Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
10 viewsIncho 2007 P7
Incho 2007 P7
Uploaded by
Beena JayThis document contains detailed solutions to problems from the INCHO 2004 P7 exam. It addresses questions about identifying the most basic species between various options, drawing the resonance structures of ozone, determining which molecules from earlier questions would have a non-zero dipole moment, explaining which of several arrangements is most stable, and using VSEPR theory to calculate valence electrons and draw the structures of SF4 and SF6 with their geometries.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Organic Chemistry II Practice Exam #3A Answer KeyDocument8 pagesOrganic Chemistry II Practice Exam #3A Answer Keyhiep237No ratings yet
- Materials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangDocument6 pagesMaterials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Scicent PPT 13 3 eDocument83 pagesScicent PPT 13 3 ear arNo ratings yet
- SI-Experimental and DFT Insights On Microflower G C3N4 BiVO4Document10 pagesSI-Experimental and DFT Insights On Microflower G C3N4 BiVO4smrutirekhaswainNo ratings yet
- Topics: Gases and ThermodynamicsDocument3 pagesTopics: Gases and ThermodynamicsHazoNo ratings yet
- Accepted Manuscript: Colloids and Surfaces A: Physicochem. Eng. AspectsDocument39 pagesAccepted Manuscript: Colloids and Surfaces A: Physicochem. Eng. AspectsSeptian Perwira YudhaNo ratings yet
- Instant Download PDF Introductory Chemistry Essentials 5th Edition Tro Test Bank Full ChapterDocument51 pagesInstant Download PDF Introductory Chemistry Essentials 5th Edition Tro Test Bank Full Chapterelalemasakia100% (4)
- Equilibria (With Solution)Document49 pagesEquilibria (With Solution)Nidhi SisodiaNo ratings yet
- A Novel Multifunctional Composite Based On Reduced Graphene Oxide, Poly-O-Phenylenediamine and Silicotungstic AcidDocument7 pagesA Novel Multifunctional Composite Based On Reduced Graphene Oxide, Poly-O-Phenylenediamine and Silicotungstic Acidelenium127No ratings yet
- The Oxford College of Engineering Department of Automobile Engineering - Lesson PlanDocument18 pagesThe Oxford College of Engineering Department of Automobile Engineering - Lesson PlanManjunatha EikilaNo ratings yet
- HS2 ND ChemistryDocument9 pagesHS2 ND ChemistrySachinNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- November 27, 2021 Wuhan: Se Ÿ KR + 2 EÿÿDocument23 pagesNovember 27, 2021 Wuhan: Se Ÿ KR + 2 EÿÿPhạm Nguyễn Minh TuấnNo ratings yet
- Germanium DioxideDocument6 pagesGermanium Dioxidelegendary tubeNo ratings yet
- Day Wise Planning For Class XII (2012-13) : Subject-ChemistryDocument3 pagesDay Wise Planning For Class XII (2012-13) : Subject-ChemistrySikander GirgoukarNo ratings yet
- Group 7 8dsDocument11 pagesGroup 7 8dsyyou12662No ratings yet
- Theory of ComputationDocument242 pagesTheory of ComputationSanjana AgarwalNo ratings yet
- CHEM 14D/Garg Spring 2015 Problem Set #6Document10 pagesCHEM 14D/Garg Spring 2015 Problem Set #6Hugh BangNo ratings yet
- ENGR 205, Spring 2012: Homework 4 (Part II) (Chapter 4)Document6 pagesENGR 205, Spring 2012: Homework 4 (Part II) (Chapter 4)iwuo4797No ratings yet
- Effect of H2O2 Addition On The Photocatalyst Properties of Ag3PO4 For Methylene Blue PhotodegradationDocument10 pagesEffect of H2O2 Addition On The Photocatalyst Properties of Ag3PO4 For Methylene Blue PhotodegradationFebiyantoNo ratings yet
