Professional Documents
Culture Documents
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Uploaded by
Yasser senhajiCopyright:
Available Formats
You might also like
- Particle Concentration and Mixing in Microdrops Driven by Focused Surface Acoustic WavesDocument10 pagesParticle Concentration and Mixing in Microdrops Driven by Focused Surface Acoustic Waveshani safariNo ratings yet
- Multiscale Phenomena in Micro Uidics and Nano Uidics: Guoqing Hu, Dongqing LiDocument12 pagesMultiscale Phenomena in Micro Uidics and Nano Uidics: Guoqing Hu, Dongqing LiHassan AbdelmoamenNo ratings yet
- Photocatalytic Tio Sol-Gel Thin Films: Optical and Morphological CharacterizationDocument13 pagesPhotocatalytic Tio Sol-Gel Thin Films: Optical and Morphological CharacterizationjvalverdegarciaNo ratings yet
- Applied Surface Science: Mohammad Hossein Mahdieh, Behzad FattahiDocument11 pagesApplied Surface Science: Mohammad Hossein Mahdieh, Behzad FattahiIntenNo ratings yet
- A Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysDocument12 pagesA Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysEduardoNo ratings yet
- Donald - Microrheology ReviewDocument7 pagesDonald - Microrheology Reviewsolice879No ratings yet
- Electro Permeablilization PDFDocument7 pagesElectro Permeablilization PDFcindy tatiana rodriguez marinNo ratings yet
- Electro PermeablilizationDocument7 pagesElectro Permeablilizationcindy tatiana rodriguez marinNo ratings yet
- Wen 2021Document18 pagesWen 2021zjNo ratings yet
- Science at Ultra-Brieftime Periods From The Attosecond To The PetawattDocument8 pagesScience at Ultra-Brieftime Periods From The Attosecond To The PetawattPocxaNo ratings yet
- 10 1002@ceat 200407122Document8 pages10 1002@ceat 200407122nadir adelNo ratings yet
- Microfluidic Desalination ReviewDocument11 pagesMicrofluidic Desalination ReviewArun EbenezerNo ratings yet
- Optical Biosensor Devices As Early Detectors of Biological and CDocument22 pagesOptical Biosensor Devices As Early Detectors of Biological and CjosesrsNo ratings yet
- Applsci 13 08240Document17 pagesApplsci 13 08240Aris NasNo ratings yet
- Micro Fluidic SystemsDocument18 pagesMicro Fluidic SystemsHua Hidari YangNo ratings yet
- Corr - Chapter1'Document4 pagesCorr - Chapter1'Costin GabrielaNo ratings yet
- Micromachines 15 00466Document21 pagesMicromachines 15 00466ARABONo ratings yet
- Quality Management System and ISO 90012008 CertificationBarriers and Challenges To SMEsEspaciosDocument20 pagesQuality Management System and ISO 90012008 CertificationBarriers and Challenges To SMEsEspaciosCRISTIAN FREDDY CACERES HUAHUISANo ratings yet
- Application of Nanoparticles in Quartz Crystal Microbalance BiosensorsDocument21 pagesApplication of Nanoparticles in Quartz Crystal Microbalance BiosensorsAdinda PutriNo ratings yet
- 2001 Hoenig - Sample - Prep - Preparation Steps in Environmental Trace Element Analysis Talanta - 2001Document18 pages2001 Hoenig - Sample - Prep - Preparation Steps in Environmental Trace Element Analysis Talanta - 2001ctimanaNo ratings yet
- Drop CastingDocument10 pagesDrop Castingtuwien08No ratings yet
- Advances in Micro-Droplets Coalescence Using Micro Uidics: Chinese Journal of Analytical Chemistry December 2015Document14 pagesAdvances in Micro-Droplets Coalescence Using Micro Uidics: Chinese Journal of Analytical Chemistry December 2015ankitamNo ratings yet
- Aplicaciones de Espectroscopía UV - ENGLISHDocument7 pagesAplicaciones de Espectroscopía UV - ENGLISHDayan AvilaNo ratings yet
- Dispersion of Nanoparticles From Organic Solvents To Polymer SolutionsDocument5 pagesDispersion of Nanoparticles From Organic Solvents To Polymer SolutionsMohamed SamyNo ratings yet
- Toward Continuous Nano-Plastic Monitoring in Water by High Frequency Impedance Measurement With Nano-Electrode ArraysDocument9 pagesToward Continuous Nano-Plastic Monitoring in Water by High Frequency Impedance Measurement With Nano-Electrode ArraysSupriya GowdaNo ratings yet
- 1 s2.0 S0925400503002661 MainDocument11 pages1 s2.0 S0925400503002661 MainzdNo ratings yet
- Unconventional Methods For Fabricating and Patterning NanostructuresDocument26 pagesUnconventional Methods For Fabricating and Patterning NanostructuresImtiazAhmedNo ratings yet
- Oder Ji 2016Document8 pagesOder Ji 2016HassanNo ratings yet
- Austin 2015Document17 pagesAustin 2015Premkumar RNo ratings yet
- J Perry Conf Proc 2005Document9 pagesJ Perry Conf Proc 2005Alex MartinezNo ratings yet
- Complementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionDocument10 pagesComplementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionhesoyamyecgaaaNo ratings yet
- Batchelor McAuley Et Al 2015 ChemistryOpenDocument37 pagesBatchelor McAuley Et Al 2015 ChemistryOpenOmar ReynosoNo ratings yet
- X Ray Crystallography For Beginners UnderstandingDocument8 pagesX Ray Crystallography For Beginners Understandinghop_parasherNo ratings yet
- Unit VDocument8 pagesUnit VhyctortyNo ratings yet
- 2012 - Lucas, Riedo - Invited Review Article Combining Scanning Probe Microscopy With Optical Spectroscopy For Applications in Biology ADocument37 pages2012 - Lucas, Riedo - Invited Review Article Combining Scanning Probe Microscopy With Optical Spectroscopy For Applications in Biology AClaudio BiaginiNo ratings yet
- Automated Total and Radioactive Strontium Separation and Preconcentration in Samples of Environmental Interest Exploiting A Lab-On-Valve SystemDocument6 pagesAutomated Total and Radioactive Strontium Separation and Preconcentration in Samples of Environmental Interest Exploiting A Lab-On-Valve Systemnohora parradoNo ratings yet
- COMPOSITE and Nano Materials SASTRA University 1st YearDocument11 pagesCOMPOSITE and Nano Materials SASTRA University 1st Yearstar100% (1)
- Nano 2Document1 pageNano 2api-3777104No ratings yet
- Journal Name: CommunicationDocument3 pagesJournal Name: CommunicationzuopengxiangNo ratings yet
- Nanofabrication by Electron Beam Lithography and Its Application A Review PDFDocument16 pagesNanofabrication by Electron Beam Lithography and Its Application A Review PDFJulio HernandoNo ratings yet
- Lab-On-A-Chip, Microfluidics and Interfacial ElectrokineticsDocument6 pagesLab-On-A-Chip, Microfluidics and Interfacial ElectrokineticsAditya RaghunandanNo ratings yet
- Nanoparticle Characterization What To MeasureDocument26 pagesNanoparticle Characterization What To MeasureMarwaya EmamNo ratings yet
- LevitadorDocument7 pagesLevitadorWagner Pereira Lima PereiraNo ratings yet
- Sensors 21 00639 v3Document19 pagesSensors 21 00639 v3lcscosta2761No ratings yet
- Vogel2005ApplPhysB PDFDocument34 pagesVogel2005ApplPhysB PDFElektronika PMFNo ratings yet
