo ratings yet
- Biodiesel ProductionDocument2 pagesBiodiesel ProductionErebus Fobos Momus ErisNo ratings yet
- Process DescriptionDocument4 pagesProcess DescriptionKen VenzonNo ratings yet
- Batch Distillation: Camila Carvajal Paula Gutiérrez Sojo Karen RomeroDocument12 pagesBatch Distillation: Camila Carvajal Paula Gutiérrez Sojo Karen RomeroCamila CarvajalNo ratings yet
- FutureChemistry AppNote3.4 FlowStart Paal-KnorrDocument4 pagesFutureChemistry AppNote3.4 FlowStart Paal-KnorrHarsh KoshtiNo ratings yet
- Carbon Nanomaterials for Advanced Energy Systems: Advances in Materials Synthesis and Device ApplicationsFrom EverandCarbon Nanomaterials for Advanced Energy Systems: Advances in Materials Synthesis and Device ApplicationsWen LuNo ratings yet
- 7 Day Ruqyah Detox Programme - Shaykh 'Adil Ibn Tahir Al-Muqbil - FacebookDocument2 pages7 Day Ruqyah Detox Programme - Shaykh 'Adil Ibn Tahir Al-Muqbil - FacebookR.RNo ratings yet
- TaucuDocument1 pageTaucuWan ZiehanNo ratings yet
- Rotograph Evo 3dDocument290 pagesRotograph Evo 3dشادي العمرNo ratings yet
- Container Type Linear Dimensions (Base Width / Overall Width × Depth × Height) RemarksDocument2 pagesContainer Type Linear Dimensions (Base Width / Overall Width × Depth × Height) RemarksBablu JiNo ratings yet
- Catalogue Inverseurs OTMDocument104 pagesCatalogue Inverseurs OTMchahbounnabil100% (1)
- Precios Febrero SocofarDocument9 pagesPrecios Febrero SocofarAdidaspuma ReplicasNo ratings yet
- School-Based Report On Incidents of Bullying SY 2020-2021Document5 pagesSchool-Based Report On Incidents of Bullying SY 2020-2021Jeraldine MayolNo ratings yet
- Advisory Committee On Human Radiation Experiments-1053 PDFDocument1,053 pagesAdvisory Committee On Human Radiation Experiments-1053 PDFsagor sagor100% (1)
- DineshPonraj 1808165 - 05 00 - 1Document4 pagesDineshPonraj 1808165 - 05 00 - 1revanth kumarNo ratings yet
- Bomba SKC 222 ManualDocument11 pagesBomba SKC 222 ManualEderson GuimaraesNo ratings yet
- Pilates For Beginners Class 1 Guide Booklet PDFDocument12 pagesPilates For Beginners Class 1 Guide Booklet PDFnakisgio5568No ratings yet
- Conval Training Material Part 1Document23 pagesConval Training Material Part 1Kumar Phanishwar50% (2)
- Taconic Road Runners Summer 08 NewsletterDocument29 pagesTaconic Road Runners Summer 08 NewslettergregorydcohenNo ratings yet
- Career Theory GottfredsonDocument2 pagesCareer Theory GottfredsonanaendamNo ratings yet
- Estimated Out-of-Pocket Expenses: Mississippi State University Travel Advance RequestDocument1 pageEstimated Out-of-Pocket Expenses: Mississippi State University Travel Advance Requestuthmankheil89No ratings yet
- Baden Alner F. DomasigIn The BeginningDocument3 pagesBaden Alner F. DomasigIn The BeginningBaden DomasigNo ratings yet
- Ruwais Refinery Expansion Project Project No. 5578 Epc Package - 6 (Non Process Building)Document32 pagesRuwais Refinery Expansion Project Project No. 5578 Epc Package - 6 (Non Process Building)Mohammed AzharNo ratings yet
- Estimation of Uric Acid Levels in SerumDocument6 pagesEstimation of Uric Acid Levels in Serumvicky_law_280% (5)
- 2 Kind of Carts. 3 Colors. 6 SizeDocument16 pages2 Kind of Carts. 3 Colors. 6 SizeAlesioNo ratings yet
- Iterenary Travel FormDocument3 pagesIterenary Travel Formsplef lguNo ratings yet
- Module 2 AnswersDocument6 pagesModule 2 AnswersJoy A. VisitacionNo ratings yet
- TP Paralift PDFDocument39 pagesTP Paralift PDFSatheesh kumarNo ratings yet
- List of CseDocument13 pagesList of CseAyni ReyesNo ratings yet
- Collision TheoryDocument38 pagesCollision TheorySaadiah MohammadNo ratings yet
- Science and Technology Grade 7 ContentDocument1 pageScience and Technology Grade 7 ContentNorjana Otto Medal-Madale67% (3)
- Behavioral Studies of Bats in Captivity: Methodology, Training, and Experimental DesignDocument20 pagesBehavioral Studies of Bats in Captivity: Methodology, Training, and Experimental DesignIslam Ausraf RajibNo ratings yet
- For Fast Vessels With Low Load Factors (1DS) : Diesel Engines10V/12V/16V 2000 M96Document2 pagesFor Fast Vessels With Low Load Factors (1DS) : Diesel Engines10V/12V/16V 2000 M96Amin ThabetNo ratings yet