- Basic Principles and Techniques of Organic ChemistryDocument15 pagesBasic Principles and Techniques of Organic Chemistryshalika42598No ratings yet
- JuvabioneDocument13 pagesJuvabionePreeti Yadav100% (1)
- Chapter - I - Atomic Structure: Additional Questions: Total - 36Document13 pagesChapter - I - Atomic Structure: Additional Questions: Total - 36kannan2030No ratings yet
- CH 5Document23 pagesCH 5balayogeshNo ratings yet
- Engineering Chemistry-1Document3 pagesEngineering Chemistry-1Supreet hiremaniNo ratings yet
- CH 212 (oRmeTT) Lec2Document8 pagesCH 212 (oRmeTT) Lec2Supun KahawaththaNo ratings yet
- University of Pretoria Universiteit Van Pretoria Department of Chemistry Departement ChemieDocument17 pagesUniversity of Pretoria Universiteit Van Pretoria Department of Chemistry Departement ChemieAneesaNo ratings yet
- Organic Photovoltaics: Materials, Device Physics, and Manufacturing TechnologiesFrom EverandOrganic Photovoltaics: Materials, Device Physics, and Manufacturing TechnologiesChristoph BrabecNo ratings yet
- A Novel Non-Enzymatic Dopamine Sensors Based On NiO-reduced Graphene Oxide Hybrid Nanosheets.Document8 pagesA Novel Non-Enzymatic Dopamine Sensors Based On NiO-reduced Graphene Oxide Hybrid Nanosheets.Đỗ MinhNo ratings yet
- Practice Exam3 PDFDocument9 pagesPractice Exam3 PDFdave lucasNo ratings yet
- StoichiometryDocument6 pagesStoichiometryvintu pvNo ratings yet
- Aldehydes and Ketones-12cDocument12 pagesAldehydes and Ketones-12cAlmira Kaye CuadraNo ratings yet
- Duragyp 13mm AA - VOC Emission Report - Indoor Air Comfort GoldDocument20 pagesDuragyp 13mm AA - VOC Emission Report - Indoor Air Comfort GoldDimitris KousoulasNo ratings yet
- JEE Main Previous Year Questions (2016 - 2023) : CHDocument73 pagesJEE Main Previous Year Questions (2016 - 2023) : CHlimitsdneNo ratings yet
- Chemical Reactions and Equations: Chapter-1Document59 pagesChemical Reactions and Equations: Chapter-1Mohit ShivhareNo ratings yet
- Cambridge Pre-U CertificateDocument12 pagesCambridge Pre-U Certificatelaksh bissoondialNo ratings yet
- Electronics 09 00650Document14 pagesElectronics 09 00650Doan Anh TuanNo ratings yet
- Jmse D 21 02352Document24 pagesJmse D 21 02352Aishik ChattopadhyayNo ratings yet
- CBSE Class 12 Chemistry Set 1 - N 2016Document14 pagesCBSE Class 12 Chemistry Set 1 - N 2016Santhosh KrishnaNo ratings yet
- Teknik Menjawab Kimia SPMDocument44 pagesTeknik Menjawab Kimia SPMFazza Rudy100% (1)
- Competing Reactions in Electrolysis Lesson ElementDocument18 pagesCompeting Reactions in Electrolysis Lesson Element23493No ratings yet
- Chem1Chap3L2-Chemical FormulasDocument16 pagesChem1Chap3L2-Chemical FormulasPatrixiah Monicah Mareight BaronNo ratings yet
- Cui2012 PDFDocument7 pagesCui2012 PDFabha abrahamNo ratings yet
- 14 Electrophilic AdditionsDocument49 pages14 Electrophilic Additionsmohammad_1102No ratings yet
- Inorganic Chemistry 2 Main Exam (3) and MemoDocument11 pagesInorganic Chemistry 2 Main Exam (3) and MemoKgasu MosaNo ratings yet
- Molecular Geometry NotesDocument5 pagesMolecular Geometry NotesAngel LaguraNo ratings yet
- 2014 Tos All Final YearDocument7 pages2014 Tos All Final YearGopi KupuchittyNo ratings yet
- 1 s2.0 S0360319920336053 MainDocument12 pages1 s2.0 S0360319920336053 MainShakoor MalikNo ratings yet
- Jurnal Modern Applied ScienceDocument5 pagesJurnal Modern Applied ScienceAndi nurwidiyahNo ratings yet
- 0620 Sow Unit 11 Redox Electrochemistry Group VIIDocument7 pages0620 Sow Unit 11 Redox Electrochemistry Group VIIPakardan TeaNo ratings yet