- Semiconductor Nanoparticles ThesisDocument5 pagesSemiconductor Nanoparticles Thesisstefanieyangmanchester100% (2)
- Sensors and Actuators B - Chemical Volume Issue 2018 (Doi 10.1016 - j.snb.2018.03.091) Yang, Ruey-Jen Fu, Lung-Ming Hou, Hui-Hsiung - Review and Perspectives On Microfluidic Flow CytometersDocument59 pagesSensors and Actuators B - Chemical Volume Issue 2018 (Doi 10.1016 - j.snb.2018.03.091) Yang, Ruey-Jen Fu, Lung-Ming Hou, Hui-Hsiung - Review and Perspectives On Microfluidic Flow CytometersMunir AliNo ratings yet
- Mathematical Modeling and Simulation of Concentration Polarization Layer in Reverse Osmosis ProcessDocument5 pagesMathematical Modeling and Simulation of Concentration Polarization Layer in Reverse Osmosis ProcessSindhuja RaghunathanNo ratings yet
- Biotechnology: Low Reynolds NumbersDocument12 pagesBiotechnology: Low Reynolds NumbersChoti 2998No ratings yet
- Nanomaterials: Origin and Future of Plasmonic Optical TweezersDocument18 pagesNanomaterials: Origin and Future of Plasmonic Optical TweezersShailendra SinghNo ratings yet
- Cnrs ThesisDocument6 pagesCnrs Thesisandreaariascoralsprings100% (2)
- Accepted Manuscript: Sensors and Actuators BDocument30 pagesAccepted Manuscript: Sensors and Actuators BSamsouma BkNo ratings yet
- 1 s2.0 S0925400507001694 MainDocument7 pages1 s2.0 S0925400507001694 MainElif ŞahinNo ratings yet
- Microfluidics Meets MEMS: Elisabeth Verpoorte Nico F. de RooijDocument24 pagesMicrofluidics Meets MEMS: Elisabeth Verpoorte Nico F. de RooijArunNo ratings yet
- AO PaperDocument14 pagesAO PaperKumar AvinashNo ratings yet
- RFDC SputteringDocument4 pagesRFDC Sputteringसुरेन्द्र वशिष्ठNo ratings yet
- Ultrasonics-A Rapid, Fully Non-Contact, Hybrid System For Generating Lamb WaveDocument9 pagesUltrasonics-A Rapid, Fully Non-Contact, Hybrid System For Generating Lamb WaveMohammad HarbNo ratings yet
- New Sensors and Processing ChainFrom EverandNew Sensors and Processing ChainJean-Hugh ThomasNo ratings yet
- Class Xii Physics PaperDocument10 pagesClass Xii Physics Papergojo satoruNo ratings yet
- Physics 22Document20 pagesPhysics 22Kazuto ShibaNo ratings yet
- Navier StokesDocument36 pagesNavier Stokesvincent02hk_57881301No ratings yet
- Cathode MaterialsDocument8 pagesCathode Materialsnajem AlsalehNo ratings yet
- Summative Assessment - II: A. Choose The Correct OptionDocument6 pagesSummative Assessment - II: A. Choose The Correct OptionSanchit MukherjeeNo ratings yet
- Chapter 3Document23 pagesChapter 3kuppler7967No ratings yet
- Brandon Swanson - Trigonometry Test ReviewDocument5 pagesBrandon Swanson - Trigonometry Test ReviewladeeshaNo ratings yet
- Stoichiometry PowerPointDocument23 pagesStoichiometry PowerPointAngelaWillson100% (1)
- Bronze c54400 SpecificationsDocument3 pagesBronze c54400 SpecificationsRam Parimalam100% (1)
- The Compressible Euler System in Two Space Dimensions: Yuxi ZhengDocument92 pagesThe Compressible Euler System in Two Space Dimensions: Yuxi ZhengchyixxNo ratings yet
- Strength of Materials - Shear StressesDocument2 pagesStrength of Materials - Shear StressesJan Alexis Monsalud0% (1)
- Lenz LessDocument8 pagesLenz LessGindac Ralea100% (1)
- Governing Equations For Turbulent FlowDocument15 pagesGoverning Equations For Turbulent FlowRenz Marion CorpuzNo ratings yet
- Comparing Various NDT TechniquesDocument4 pagesComparing Various NDT TechniquesfenasikerimNo ratings yet
- 02 E1 Sticky TapeDocument6 pages02 E1 Sticky TapeRaviNo ratings yet
- Electric Field: Physics 16Document19 pagesElectric Field: Physics 16clndneNo ratings yet
- 2003, 94665723-New-energy-technologies-Issue-12 PDFDocument81 pages2003, 94665723-New-energy-technologies-Issue-12 PDFJosefina SilveyraNo ratings yet
- Problem Set: Strain and Thermal StressesDocument5 pagesProblem Set: Strain and Thermal StressesClint Charles P. BrutasNo ratings yet
- Topic 7.3: The Structure of MatterDocument3 pagesTopic 7.3: The Structure of MatterJerry DNo ratings yet
- 13 08 Divergence THM PDFDocument6 pages13 08 Divergence THM PDFMohamed Jamal AmanullahNo ratings yet
- Iesc 104Document11 pagesIesc 104Ty GravesNo ratings yet
- New Ideas That Changed EINSTEIN's TheoryDocument97 pagesNew Ideas That Changed EINSTEIN's TheorymobilecrackersNo ratings yet
- Cswip QbankDocument23 pagesCswip QbankFownoon100% (1)
- MSE 301 1stLongExam Chapter3Document2 pagesMSE 301 1stLongExam Chapter3Ilove PioneeringNo ratings yet
- Tension in A CordDocument4 pagesTension in A Cordkaveh-bahiraeeNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Ex 3Document3 pagesEx 3api-3708873No ratings yet
- Composite Materials and StructuresDocument35 pagesComposite Materials and StructuresGundoju SreenivasNo ratings yet
- Gatechemical 2012Document20 pagesGatechemical 2012Venkatesh ChNo ratings yet
- SW#2 Integrals of Trigonometric FunctionsDocument1 pageSW#2 Integrals of Trigonometric FunctionsRachel MonesNo ratings yet
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Uploaded by
Yasser senhajiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Airborne Chemistry: Acoustic Levitation in Chemical Analysis
Uploaded by
Yasser senhajiCopyright:
Available Formats
Anal Bioanal Chem (2004) 378 : 1704–1709 1704
DOI 10.1007/s00216-003-2403-2
REVIEW
Sabina Santesson · Staffan Nilsson
Airborne chemistry: acoustic levitation in chemical analysis
Received: 5 September 2003 / Revised: 3 November 2003 / Accepted: 5 November 2003 / Published online: 5 February 2004
© Springer-Verlag 2004
Abstract This review with 60 references describes a unique
path to miniaturisation, that is, the use of acoustic levita- Introduction
tion in analytical and bioanalytical chemistry applications. Advances in understanding how cells differentiate, function
Levitation of small volumes of sample by means of a levi- and interact are vital in the search for new therapeutic targets
tation technique can be used as a way to avoid solid walls and pharmaceuticals. The “omics” research areas, compris-
around the sample, thus circumventing the main problem ing genomics, proteomics, crystallomics, organellomics,
of miniaturisation, the unfavourable surface-to-volume ra- metabolomics, and cellomics, aim at defining the status of
tio. Different techniques for sample levitation have been every gene, protein, organelle, and metabolic profile in a
developed and improved. Of the levitation techniques de- given cell under different conditions. Due to the growing
scribed, acoustic or ultrasonic levitation fulfils all require- number of “omics” research projects, development of user-
ments for analytical chemistry applications. This technique friendly, sensitive and biocompatible miniaturisation tech-
has previously been used to study properties of molten ma- nologies is of the utmost concern for applications in biology,
