Professional Documents
Culture Documents
Energy Efficiency Worksheet and Answers
Energy Efficiency Worksheet and Answers
Uploaded by
Женя МарченкоCopyright:
Available Formats
You might also like
- Kinetic and Potential Energy Worksheet Name - PDFDocument2 pagesKinetic and Potential Energy Worksheet Name - PDFsarahNo ratings yet
- Energy Systems Phet WorksheetDocument5 pagesEnergy Systems Phet Worksheetapi-203432401No ratings yet
- Chapter 5 Worksheets KeyDocument4 pagesChapter 5 Worksheets KeyMikhaela TorresNo ratings yet
- Leah Hartsock: Gravitational-Potential WorkDocument3 pagesLeah Hartsock: Gravitational-Potential WorkRHEAMAE GALLEGONo ratings yet
- Conservation of Energy: Potential Energy and Kinetic EnergyDocument5 pagesConservation of Energy: Potential Energy and Kinetic EnergyDewiNo ratings yet
- Che 311 - Specific Heats WorksheetDocument5 pagesChe 311 - Specific Heats WorksheetKier Deo NitafanNo ratings yet
- Energy Transformation GameDocument1 pageEnergy Transformation GameRitesh Sinha75% (4)
- Energy Transformation Ws PDFDocument4 pagesEnergy Transformation Ws PDFAndrea Jarani LinezoNo ratings yet
- Energy Efficiency WorksheetDocument3 pagesEnergy Efficiency Worksheetsupratik30No ratings yet
- Worksheet 1 - Energy EfficiencyDocument3 pagesWorksheet 1 - Energy EfficiencyJerell alexanderNo ratings yet
- Energy Stores and Transfers WorksheetDocument3 pagesEnergy Stores and Transfers WorksheetAndreas LambriasNo ratings yet
- Name: - Class: - DateDocument4 pagesName: - Class: - Dateaniahsefa11No ratings yet
- EfficiencyDocument2 pagesEfficiencyDanielGuimarãesCosta100% (1)
- Phyiscs Sankey Diagram WorksheetDocument1 pagePhyiscs Sankey Diagram WorksheetCosmos WithmeNo ratings yet
- Energy Efficiency WorksheetDocument2 pagesEnergy Efficiency WorksheetBen MasonNo ratings yet
- Sankey Diagram AnswersDocument7 pagesSankey Diagram AnswersSean TangNo ratings yet
- 3.4 Power & EfficiencyDocument2 pages3.4 Power & EfficiencyCurtis Collins50% (2)
- Worksheet For WorkDocument3 pagesWorksheet For Workreielleceana07No ratings yet
- 6 - Efficiency: Unit 7: Work, Energy and PowerDocument2 pages6 - Efficiency: Unit 7: Work, Energy and PowerEraser QueenNo ratings yet
- Physical Science Worksheet Conservation of Energy #2: KE MV GPE MGH Me Ke + GpeDocument4 pagesPhysical Science Worksheet Conservation of Energy #2: KE MV GPE MGH Me Ke + GpeJudy MelegritoNo ratings yet
- Work and Power Practice 2 AnswersDocument4 pagesWork and Power Practice 2 AnswersJanelleNo ratings yet
- Heat Capacity and Latent Heat QuestionsDocument2 pagesHeat Capacity and Latent Heat QuestionstuvvacNo ratings yet
- Maliyah Winston - Kinetic and Potential Energy WorksheetDocument3 pagesMaliyah Winston - Kinetic and Potential Energy Worksheetkaty collinsNo ratings yet
- Work and Power Ws 2Document2 pagesWork and Power Ws 2debbyhooi100% (3)
- Energy Transfer: Taking The Heat: PhysicsDocument18 pagesEnergy Transfer: Taking The Heat: PhysicsSaima HoqueNo ratings yet
- LP Energy BasicsDocument6 pagesLP Energy BasicsJedNo ratings yet
- Worksheet - Work and Power ProblemsDocument2 pagesWorksheet - Work and Power ProblemsDaisy Soriano PrestozaNo ratings yet
- Impulse and Momentum QuestionsDocument2 pagesImpulse and Momentum Questionsapi-301275445100% (1)
- CH Sankey Diagram WorksheetDocument1 pageCH Sankey Diagram WorksheetschlemielzNo ratings yet
- Work-Energy-Power QuestionsDocument5 pagesWork-Energy-Power QuestionsIftikhar Ahmed100% (1)