- Basic PDFDocument61 pagesBasic PDFGopinath B L Naidu0% (2)
- Series-Parallel Reduction Method: By: Ms. Noor Farhana Halil Binti Abdul RazakDocument61 pagesSeries-Parallel Reduction Method: By: Ms. Noor Farhana Halil Binti Abdul RazakDarveen AslenzNo ratings yet
- Copper-Catalyzed Cross-Coupling Interrupted by An Opportunistic Smiles Rearrangement: An Efficient Domino Approach To DibenzoxazepinonesDocument6 pagesCopper-Catalyzed Cross-Coupling Interrupted by An Opportunistic Smiles Rearrangement: An Efficient Domino Approach To DibenzoxazepinonesMuhammad AyazNo ratings yet
- 1 s2.0 S240584402101570X MainDocument9 pages1 s2.0 S240584402101570X MainOumaima BlNo ratings yet
- University of Pretoria: Universiteit VAN Pretoria Outeursreg Voorbehou 'Document10 pagesUniversity of Pretoria: Universiteit VAN Pretoria Outeursreg Voorbehou 'AneesaNo ratings yet
- Photocatalytic Degradation of The Light Sensitive Organic DyesDocument10 pagesPhotocatalytic Degradation of The Light Sensitive Organic DyesĐức Anh Nguyễn DuyNo ratings yet
- Russian Olympiads Organic CompilationDocument43 pagesRussian Olympiads Organic CompilationKiên TrầnNo ratings yet
- Integrated Master in Chemical EngineeringDocument74 pagesIntegrated Master in Chemical EngineeringRestia Eka PuspitaNo ratings yet
- Oxoacids of PhosphorusDocument12 pagesOxoacids of PhosphorusBeena JayNo ratings yet
- TrialDocument1 pageTrialBeena JayNo ratings yet
- Drop 13 SeprotationDocument16 pagesDrop 13 SeprotationBeena JayNo ratings yet
- Droplect 12 ThdecDocument20 pagesDroplect 12 ThdecBeena JayNo ratings yet
- Circular MotionDocument21 pagesCircular MotionBeena JayNo ratings yet
- Equivalent Concept - Titration APSPDocument20 pagesEquivalent Concept - Titration APSPBeena JayNo ratings yet
- ChemistryDocument108 pagesChemistryBeena JayNo ratings yet
- Zelda MinihuntDocument3 pagesZelda MinihuntBeena JayNo ratings yet
- DocScanner Jan 27, 2023 6-36 PMDocument1 pageDocScanner Jan 27, 2023 6-36 PMBeena JayNo ratings yet
- 12th Chemistry Journal V2 - Eng (VisionPapers - In)Document117 pages12th Chemistry Journal V2 - Eng (VisionPapers - In)Beena JayNo ratings yet
- 12th Chemistry Lab Manual (EM) (VisionPapers - In)Document124 pages12th Chemistry Lab Manual (EM) (VisionPapers - In)Beena Jay100% (1)
- Current Electricity ExerciseDocument36 pagesCurrent Electricity ExerciseBeena JayNo ratings yet
- 6 by 6 Mathdoku: (Hard No. 25 and 26)Document2 pages6 by 6 Mathdoku: (Hard No. 25 and 26)Beena JayNo ratings yet
- Course - Planner - Prakhar-III With Test GridDocument2 pagesCourse - Planner - Prakhar-III With Test GridBeena JayNo ratings yet
- Reported Speech Tense Changes ChartDocument1 pageReported Speech Tense Changes ChartBeena Jay100% (1)
- O8sql - 4Document220 pagesO8sql - 4Beena JayNo ratings yet
- RDSharmaClass9thSolutions PDFDocument2 pagesRDSharmaClass9thSolutions PDFBeena JayNo ratings yet
Incho 2007 P7
Incho 2007 P7
Uploaded by
Beena Jay0 ratings0% found this document useful (0 votes)
10 views13 pagesThis document contains detailed solutions to problems from the INCHO 2004 P7 exam. It addresses questions about identifying the most basic species between various options, drawing the resonance structures of ozone, determining which molecules from earlier questions would have a non-zero dipole moment, explaining which of several arrangements is most stable, and using VSEPR theory to calculate valence electrons and draw the structures of SF4 and SF6 with their geometries.