terials and the equilibrium shapeand stability of liquid drops. biomedicine, biotechnology and chemical and pharmaceuti-
Temperature and mass transfer in levitated drops have also cal analysis. There is now a lack of analytical systems com-
been described, as have crystallisation and microgravity bining high sample throughput with the ability to extract
applications. high-quality information from biological and biochemical
The airborne analytical system described here is equipped events. One main quality of such a system would be minia-
with different and exchangeable remote detection sys- turisation in order to keep sample and chemical consumption
tems. The levitated drops are normally in the 100 nL–2 µL low. This is imperative if sample access is limited (e.g. in
volume range and additions to the levitated drop can be single-cell analysis and early analysis of drug candidates).
made in the pL-volume range. Chip-based analytical systems have partly achieved this goal
The use of levitated drops in analytical and bioanalyti- and mostly involve electrophoretically driven flows, that is
cal chemistry offers several benefits. Several remote de- electrophoresis and electrochromatography [1, 2, 3]. There
tection systems are compatible with acoustic levitation, in- have been a number of recent advances in the fields of minia-
cluding fluorescence imaging detection, right angle light turised reaction and separation systems, including the con-
scattering, Raman spectroscopy, and X-ray diffraction. Ap- struction of fully integrated ‘lab-on-a-chip’ systems [4, 5].
plications include liquid/liquid extractions, solvent exchange, Many techniques for studying dynamic events at the single-
analyte enrichment, single-cell analysis, cell–cell commu- cell level have been developed, including fluorescence imag-
nication studies, precipitation screening of proteins to es- ing techniques [6, 7], capillary electrophoresis [8, 9], flow
tablish nucleation conditions, and crystallisation of pro- cytometry [2] and capillary liquid chromatography [10].
teins and pharmaceuticals. However successful in certain applications, miniaturisa-
tion and single-cell studies in such systems have their own
difficulties [11]. Among the most serious drawbacks is the
risk of analyte adsorption to walls and interfaces [12, 13, 14,
15, 16, 17], a problem multiplied in small-dimensional ana-
lytic systems where volumes in the picolitre (pL) or nanolitre
(nL) range are used. Another problem is optical interference
S. Santesson · S. Nilsson (✉) at the walls of the sample container hampering detection
Technical Analytical Chemistry, Lund University,
P.O. Box 124, 221 00 Lund, Sweden [15], especially since such low amounts of analytes are being
Tel.: +46 46 2228177, Fax: +46 46 2224525, used. Therefore, future advances in these areas require the
e-mail: Staffan.Nilsson@teknlk.lth.se development of analytical techniques capable of circumvent-
1705
ing the use of contacting walls and thereby avoiding these netic field. Electrons in such materials rearrange their or-
problems and yet able to monitor minute cellular changes in- bits slightly so that they expel the external field. As a re-
duced by ligands, drugs, activators, or inhibitors. sult, diamagnetic materials repel and are repelled by
strong magnetic fields. Superconductors are ideal diamag-
netics and completely expel magnetic field at low temper-
Levitation techniques atures. But an object does not need to be superconducting
to levitate if placed in a strong enough magnetic field
A number of physical effects allow free floatation, levita- [27]. Use of magnetic field strengths of about 10 Tesla has
tion, of solid and liquid matter. Levitation of small vol- been described, and water droplets and even frogs have
umes of sample by means of a levitation technique can be been levitated in this way at Nijmegen Magnetics Labora-
used as a way to avoid solid walls around the sample. The tory in the Netherlands [21].
only contacting surface is thus the surrounding medium,
which is commonly air. The use of levitated drops ex-
hibits the same miniaturisation benefits as the chip ap- Electrostatic levitation
proach, such as diversity of application and low reagent
and sample consumption. In addition to the obvious ad- In an electrostatic levitation system, a charged drop is lev-
vantage of levitation for preventing the chemical and ther- itated by applying electrostatic fields from a set of prop-
mal contamination that accompanies contact between erly arranged electrodes. The sample is positioned by ac-
drops and external objects, the use of levitated drops also tively controlling an applied electrostatic field. A large
has the added advantage of increased sensitivity of detec- levitation force is required to compensate for the gravita-
tion, since no walls disturb detection [12, 15]. tional pull, which in practice means large sample charges
Different techniques for sample levitation have previ- and a strong applied electric field (or large potential dif-
ously been developed and improved [18]. These techniques ference between electrodes). The requirement for a high-
include optical levitation [19], electrostatic levitation [20], voltage output can make this approach somewhat incon-
aerodynamic levitation [18], diamagnetic levitation [21] and venient. Electrostatic levitation has, among other things,
acoustic levitation [12]. found use in protein crystal growth [28] and X-ray scat-
However, for a levitation technique to be useful as a tering studies of metallic liquids [29].
general, bioanalytical technique, it must combine biologi-
cal compatibility with ease of sample handling such as
stable sample position, easy access to the sample, a wide Aerodynamic levitation
range of sample volumes and low costs for supply and op-
eration [12]. In contrast to the other levitation techniques, Aerodynamic levitation is achieved by lifting spherical
acoustic levitation also has the advantage of not requiring specimens by a fluid jet. The divergence of the jet leads to
any specific physical properties (e.g. electric charge or a a decreasing drag with increasing height, which gives sta-
certain refractive index) of the sample. Consequently al- bility in the vertical direction. In the transverse direction
most all substances whether they are solids or liquids can stable levitation is achieved because the jet is deflected to-
be acoustically levitated. wards an off-axis specimen. Aerodynamic levitation has
found much use in industrial applications and for levitat-
ing solid spheres. Vibrational instabilities have been de-
Optical levitation scribed for aerodynamic levitation of liquids [22].