- Energy, Energy Transfer, and General Energy Analysis: Fundamentals of Thermal-Fluid SciencesDocument33 pagesEnergy, Energy Transfer, and General Energy Analysis: Fundamentals of Thermal-Fluid SciencesJoelle KharratNo ratings yet
- GPE and KE Worksheet #1Document2 pagesGPE and KE Worksheet #1Mohd FaridNo ratings yet
- CBSE Class 10 Physics Electricity Worksheet Set ADocument2 pagesCBSE Class 10 Physics Electricity Worksheet Set AlalitNo ratings yet
- Work Power EnergyDocument28 pagesWork Power Energymarife gupaalNo ratings yet
- Work and Power Worksheet KeyDocument1 pageWork and Power Worksheet KeyBahareh D. Deramus100% (2)
- Pearson - Science - 8 - SB - ENERGY Chapter - 5 - Unit - 5.1Document8 pagesPearson - Science - 8 - SB - ENERGY Chapter - 5 - Unit - 5.1loupoo80No ratings yet
- Lab 4 Calorimetry LabDocument6 pagesLab 4 Calorimetry Labapi-458764744No ratings yet
- Wasted Energy and EfficiencyDocument2 pagesWasted Energy and EfficiencyphydotsiNo ratings yet
- Worksheet Energy Changes Chemical Reactions ks3Document5 pagesWorksheet Energy Changes Chemical Reactions ks3trical27 tricalNo ratings yet
- Kinetic and Potential Energy Worksheet PDFDocument3 pagesKinetic and Potential Energy Worksheet PDFMya BNo ratings yet
- Calculating Kinetic and Potential EnergyDocument1 pageCalculating Kinetic and Potential EnergySietina Villanueva100% (1)
- Work and Power Practice ProblemsDocument1 pageWork and Power Practice ProblemsmagiclcjNo ratings yet
- Physics Worksheet For Grade - 10 (Part - 2) Ebenezer School (From K-G Up To Preparatory)Document6 pagesPhysics Worksheet For Grade - 10 (Part - 2) Ebenezer School (From K-G Up To Preparatory)YishakNo ratings yet
- Kinetic and Potential Energy WSDocument3 pagesKinetic and Potential Energy WSKiyu ImanNo ratings yet
- Calculating Work WorksheetDocument1 pageCalculating Work WorksheetMarivic Tanque SedayonNo ratings yet
- Latent Heat QuestionsDocument2 pagesLatent Heat QuestionsSatria HalimNo ratings yet
- Momentum and ImpulseDocument10 pagesMomentum and ImpulseYuniar AmaliaNo ratings yet
- Heat TransferDocument1 pageHeat TransferFernan SibugNo ratings yet
- 3.first Law of ThermodynamicsDocument5 pages3.first Law of ThermodynamicsVarun dhawanNo ratings yet
- Power, Work and Force IIDocument4 pagesPower, Work and Force IIchpwalkerNo ratings yet
- Power, Work, and Force IDocument4 pagesPower, Work, and Force Ichpwalker100% (4)
- Sankey DiagramsDocument2 pagesSankey Diagramsphydotsi100% (2)
- Conservation of Energy QuizDocument2 pagesConservation of Energy QuizJay Noma RamosNo ratings yet
- Kinetic and Potential Energy Worksheet Name: - KEYDocument3 pagesKinetic and Potential Energy Worksheet Name: - KEYBreana Shaw100% (1)
- Work Energy 1Document11 pagesWork Energy 1Bahril IlmiwanNo ratings yet
- Density Calculations ks3 ks4Document2 pagesDensity Calculations ks3 ks4sesma1No ratings yet
- 8.5 Final Review Key PDFDocument4 pages8.5 Final Review Key PDFAlex VongNo ratings yet
- 60 Mins Mains Electricity Exam Qs B+ With AnswersDocument20 pages60 Mins Mains Electricity Exam Qs B+ With AnswersmadhujayanNo ratings yet
- Ippe Round 1Document21 pagesIppe Round 1twometersNo ratings yet
- Problem Set 1-Special Term-Thermo and Fluid MechanicsDocument2 pagesProblem Set 1-Special Term-Thermo and Fluid MechanicsChristian Husmillo ValenzuelaNo ratings yet
Energy Efficiency Worksheet and Answers
Energy Efficiency Worksheet and Answers
Uploaded by
Женя МарченкоOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Energy Efficiency Worksheet and Answers
Energy Efficiency Worksheet and Answers
Uploaded by
Женя МарченкоCopyright:
Available Formats
Energy efficiency
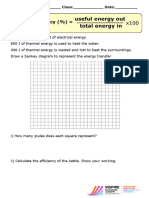
1 A 100 W light bulb produces 8 W of light.
a What happens to the other 92 W?
b Calculate its efficiency.
2 A 25 W low-energy light bulb produces 15 W of light. Calculate its efficiency.
3. An energy efficient bulb transfers 40% of the energy as useful light energy.
How much energy is transferred as heat?
4. A team of scientists test an “old banger” car. They find out that only 10% of the energy is transferred as
kinetic energy and 70% is transferred as heat energy. If the useful energy output was 300 000J, what was
the energy input (in J)?
5. A wood-burning power station is 12 % efficient. Calculate the input power necessary to produce an
output power of 1500 MW.
6.A new energy-saving kettle is claimed to be 95 % efficient. It needs 190 000 J of energy to boil a kettle full
of water at room temperature [190 000J has to be transferred to the water].
a How much energy will the kettle take from the mains?
b What happens to the rest of the energy?
Energy efficiency
1 A 100 W light bulb produces 8 W of light.
a What happens to the other 92 W? Lost as heat
b Calculate its efficiency.
8%
2. A 25 W low-energy light bulb produces 15 W of light. Calculate its efficiency.
60%
3. An energy efficient bulb transfers 40% of the energy as useful light energy.
How much energy is transferred as heat?
60%
4. A team of scientists test an “old banger” car. They find out that only 10% of the energy is transferred as
kinetic energy and 70% is transferred as heat energy. If the useful energy output was 300 000J, what was
the energy input (in J)?
3 000 000J
5. A wood-burning power station is 12 % efficient. Calculate the input power necessary to produce an
output power of 1500 MW.
12500 MW
6.A new energy-saving kettle is claimed to be 95 % efficient. It needs 190 000 J of energy to boil a kettle full
of water at room temperature [190 000J has to be transferred to the water].
a How much energy will the kettle take from the mains? 200 000J
b What happens to the rest of the energy? Lost as heat to surroundings
You might also like
- Kinetic and Potential Energy Worksheet Name - PDFDocument2 pagesKinetic and Potential Energy Worksheet Name - PDFsarahNo ratings yet
- Energy Systems Phet WorksheetDocument5 pagesEnergy Systems Phet Worksheetapi-203432401No ratings yet
- Chapter 5 Worksheets KeyDocument4 pagesChapter 5 Worksheets KeyMikhaela TorresNo ratings yet
- Leah Hartsock: Gravitational-Potential WorkDocument3 pagesLeah Hartsock: Gravitational-Potential WorkRHEAMAE GALLEGONo ratings yet
- Conservation of Energy: Potential Energy and Kinetic EnergyDocument5 pagesConservation of Energy: Potential Energy and Kinetic EnergyDewiNo ratings yet
- Che 311 - Specific Heats WorksheetDocument5 pagesChe 311 - Specific Heats WorksheetKier Deo NitafanNo ratings yet
- Energy Transformation GameDocument1 pageEnergy Transformation GameRitesh Sinha75% (4)
- Energy Transformation Ws PDFDocument4 pagesEnergy Transformation Ws PDFAndrea Jarani LinezoNo ratings yet