Original Description:
Original Title
INCHO__2007_P7
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains detailed solutions to problems from the INCHO 2004 P7 exam. It addresses questions about identifying the most basic species between various options, drawing the resonance structures of ozone, determining which molecules from earlier questions would have a non-zero dipole moment, explaining which of several arrangements is most stable, and using VSEPR theory to calculate valence electrons and draw the structures of SF4 and SF6 with their geometries.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
10 views13 pagesIncho 2007 P7
Incho 2007 P7
Uploaded by
Beena JayThis document contains detailed solutions to problems from the INCHO 2004 P7 exam. It addresses questions about identifying the most basic species between various options, drawing the resonance structures of ozone, determining which molecules from earlier questions would have a non-zero dipole moment, explaining which of several arrangements is most stable, and using VSEPR theory to calculate valence electrons and draw the structures of SF4 and SF6 with their geometries.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 13
INCHO 2004 P7
Detailed Solution
GRAMOLY
January 15, 2022
GRAMOLY INCHO 2004 P7 January 15, 2022 1 / 13
Outline
1 INCHO 2004 P7
7.1
7.2
7.3
7.4
7.5
GRAMOLY INCHO 2004 P7 January 15, 2022 2 / 13
7.1
GRAMOLY INCHO 2004 P7 January 15, 2022 3 / 13
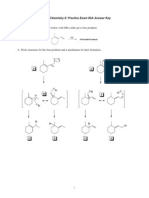
Solution to 7.1
Figure: HCN and N2 H4
Figure: CO2 and (CO3 )2−
GRAMOLY INCHO 2004 P7 January 15, 2022 4 / 13
Solution to 7.1
Most basic species = N2 H4
Because 1 lone pair is present on Nitrogen and moreover all other species
either have lone pair on oxygen or on more electronegative Nitrogen due to
which lone pair giving tendency ↓
GRAMOLY INCHO 2004 P7 January 15, 2022 5 / 13
7.2
Draw the resonance structure of ozone molecule.
GRAMOLY INCHO 2004 P7 January 15, 2022 6 / 13
Solution to 7.2
GRAMOLY INCHO 2004 P7 January 15, 2022 7 / 13
7.3
Which of the molecules in 7.1 and 7.2 may be expected to have a
non-zero dipole moment?
GRAMOLY INCHO 2004 P7 January 15, 2022 8 / 13
Solution to 7.3
Molecules with non-zero dipole: (HCN,N2 H4 , O3 )
GRAMOLY INCHO 2004 P7 January 15, 2022 9 / 13
7.4
GRAMOLY INCHO 2004 P7 January 15, 2022 10 / 13
Solution to 7.4
A is most stable because:
All Octets are complete.
No element is expending it’s capability to form minimum number of
bonds.
-ve is on most electronegative atom (i.e Oxygen)
GRAMOLY INCHO 2004 P7 January 15, 2022 11 / 13
7.5
Using VSEPR theory, calculate the total valence shell electrons in SF4 and
SF6 molecules. Draw structures with appropriate geometry of these
molecules and indicate the location/s of lone pair/s wherever present.