Optical levitation [22] is achieved by using a high numer-
ical aperture lens to sharply focus a laser beam. By illu- Acoustic levitation
minating an object by such a focused laser beam, the ob-
ject is subjected to a scattering force that pushes it for- The phenomenon that small samples can be levitated in
ward into the direction of the energy flux. A gradient the nodal points of a standing ultrasonic wave was first
force is also exerted to pull the object towards regions with described in 1933 [30]. The theory behind this phenome-
high electrical density (generally the focal region). In re- non is well understood [22, 31]. A standing wave with
gions where the two forces cancel each other particles may equally spaced nodes and antinodes is created by multiple
be trapped at a stationary three-dimensional (3D) position reflections between an ultrasonic radiator and a solid re-
[23]. Examples of applications are 3D cellular and intra- flector. Figure 1 shows a levitated water drop (i.e. “wall-
cellular micromanipulation [24, 25] and cell sorting [26]. less” test tube) positioned in such a nodal point between
the ultrasonic transducer and the reflector.
Acoustic levitation has been used to study properties of
Diamagnetic levitation molten materials and the equilibrium shape [32] and sta-
bility of liquid drops. The technique has been employed
Levitation can be achieved without the need for energy in- for density measurements [33] and in the study of surface-
put by the use of diamagnetism. Diamagnetism refers to the tension-dominated fluid dynamic phenomena with appli-
ability of a material to expel a portion of an external mag- cations in microgravity [27, 28]. Streaming flows associ-
1706
Fig. 2 Dispenser droplet addition to the levitated drop. The dis-
penser trajectory is seen as the thin white line connecting the dis-
penser nozzle on the right with the levitated drop
Fig. 1 A 500-nLwater drop levitated in a node in a standing wave
created between an ultrasonic transducer (bottom) and a solid re-
flector (top)
ated with ultrasonic levitators [34] and effects of acoustic
streaming [35] have been discussed, as have flowfield
characteristics of aerodynamic acoustic levitators [36] and
effects of acoustic streaming [35]. Temperature and mass
transfer in levitated drops have also been described [32,
33].
Extensive studies have also been performed on evapo-
ration [37, 38] and drying [39] of acoustically levitated
droplets.
Airborne chemistry
Applications
Acoustic levitation has begun to attract interest for use in
analytical and bioanalytical chemistry applications. In 1982,
Apfel and co-workers discussed the development of
acoustic levitation for measuring the mechanical proper-
ties, namely density, compressibility, and sound velocity,
of biological materials [40]. Later, Neidhart and co-work-
ers reported their first experiences with the technique of
acoustical levitation of droplets in the field of analytical Fig. 3 Two-phase (aq./org.) system in levitated drop. The aqueous
and atmospheric chemistry [12]. They performed the fol- phase is in yellow and the organic phase in orange
lowing common experiments of sample preparation pro-
cedures in levitated drops: acid–base titrations, liquid/liq-
uid extractions, solvent exchange and analyte enrichment 44, 45] has been used in our laboratory for bioanalytical
by evaporation of the solvent. They also reported the for- purposes. This airborne analytical system [15, 39, 40, 41,
mation and growth of ice particles and trapping of heavy 42] is equipped with different and exchangeable remote
gases [41] in stationary ultrasonic fields as well as sample detection systems. The levitated drops are normally in the
preparation prior to gas chromatography [12]. Klockow 100 nL–2 µL volume range and additions to the levitated
and co-workers have performed titrations and crystallisa- drop can be made in the pL-volume range. It has been
tion experiments [42] and investigated phase transfer and shown that the airborne system can be successfully used
freezing processes on acoustically levitated aqueous droplets for single-cell analysis, [15, 39] crystallisation studies of
[43]. pharmaceuticals [46] and proteins [47] as well as for sam-
A basic analytical system (Fig. 2) consisting of an acoustic ple enrichment prior to capillary electrophoresis/capillary
levitator combined with flow-through pL dispensers [14, electrochromatography (CE/CEC) analysis [14].
1707
For the single-cell experiments, a buffer drop contain- Sample handling
ing living cells is levitated and different substances are
presented to the cells using the pL flow-through dis- Accurate free hand positioning of an acoustically levitated
pensers. The cell response (or lack of response) is then drop requires some practice. Basically any kind of sy-
monitored using fluorescence imaging detection. In our ringe, pipette or capillary may be used for drop position-
laboratory, methods for liquid/liquid cell organelle extrac- ing in the ultrasonic field [12], provided that small enough
tions in levitated drops are presently being developed. drops can be produced. To aid drop detachment from the
Figure 3 shows an aqueous/organic two-phase system in a tip, the tip can be coated with a hydrophobic substance.
levitated drop. This approach will allow the study at the Difficulties in drop detachment also increase with de-
single-cell level of the up-and-down regulation of intra- creased surface tension and/or viscosity of the liquid. Op-
cellular molecules in response to environmental factors erating at increased ultrasonic power during the position-
such as other cells, substances produced by other cells, or ing also enhances drop detachment. After positioning the
drugs. In the levitated drop, cells are first stimulated/in- levitated drop may perform oscillations, but it can be
hibited and then lysed by addition of an organic solvent. readily stabilised by adjusting the distance between the
The organic solvent also creates a two-phase system into transducer and the reflector and by setting the ultrasonic
which the cells release their intracellular molecules that power properly [14, 15].
are extracted to one of the phases. The use of affinity Sample delivery can also be achieved by using a non-
aqueous two-phase systems, currently under development contact delivery device (e.g. some variety of ink-jet print-
in our laboratory, would further allow isolation of subcel- ing technology which allows droplets to fly freely over a
lular fractions from single cells by affinity two-phase par- distance). The volumetric accuracy of ink-jet dispensing
titioning. is not affected by how the fluid wets a substrate and the
Levitation has also attracted substantial interest for pro- fluid source cannot be contaminated by the substrate. One
tein crystallisation, especially with respect to micrograv- example of such a device is the flow-through droplet dis-
ity applications. Ishikawa and Komada have discussed the penser used in our laboratory, which was developed in-
development of acoustic and electrostatic levitators for con- house at the department of Electrical Measurements, Lund
tainerless protein crystallization [48]. Rhim and Chung University, Sweden. The main advantages of the flow-
used an electrostatic multi-drop levitation system [49] and through dispenser are the possibility to dispense samples
Chung and Trinh used an ultrasonic–electrostatic hybrid from flowing liquids, the high-precision non-contact
levitator for growing protein crystals from levitated drops mode of sample supply, the small size of the droplets
with the aim to create controlled crystal growth conditions (pL-volume range) and the high droplet ejection frequency,
which would reproduce some of the aspects of a low-grav- up to 9 kHz [14, 51].
ity environment [28]. In our laboratory, a screening method After analysis in the levitated drop, samples can be re-
based on acoustically levitated drops was developed for moved from the levitator by using the same type of sy-
the study of precipitation of proteins for crystallisation ringe/micropipette/capillary used to position them there.
purposes [47]. At present, crystals are mostly grown using Removing aliquots from the levitated drop is best
trial-and-error procedures, and protocols that rapidly screen achieved by using a very thin tip, since otherwise the lev-
for the crystal nucleation step are rare. Our approach is to itated drop is most likely to catch on the tip and be pulled
minimise the consumption of protein material while search- out of the node [52]. A nano-tip suitable for withdrawing
ing for the nucleation conditions in a rational manner. Lev- aliquots from the drop has been developed in our labora-
itated drops of known protein concentration are injected tory [53]. If the whole drop is to be removed, it can either
with crystallising agents using piezoelectric flow-through be caught on the tip and placed in a microtiter plate for
dispensers and calculations are performed giving the con- observation under microscope, or introduced in for exam-
centrations of all components in the drop at any time dur- ple a capillary for further analysis [52].
ing an experiment. Protein precipitation is monitored us-
ing right-angle light scattering. Precipitation diagrams are
then constructed giving the protein/crystallising agent Detection systems
concentration boundaries between the minimum and the
maximum detectable protein precipitation. This approach In order to truly benefit from the use of levitated drops,
is especially suitable if previous screening for nucleation remote and non-invasive detection protocols are impor-
conditions of a protein has failed [47]. Recently, some in- tant. This can be achieved by using varieties of spectros-
teresting microfluidic approaches to screening of protein copy [42]. Neidhart and co-workers used a diode array
nucleation conditions have been described [50], which spectrometer designed for use with optical fibres for ab-
utilize even smaller volumes than the levitated approach sorption and fluorescence measurements [13]. A method
developed in our laboratory. However, the authors claim for non-contact fluorescence thermometry of acoustically
that their system is especially suited for the optimisation levitated water drops was developed by Seaver and Peele
step in crystallization. These two approaches, levitation [54]. Fluorescence imaging detection based on previous
and microfluidic, could therefore be complementary in the work on real-time fluorescence imaging for capillary elec-
search for protein nucleation conditions. trophoresis [55, 56] was also used by our group for single-
cell analysis [15, 39]. The experimental set-up is shown in
1708
Fig. 4 Instrumental set-up for
airborne cell experiments using
fluorescence imaging detection
consisting of a Hg lamp, an
optical fibre, two alternating in-
terference filters (405 and
435 nm), a lens, an acoustic
levitator, continuous flow-
through droplet dispensers,
a lens system, another interfer-
ence filter (510 nm), a CCD
camera and a computer
Fig. 4. The main aim has been to develop a method able to
detect single-cell responses from fat cells and other cell Conclusions
types involved in fat metabolism, thus making possible a
search for differences between individual cells and cell re- The use of acoustically levitated drops in analytical and
sponses in order to aid in the understanding of diabetes bioanalytical chemistry offers several benefits. Several re-
and related diseases. mote detection systems are compatible with acoustic levi-
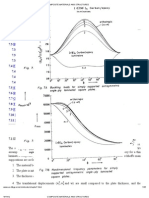
Right-angle light-scattering detection was applied by tation, including fluorescence imaging detection, right-an-
our group to the study of precipitation of proteins for crys- gle light scattering, Raman spectroscopy, and X-ray dif-
tallisation purposes [47]. Biswas described solidification fraction. Applications include liquid/liquid extractions, sol-
of acoustically levitated crystals using Raman spectros- vent exchange, analyte enrichment, single-cell analysis,
copy [57]. Raman spectroscopy was also employed by our cell–cell communication studies, precipitation screening
group for studies of crystallization processes in levitated of proteins to establish nucleation conditions, and crys-
drops [46]. Crystallization studies on the model com- tallisation of proteins and pharmaceuticals. The diversity
pounds benzamide and indomethacin resulted in the for- of applications possible, the biological compatibility, the
mation of two crystal modifications for each compound, small volumes, and the absence of contacting surfaces are
suggesting that this methodology may be useful for inves- all important features and strongly support the further use
tigation of polymorphs. Surface-enhanced Raman scatter- and development of this new approach in bioanalytical
ing (SERS) detection in combination with levitated drops chemistry. A growing area of science utilising levitation
was demonstrated by our group [46]. Lendl and co-work- systems can be foreseen, especially in the different “omics”
ers used FT-Raman spectroscopy for in situ synthesis and research areas where high-quality information is neces-
application of SERS-active Ag-sols for trace analysis sary.
[58]. In the future, several important applications of the Acknowledgements The authors are indebted to the following
combination between Raman spectroscopy and acoustic collaborators: Prof Thomas Laurell and Docent Johan Nilsson,
drop levitation are to be expected. Electrical Measurements, Lund Institute of Technology, Sweden,
Tests have been performed by our group to evaluate for the flow-through pL dispensers; Dr Thomas Johansson, Atomic