GRAMOLY INCHO 2004 P7 January 15, 2022 12 / 13
Solution to 7.5
Figure: SF4 and SF6
GRAMOLY INCHO 2004 P7 January 15, 2022 13 / 13
You might also like
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Organic Chemistry II Practice Exam #3A Answer KeyDocument8 pagesOrganic Chemistry II Practice Exam #3A Answer Keyhiep237No ratings yet
- Materials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangDocument6 pagesMaterials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Scicent PPT 13 3 eDocument83 pagesScicent PPT 13 3 ear arNo ratings yet
- SI-Experimental and DFT Insights On Microflower G C3N4 BiVO4Document10 pagesSI-Experimental and DFT Insights On Microflower G C3N4 BiVO4smrutirekhaswainNo ratings yet
- Topics: Gases and ThermodynamicsDocument3 pagesTopics: Gases and ThermodynamicsHazoNo ratings yet
- Accepted Manuscript: Colloids and Surfaces A: Physicochem. Eng. AspectsDocument39 pagesAccepted Manuscript: Colloids and Surfaces A: Physicochem. Eng. AspectsSeptian Perwira YudhaNo ratings yet
- Instant Download PDF Introductory Chemistry Essentials 5th Edition Tro Test Bank Full ChapterDocument51 pagesInstant Download PDF Introductory Chemistry Essentials 5th Edition Tro Test Bank Full Chapterelalemasakia100% (4)
- Equilibria (With Solution)Document49 pagesEquilibria (With Solution)Nidhi SisodiaNo ratings yet
- A Novel Multifunctional Composite Based On Reduced Graphene Oxide, Poly-O-Phenylenediamine and Silicotungstic AcidDocument7 pagesA Novel Multifunctional Composite Based On Reduced Graphene Oxide, Poly-O-Phenylenediamine and Silicotungstic Acidelenium127No ratings yet
- The Oxford College of Engineering Department of Automobile Engineering - Lesson PlanDocument18 pagesThe Oxford College of Engineering Department of Automobile Engineering - Lesson PlanManjunatha EikilaNo ratings yet
- HS2 ND ChemistryDocument9 pagesHS2 ND ChemistrySachinNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- November 27, 2021 Wuhan: Se Ÿ KR + 2 EÿÿDocument23 pagesNovember 27, 2021 Wuhan: Se Ÿ KR + 2 EÿÿPhạm Nguyễn Minh TuấnNo ratings yet
- Germanium DioxideDocument6 pagesGermanium Dioxidelegendary tubeNo ratings yet
- Day Wise Planning For Class XII (2012-13) : Subject-ChemistryDocument3 pagesDay Wise Planning For Class XII (2012-13) : Subject-ChemistrySikander GirgoukarNo ratings yet
- Group 7 8dsDocument11 pagesGroup 7 8dsyyou12662No ratings yet
- Theory of ComputationDocument242 pagesTheory of ComputationSanjana AgarwalNo ratings yet
- CHEM 14D/Garg Spring 2015 Problem Set #6Document10 pagesCHEM 14D/Garg Spring 2015 Problem Set #6Hugh BangNo ratings yet
- ENGR 205, Spring 2012: Homework 4 (Part II) (Chapter 4)Document6 pagesENGR 205, Spring 2012: Homework 4 (Part II) (Chapter 4)iwuo4797No ratings yet
- Effect of H2O2 Addition On The Photocatalyst Properties of Ag3PO4 For Methylene Blue PhotodegradationDocument10 pagesEffect of H2O2 Addition On The Photocatalyst Properties of Ag3PO4 For Methylene Blue PhotodegradationFebiyantoNo ratings yet
- Basic Principles and Techniques of Organic ChemistryDocument15 pagesBasic Principles and Techniques of Organic Chemistryshalika42598No ratings yet