Physics, Lund Institute of Technology, Sweden, for work on de-
the use of acoustically levitated drops for liquid X-ray dif- tection systems and computer programs; Dr Eila S. Cedergren-
fraction [59]. The tests were performed at the crystallog- Zeppezauer, for vital collaboration on the protein precipitation ap-
raphy beamline I711 at the MAX II synchrotron in Lund, plication; Prof Eva Degerman, Molecular Signalling, Cell and Mo-
Sweden. The results showed that a droplet of liquid and lecular Biology, Lund University, Sweden, for collaboration on
solid (powder) samples can be kept in an X-ray beam for adipocytes and lipolysis; Prof Patrik Rorsman, Molecular and Cel-
lular Physiology, Lund University, Sweden, for pancreatic beta-
sufficient time to allow collection of the X-ray diffraction cells and cell–cell communication; Dr Jonas Johansson, Analytical
pattern. X-ray scattering from levitated liquids has previ- R&D, AstraZeneca R&D Mölndal, Sweden, for collaboration on
ously been extensively described for several other levita- detection systems, especially Raman spectroscopy, and Dr Lynne
tion techniques [60]. S. Taylor, Analytical R&D, AstraZeneca R&D Mölndal, Sweden,
for collaboration on Raman detection of crystal polymorphs; Prof
Michael Sepaniak, Department of Chemistry, University of Ten-
nessee, Knoxville, TN, USA, for collaboration on SERS detection;
Yngve Cerenius, Molecular Biophysics, Lund University, Lund;
1709
Prof Åke Oskarsson, Inorganic Chemistry, Lund University, Swe- 29. Kelton KF, Lee GW, Gangopadhyay AK, Hyers RW, Rathz TJ,
den, and Lars Kloo, Inorganic Chemistry, Royal Institute of Tech- Rogers JR, Robinson MB, Robinson DS (2003) Phys Rev Lett
nology, Stockholm, Sweden for collaboration on the X-ray diffrac- 90:Art No 195504
tion. 30. Bücks K, Müller HZ (1933) Phys 84:75–86
31. Lierke EG (1996) Acustica 82:220–237
32. Trinh EH, Hsu CJ (1986) J Acoust Soc Am 79:1335–1338
33. Trinh EH, Hsu CJ (1986) J Acoust Soc Am 80:1757–1761
References 34. Trinh EH, Robey JL (1994) Phys Fluids 6:3567–3579
35. Kawahara N, Yarin AL, Brenn G, Kastner O, Durst F (2000)
1. Bruin GJM (2000) Electrophoresis 21:3931–3951 Phys Fluids 12:912–923
2. McClain MA, Culbertson CT, Jacobson SC, Ramsey JM (2001) 36. Yarin AL, Brenn G, Keller J, Pfaffenlehner M, Ryssel E, Tro-
Anal Chem 73:5334–5338 pea C (1997) Phys Fluids 9:3300–3314
3. Li JJ, Thibault P, Bings NH, Skinner CD, Wang C, Colyer C, 37. Trinh EH, Marston PL, Robey JL (1998) J Colloid Interface
Harrison J (1999) Anal Chem 71:3036–3045 Sci 124:95–103
4. Krishnan M, Namasivayam V, Lin RS, Pal R, Burns MA 38. Bayazitoglu Y, Mitchell GF (1995) J Thermophys Heat Trans-
(2001) Curr Opin Biotech 12:92–98 fer 9:694–701
5. Manz A, Eijkel JCT (2001) Pure Appl Chem 73:1555–1561 39. Yarin AL, Brenn G, Kastner O, Tropea C (2002) Phys Fluids
6. Yeung ES (1999) Anal Chem 71:A522–A529 14:2289–2298
7. Tong W, Yeung ES (1998) Appl Spectrosc 52:407–413 40. Weiser MH, Apfel RE (1982) J Acoust Soc Am 71:1261–1268
8. Krylov SN, Arriaga EA, Chan NWC, Dovichi NJ, Palcic MM 41. Tuckermann R, Neidhart B, Lierke EG, Bauerecker S (2002)
(2000) Anal Biochem 283 Chem Phys Lett 363:349–354
9. de Mello AJ (2003) Lab Chip 3:29N–34N 42. Davies AN, Jacob P, Stockhaus A, Kuckuk R, Hill W, Hergen-
10. Chen G, Ewing AG (1997) Crit Rev Neurobiol 11:59–90 roder R, Zybin A, Klockow D (2000) Appl Spectrosc 54:1831–
11. Cannon DM, Winograd N, Ewing AG (2000) Annu Rev Bioph 1836
Biom 29:239–263 43. Jacob P, Stockhaus A, Hergenroder R, Klockow D (2001) Fre-
12. Welter E, Neidhart B (1997) Fresenius J Anal Chem 357:345– senius J Anal Chem 371:726–733
350 44. Yarin AL, Brenn G, Kastner O, Rensink D, Tropea C (1999)
13. Rohling O, Weitkamp C, Neidhart B (2000) Fresenius J Anal J Fluid Mech 399:151–204
Chem 368:125–129 45. Yarin AL, Brenn G, Rensink D (2002) Int J Heat Fluid Fl 23:
14. Petersson M, Nilsson J, Wallman L, Laurell T, Johansson J, 471–486
Nilsson S (1998) J Chromatogr B 714:39–46 46. Santesson S, Johansson J, Taylor LS, Levander I, Fox S, Sepa-
15. Santesson S, Andersson M, Degerman E, Johansson T, Nilsson niak M, Nilsson S (2003) Anal Chem 75:2177–2180
J, Nilsson S (2000) Anal Chem 72:3412–3418 47. Santesson S, Cedergren-Zeppezauer ES, Johansson T, Laurell
16. Nilsson S, Santesson S, Degerman E, Cedergren E, Johansson T, Nilsson J, Nilsson S (2003) Anal Chem 75:1733–1740
T, Nilsson J, Johansson J (2000) Proceedings of SPIE, scan- 48. Ishikawa Y, Komada S (1993) FUJITSU Sci Tech J 29:330–
ning and force microscopies for biomedical applications II. San 338
José, California, USA 2000, pp 81–90 49. Rhim W, Chung S (1991) J Cryst Growth 110:293–301
17. Nilsson S, Santesson S, Degerman E, Johansson T, Laurell T, 50. Zheng B, Roach LS, Ismagilov RF (2003) J Am Chem Soc
Nilsson J, Johansson J (2000) Micro total analysis systems. 125:11170–11171
Kluwer, The Netherlands, pp 19–24 51. Santesson S, Johansson T, Nilsson J, Degerman E, Rorsman P,
18. Lierke EG (1995) Forsch Ingenieurwes 61:201–216 Viberg P, Spégel P, Nilsson S (2001) ASMS, Chicago
19. Hoffmann HG, Lentz E, Schrader B (1993) Rev Sci Instrum 52. Santesson S (2002) Licentiate Thesis, Lund University, Lund
64:823–824 53. Santesson S, Barinaga-Rementeria Ramírez I, Viberg P, Jergil
20. Barnes MD, Ng KC, Whitten WB, Ramsey JM (1993) Anal B, Nilsson S (2003) Anal Chem 78:303-308
Chem 65:2360–2365 54. Seaver M, Peele JR (1990) Appl Optics 29:4956–4961
21. Berry MV, Geim AK (1997) Eur J Phys 18:307–313 55. Johansson J, Witte DT, Larsson M, Nilsson S (1996) Anal
22. Brandt EH (1989) Science 243:349–355 Chem 68:2766–2770
23. Nahmias YK, Odde DJ (2002) IEEE J Quantum Electron 38: 56. Johansson T, Petersson M, Johansson J, Nilsson S (1999) Anal
131–141 Chem 71:4190
24. Visscher K, Brakenhoff GJ, Krol JJ (1993) Cytometry 14:105– 57. Biswas A (1995) J Cryst Growth 147:155–164
114 58. Leopold N, Haberkorn M, Laurell T, Nilsson J, Baena JR,