- JuvabioneDocument13 pagesJuvabionePreeti Yadav100% (1)
- Chapter - I - Atomic Structure: Additional Questions: Total - 36Document13 pagesChapter - I - Atomic Structure: Additional Questions: Total - 36kannan2030No ratings yet
- CH 5Document23 pagesCH 5balayogeshNo ratings yet
- Engineering Chemistry-1Document3 pagesEngineering Chemistry-1Supreet hiremaniNo ratings yet
- CH 212 (oRmeTT) Lec2Document8 pagesCH 212 (oRmeTT) Lec2Supun KahawaththaNo ratings yet
- University of Pretoria Universiteit Van Pretoria Department of Chemistry Departement ChemieDocument17 pagesUniversity of Pretoria Universiteit Van Pretoria Department of Chemistry Departement ChemieAneesaNo ratings yet
- Organic Photovoltaics: Materials, Device Physics, and Manufacturing TechnologiesFrom EverandOrganic Photovoltaics: Materials, Device Physics, and Manufacturing TechnologiesChristoph BrabecNo ratings yet
- A Novel Non-Enzymatic Dopamine Sensors Based On NiO-reduced Graphene Oxide Hybrid Nanosheets.Document8 pagesA Novel Non-Enzymatic Dopamine Sensors Based On NiO-reduced Graphene Oxide Hybrid Nanosheets.Đỗ MinhNo ratings yet
- Practice Exam3 PDFDocument9 pagesPractice Exam3 PDFdave lucasNo ratings yet
- StoichiometryDocument6 pagesStoichiometryvintu pvNo ratings yet
- Aldehydes and Ketones-12cDocument12 pagesAldehydes and Ketones-12cAlmira Kaye CuadraNo ratings yet
- Duragyp 13mm AA - VOC Emission Report - Indoor Air Comfort GoldDocument20 pagesDuragyp 13mm AA - VOC Emission Report - Indoor Air Comfort GoldDimitris KousoulasNo ratings yet
- JEE Main Previous Year Questions (2016 - 2023) : CHDocument73 pagesJEE Main Previous Year Questions (2016 - 2023) : CHlimitsdneNo ratings yet
- Chemical Reactions and Equations: Chapter-1Document59 pagesChemical Reactions and Equations: Chapter-1Mohit ShivhareNo ratings yet
- Cambridge Pre-U CertificateDocument12 pagesCambridge Pre-U Certificatelaksh bissoondialNo ratings yet
- Electronics 09 00650Document14 pagesElectronics 09 00650Doan Anh TuanNo ratings yet
- Jmse D 21 02352Document24 pagesJmse D 21 02352Aishik ChattopadhyayNo ratings yet
- CBSE Class 12 Chemistry Set 1 - N 2016Document14 pagesCBSE Class 12 Chemistry Set 1 - N 2016Santhosh KrishnaNo ratings yet
- Teknik Menjawab Kimia SPMDocument44 pagesTeknik Menjawab Kimia SPMFazza Rudy100% (1)
- Competing Reactions in Electrolysis Lesson ElementDocument18 pagesCompeting Reactions in Electrolysis Lesson Element23493No ratings yet
- Chem1Chap3L2-Chemical FormulasDocument16 pagesChem1Chap3L2-Chemical FormulasPatrixiah Monicah Mareight BaronNo ratings yet
- Cui2012 PDFDocument7 pagesCui2012 PDFabha abrahamNo ratings yet
- 14 Electrophilic AdditionsDocument49 pages14 Electrophilic Additionsmohammad_1102No ratings yet
- Inorganic Chemistry 2 Main Exam (3) and MemoDocument11 pagesInorganic Chemistry 2 Main Exam (3) and MemoKgasu MosaNo ratings yet
- Molecular Geometry NotesDocument5 pagesMolecular Geometry NotesAngel LaguraNo ratings yet
- 2014 Tos All Final YearDocument7 pages2014 Tos All Final YearGopi KupuchittyNo ratings yet
- 1 s2.0 S0360319920336053 MainDocument12 pages1 s2.0 S0360319920336053 MainShakoor MalikNo ratings yet