25. Konig K (1998) Cell Mol Biol 44:721–733 Frank J, Lendl B (2003) Anal Chem 75:2166–2171
26. Grover SC, Skirtach AG, Gauthier RC, Grover CP (2001) 59. Cerenius Y, Oskarsson Å, Santesson S, Nilsson S, Kloo LJ
J Biomed Opt 6:14–22 (2003) Appl Crystallogr 36:163–164
27. Simon MD, Geim AK (2000) J Appl Phys 87:6200–6204 60. Krishnan S, Price DL (2000) J Phys Condens Matter 12:R145–
28. Chung SK, Trinh EH (1998) J Cryst Growth 194:384–397 R176
You might also like
- Particle Concentration and Mixing in Microdrops Driven by Focused Surface Acoustic WavesDocument10 pagesParticle Concentration and Mixing in Microdrops Driven by Focused Surface Acoustic Waveshani safariNo ratings yet
- Multiscale Phenomena in Micro Uidics and Nano Uidics: Guoqing Hu, Dongqing LiDocument12 pagesMultiscale Phenomena in Micro Uidics and Nano Uidics: Guoqing Hu, Dongqing LiHassan AbdelmoamenNo ratings yet
- Photocatalytic Tio Sol-Gel Thin Films: Optical and Morphological CharacterizationDocument13 pagesPhotocatalytic Tio Sol-Gel Thin Films: Optical and Morphological CharacterizationjvalverdegarciaNo ratings yet
- Applied Surface Science: Mohammad Hossein Mahdieh, Behzad FattahiDocument11 pagesApplied Surface Science: Mohammad Hossein Mahdieh, Behzad FattahiIntenNo ratings yet
- A Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysDocument12 pagesA Molecular Insight Into The Electro Transfer of - 2016 - Biochimica Et BiophysEduardoNo ratings yet
- Donald - Microrheology ReviewDocument7 pagesDonald - Microrheology Reviewsolice879No ratings yet
- Electro Permeablilization PDFDocument7 pagesElectro Permeablilization PDFcindy tatiana rodriguez marinNo ratings yet
- Electro PermeablilizationDocument7 pagesElectro Permeablilizationcindy tatiana rodriguez marinNo ratings yet
- Wen 2021Document18 pagesWen 2021zjNo ratings yet
- Science at Ultra-Brieftime Periods From The Attosecond To The PetawattDocument8 pagesScience at Ultra-Brieftime Periods From The Attosecond To The PetawattPocxaNo ratings yet
- 10 1002@ceat 200407122Document8 pages10 1002@ceat 200407122nadir adelNo ratings yet
- Microfluidic Desalination ReviewDocument11 pagesMicrofluidic Desalination ReviewArun EbenezerNo ratings yet
- Optical Biosensor Devices As Early Detectors of Biological and CDocument22 pagesOptical Biosensor Devices As Early Detectors of Biological and CjosesrsNo ratings yet
- Applsci 13 08240Document17 pagesApplsci 13 08240Aris NasNo ratings yet
- Micro Fluidic SystemsDocument18 pagesMicro Fluidic SystemsHua Hidari YangNo ratings yet
- Corr - Chapter1'Document4 pagesCorr - Chapter1'Costin GabrielaNo ratings yet
- Micromachines 15 00466Document21 pagesMicromachines 15 00466ARABONo ratings yet
- Quality Management System and ISO 90012008 CertificationBarriers and Challenges To SMEsEspaciosDocument20 pagesQuality Management System and ISO 90012008 CertificationBarriers and Challenges To SMEsEspaciosCRISTIAN FREDDY CACERES HUAHUISANo ratings yet
- Application of Nanoparticles in Quartz Crystal Microbalance BiosensorsDocument21 pagesApplication of Nanoparticles in Quartz Crystal Microbalance BiosensorsAdinda PutriNo ratings yet
- 2001 Hoenig - Sample - Prep - Preparation Steps in Environmental Trace Element Analysis Talanta - 2001Document18 pages2001 Hoenig - Sample - Prep - Preparation Steps in Environmental Trace Element Analysis Talanta - 2001ctimanaNo ratings yet
- Drop CastingDocument10 pagesDrop Castingtuwien08No ratings yet
- Advances in Micro-Droplets Coalescence Using Micro Uidics: Chinese Journal of Analytical Chemistry December 2015Document14 pagesAdvances in Micro-Droplets Coalescence Using Micro Uidics: Chinese Journal of Analytical Chemistry December 2015ankitamNo ratings yet
- Aplicaciones de Espectroscopía UV - ENGLISHDocument7 pagesAplicaciones de Espectroscopía UV - ENGLISHDayan AvilaNo ratings yet
- Dispersion of Nanoparticles From Organic Solvents To Polymer SolutionsDocument5 pagesDispersion of Nanoparticles From Organic Solvents To Polymer SolutionsMohamed SamyNo ratings yet
- Toward Continuous Nano-Plastic Monitoring in Water by High Frequency Impedance Measurement With Nano-Electrode ArraysDocument9 pagesToward Continuous Nano-Plastic Monitoring in Water by High Frequency Impedance Measurement With Nano-Electrode ArraysSupriya GowdaNo ratings yet
- 1 s2.0 S0925400503002661 MainDocument11 pages1 s2.0 S0925400503002661 MainzdNo ratings yet
- Unconventional Methods For Fabricating and Patterning NanostructuresDocument26 pagesUnconventional Methods For Fabricating and Patterning NanostructuresImtiazAhmedNo ratings yet
- Oder Ji 2016Document8 pagesOder Ji 2016HassanNo ratings yet
- Austin 2015Document17 pagesAustin 2015Premkumar RNo ratings yet
- J Perry Conf Proc 2005Document9 pagesJ Perry Conf Proc 2005Alex MartinezNo ratings yet
- Complementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionDocument10 pagesComplementary Split-Ring Resonator With Improved Dielectric Spatial ResolutionhesoyamyecgaaaNo ratings yet
- Batchelor McAuley Et Al 2015 ChemistryOpenDocument37 pagesBatchelor McAuley Et Al 2015 ChemistryOpenOmar ReynosoNo ratings yet
- X Ray Crystallography For Beginners UnderstandingDocument8 pagesX Ray Crystallography For Beginners Understandinghop_parasherNo ratings yet
- Unit VDocument8 pagesUnit VhyctortyNo ratings yet
- 2012 - Lucas, Riedo - Invited Review Article Combining Scanning Probe Microscopy With Optical Spectroscopy For Applications in Biology ADocument37 pages2012 - Lucas, Riedo - Invited Review Article Combining Scanning Probe Microscopy With Optical Spectroscopy For Applications in Biology AClaudio BiaginiNo ratings yet
- Automated Total and Radioactive Strontium Separation and Preconcentration in Samples of Environmental Interest Exploiting A Lab-On-Valve SystemDocument6 pagesAutomated Total and Radioactive Strontium Separation and Preconcentration in Samples of Environmental Interest Exploiting A Lab-On-Valve Systemnohora parradoNo ratings yet
- COMPOSITE and Nano Materials SASTRA University 1st YearDocument11 pagesCOMPOSITE and Nano Materials SASTRA University 1st Yearstar100% (1)
- Nano 2Document1 pageNano 2api-3777104No ratings yet
- Journal Name: CommunicationDocument3 pagesJournal Name: CommunicationzuopengxiangNo ratings yet
- Nanofabrication by Electron Beam Lithography and Its Application A Review PDFDocument16 pagesNanofabrication by Electron Beam Lithography and Its Application A Review PDFJulio HernandoNo ratings yet
- Lab-On-A-Chip, Microfluidics and Interfacial ElectrokineticsDocument6 pagesLab-On-A-Chip, Microfluidics and Interfacial ElectrokineticsAditya RaghunandanNo ratings yet
- Nanoparticle Characterization What To MeasureDocument26 pagesNanoparticle Characterization What To MeasureMarwaya EmamNo ratings yet
- LevitadorDocument7 pagesLevitadorWagner Pereira Lima PereiraNo ratings yet
- Sensors 21 00639 v3Document19 pagesSensors 21 00639 v3lcscosta2761No ratings yet
- Vogel2005ApplPhysB PDFDocument34 pagesVogel2005ApplPhysB PDFElektronika PMFNo ratings yet
- Semiconductor Nanoparticles ThesisDocument5 pagesSemiconductor Nanoparticles Thesisstefanieyangmanchester100% (2)
- Sensors and Actuators B - Chemical Volume Issue 2018 (Doi 10.1016 - j.snb.2018.03.091) Yang, Ruey-Jen Fu, Lung-Ming Hou, Hui-Hsiung - Review and Perspectives On Microfluidic Flow CytometersDocument59 pagesSensors and Actuators B - Chemical Volume Issue 2018 (Doi 10.1016 - j.snb.2018.03.091) Yang, Ruey-Jen Fu, Lung-Ming Hou, Hui-Hsiung - Review and Perspectives On Microfluidic Flow CytometersMunir AliNo ratings yet
- Mathematical Modeling and Simulation of Concentration Polarization Layer in Reverse Osmosis ProcessDocument5 pagesMathematical Modeling and Simulation of Concentration Polarization Layer in Reverse Osmosis ProcessSindhuja RaghunathanNo ratings yet
- Biotechnology: Low Reynolds NumbersDocument12 pagesBiotechnology: Low Reynolds NumbersChoti 2998No ratings yet
- Nanomaterials: Origin and Future of Plasmonic Optical TweezersDocument18 pagesNanomaterials: Origin and Future of Plasmonic Optical TweezersShailendra SinghNo ratings yet
- Cnrs ThesisDocument6 pagesCnrs Thesisandreaariascoralsprings100% (2)
- Accepted Manuscript: Sensors and Actuators BDocument30 pagesAccepted Manuscript: Sensors and Actuators BSamsouma BkNo ratings yet
- 1 s2.0 S0925400507001694 MainDocument7 pages1 s2.0 S0925400507001694 MainElif ŞahinNo ratings yet
- Microfluidics Meets MEMS: Elisabeth Verpoorte Nico F. de RooijDocument24 pagesMicrofluidics Meets MEMS: Elisabeth Verpoorte Nico F. de RooijArunNo ratings yet
- AO PaperDocument14 pagesAO PaperKumar AvinashNo ratings yet
- RFDC SputteringDocument4 pagesRFDC Sputteringसुरेन्द्र वशिष्ठNo ratings yet
- Ultrasonics-A Rapid, Fully Non-Contact, Hybrid System For Generating Lamb WaveDocument9 pagesUltrasonics-A Rapid, Fully Non-Contact, Hybrid System For Generating Lamb WaveMohammad HarbNo ratings yet
- New Sensors and Processing ChainFrom EverandNew Sensors and Processing ChainJean-Hugh ThomasNo ratings yet
- Class Xii Physics PaperDocument10 pagesClass Xii Physics Papergojo satoruNo ratings yet
- Physics 22Document20 pagesPhysics 22Kazuto ShibaNo ratings yet
- Navier StokesDocument36 pagesNavier Stokesvincent02hk_57881301No ratings yet
- Cathode MaterialsDocument8 pagesCathode Materialsnajem AlsalehNo ratings yet
- Summative Assessment - II: A. Choose The Correct OptionDocument6 pagesSummative Assessment - II: A. Choose The Correct OptionSanchit MukherjeeNo ratings yet
- Chapter 3Document23 pagesChapter 3kuppler7967No ratings yet
- Brandon Swanson - Trigonometry Test ReviewDocument5 pagesBrandon Swanson - Trigonometry Test ReviewladeeshaNo ratings yet
- Stoichiometry PowerPointDocument23 pagesStoichiometry PowerPointAngelaWillson100% (1)
- Bronze c54400 SpecificationsDocument3 pagesBronze c54400 SpecificationsRam Parimalam100% (1)
- The Compressible Euler System in Two Space Dimensions: Yuxi ZhengDocument92 pagesThe Compressible Euler System in Two Space Dimensions: Yuxi ZhengchyixxNo ratings yet
- Strength of Materials - Shear StressesDocument2 pagesStrength of Materials - Shear StressesJan Alexis Monsalud0% (1)
- Lenz LessDocument8 pagesLenz LessGindac Ralea100% (1)
- Governing Equations For Turbulent FlowDocument15 pagesGoverning Equations For Turbulent FlowRenz Marion CorpuzNo ratings yet
- Comparing Various NDT TechniquesDocument4 pagesComparing Various NDT TechniquesfenasikerimNo ratings yet
- 02 E1 Sticky TapeDocument6 pages02 E1 Sticky TapeRaviNo ratings yet
- Electric Field: Physics 16Document19 pagesElectric Field: Physics 16clndneNo ratings yet
- 2003, 94665723-New-energy-technologies-Issue-12 PDFDocument81 pages2003, 94665723-New-energy-technologies-Issue-12 PDFJosefina SilveyraNo ratings yet
- Problem Set: Strain and Thermal StressesDocument5 pagesProblem Set: Strain and Thermal StressesClint Charles P. BrutasNo ratings yet
- Topic 7.3: The Structure of MatterDocument3 pagesTopic 7.3: The Structure of MatterJerry DNo ratings yet
- 13 08 Divergence THM PDFDocument6 pages13 08 Divergence THM PDFMohamed Jamal AmanullahNo ratings yet
- Iesc 104Document11 pagesIesc 104Ty GravesNo ratings yet
- New Ideas That Changed EINSTEIN's TheoryDocument97 pagesNew Ideas That Changed EINSTEIN's TheorymobilecrackersNo ratings yet
- Cswip QbankDocument23 pagesCswip QbankFownoon100% (1)
- MSE 301 1stLongExam Chapter3Document2 pagesMSE 301 1stLongExam Chapter3Ilove PioneeringNo ratings yet
- Tension in A CordDocument4 pagesTension in A Cordkaveh-bahiraeeNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Ex 3Document3 pagesEx 3api-3708873No ratings yet
- Composite Materials and StructuresDocument35 pagesComposite Materials and StructuresGundoju SreenivasNo ratings yet
- Gatechemical 2012Document20 pagesGatechemical 2012Venkatesh ChNo ratings yet
- SW#2 Integrals of Trigonometric FunctionsDocument1 pageSW#2 Integrals of Trigonometric FunctionsRachel MonesNo ratings yet