- Jurnal Modern Applied ScienceDocument5 pagesJurnal Modern Applied ScienceAndi nurwidiyahNo ratings yet
- 0620 Sow Unit 11 Redox Electrochemistry Group VIIDocument7 pages0620 Sow Unit 11 Redox Electrochemistry Group VIIPakardan TeaNo ratings yet
- Basic PDFDocument61 pagesBasic PDFGopinath B L Naidu0% (2)
- Series-Parallel Reduction Method: By: Ms. Noor Farhana Halil Binti Abdul RazakDocument61 pagesSeries-Parallel Reduction Method: By: Ms. Noor Farhana Halil Binti Abdul RazakDarveen AslenzNo ratings yet
- Copper-Catalyzed Cross-Coupling Interrupted by An Opportunistic Smiles Rearrangement: An Efficient Domino Approach To DibenzoxazepinonesDocument6 pagesCopper-Catalyzed Cross-Coupling Interrupted by An Opportunistic Smiles Rearrangement: An Efficient Domino Approach To DibenzoxazepinonesMuhammad AyazNo ratings yet
- 1 s2.0 S240584402101570X MainDocument9 pages1 s2.0 S240584402101570X MainOumaima BlNo ratings yet
- University of Pretoria: Universiteit VAN Pretoria Outeursreg Voorbehou 'Document10 pagesUniversity of Pretoria: Universiteit VAN Pretoria Outeursreg Voorbehou 'AneesaNo ratings yet
- Photocatalytic Degradation of The Light Sensitive Organic DyesDocument10 pagesPhotocatalytic Degradation of The Light Sensitive Organic DyesĐức Anh Nguyễn DuyNo ratings yet
- Russian Olympiads Organic CompilationDocument43 pagesRussian Olympiads Organic CompilationKiên TrầnNo ratings yet
- Integrated Master in Chemical EngineeringDocument74 pagesIntegrated Master in Chemical EngineeringRestia Eka PuspitaNo ratings yet
- Oxoacids of PhosphorusDocument12 pagesOxoacids of PhosphorusBeena JayNo ratings yet
- TrialDocument1 pageTrialBeena JayNo ratings yet
- Drop 13 SeprotationDocument16 pagesDrop 13 SeprotationBeena JayNo ratings yet
- Droplect 12 ThdecDocument20 pagesDroplect 12 ThdecBeena JayNo ratings yet
- Circular MotionDocument21 pagesCircular MotionBeena JayNo ratings yet
- Equivalent Concept - Titration APSPDocument20 pagesEquivalent Concept - Titration APSPBeena JayNo ratings yet
- ChemistryDocument108 pagesChemistryBeena JayNo ratings yet
- Zelda MinihuntDocument3 pagesZelda MinihuntBeena JayNo ratings yet
- DocScanner Jan 27, 2023 6-36 PMDocument1 pageDocScanner Jan 27, 2023 6-36 PMBeena JayNo ratings yet
- 12th Chemistry Journal V2 - Eng (VisionPapers - In)Document117 pages12th Chemistry Journal V2 - Eng (VisionPapers - In)Beena JayNo ratings yet
- 12th Chemistry Lab Manual (EM) (VisionPapers - In)Document124 pages12th Chemistry Lab Manual (EM) (VisionPapers - In)Beena Jay100% (1)
- Current Electricity ExerciseDocument36 pagesCurrent Electricity ExerciseBeena JayNo ratings yet
- 6 by 6 Mathdoku: (Hard No. 25 and 26)Document2 pages6 by 6 Mathdoku: (Hard No. 25 and 26)Beena JayNo ratings yet
- Course - Planner - Prakhar-III With Test GridDocument2 pagesCourse - Planner - Prakhar-III With Test GridBeena JayNo ratings yet
- Reported Speech Tense Changes ChartDocument1 pageReported Speech Tense Changes ChartBeena Jay100% (1)
- O8sql - 4Document220 pagesO8sql - 4Beena JayNo ratings yet
- RDSharmaClass9thSolutions PDFDocument2 pagesRDSharmaClass9thSolutions PDFBeena JayNo ratings yet