- Energy Efficiency WorksheetDocument3 pagesEnergy Efficiency Worksheetsupratik30No ratings yet
- Worksheet 1 - Energy EfficiencyDocument3 pagesWorksheet 1 - Energy EfficiencyJerell alexanderNo ratings yet
- Energy Stores and Transfers WorksheetDocument3 pagesEnergy Stores and Transfers WorksheetAndreas LambriasNo ratings yet
- Name: - Class: - DateDocument4 pagesName: - Class: - Dateaniahsefa11No ratings yet
- EfficiencyDocument2 pagesEfficiencyDanielGuimarãesCosta100% (1)
- Phyiscs Sankey Diagram WorksheetDocument1 pagePhyiscs Sankey Diagram WorksheetCosmos WithmeNo ratings yet
- Energy Efficiency WorksheetDocument2 pagesEnergy Efficiency WorksheetBen MasonNo ratings yet
- Sankey Diagram AnswersDocument7 pagesSankey Diagram AnswersSean TangNo ratings yet
- 3.4 Power & EfficiencyDocument2 pages3.4 Power & EfficiencyCurtis Collins50% (2)
- Worksheet For WorkDocument3 pagesWorksheet For Workreielleceana07No ratings yet
- 6 - Efficiency: Unit 7: Work, Energy and PowerDocument2 pages6 - Efficiency: Unit 7: Work, Energy and PowerEraser QueenNo ratings yet
- Physical Science Worksheet Conservation of Energy #2: KE MV GPE MGH Me Ke + GpeDocument4 pagesPhysical Science Worksheet Conservation of Energy #2: KE MV GPE MGH Me Ke + GpeJudy MelegritoNo ratings yet
- Work and Power Practice 2 AnswersDocument4 pagesWork and Power Practice 2 AnswersJanelleNo ratings yet
- Heat Capacity and Latent Heat QuestionsDocument2 pagesHeat Capacity and Latent Heat QuestionstuvvacNo ratings yet
- Maliyah Winston - Kinetic and Potential Energy WorksheetDocument3 pagesMaliyah Winston - Kinetic and Potential Energy Worksheetkaty collinsNo ratings yet
- Work and Power Ws 2Document2 pagesWork and Power Ws 2debbyhooi100% (3)
- Energy Transfer: Taking The Heat: PhysicsDocument18 pagesEnergy Transfer: Taking The Heat: PhysicsSaima HoqueNo ratings yet
- LP Energy BasicsDocument6 pagesLP Energy BasicsJedNo ratings yet
- Worksheet - Work and Power ProblemsDocument2 pagesWorksheet - Work and Power ProblemsDaisy Soriano PrestozaNo ratings yet
- Impulse and Momentum QuestionsDocument2 pagesImpulse and Momentum Questionsapi-301275445100% (1)
- CH Sankey Diagram WorksheetDocument1 pageCH Sankey Diagram WorksheetschlemielzNo ratings yet
- Work-Energy-Power QuestionsDocument5 pagesWork-Energy-Power QuestionsIftikhar Ahmed100% (1)
- Energy, Energy Transfer, and General Energy Analysis: Fundamentals of Thermal-Fluid SciencesDocument33 pagesEnergy, Energy Transfer, and General Energy Analysis: Fundamentals of Thermal-Fluid SciencesJoelle KharratNo ratings yet
- GPE and KE Worksheet #1Document2 pagesGPE and KE Worksheet #1Mohd FaridNo ratings yet
- CBSE Class 10 Physics Electricity Worksheet Set ADocument2 pagesCBSE Class 10 Physics Electricity Worksheet Set AlalitNo ratings yet
- Work Power EnergyDocument28 pagesWork Power Energymarife gupaalNo ratings yet
- Work and Power Worksheet KeyDocument1 pageWork and Power Worksheet KeyBahareh D. Deramus100% (2)
- Pearson - Science - 8 - SB - ENERGY Chapter - 5 - Unit - 5.1Document8 pagesPearson - Science - 8 - SB - ENERGY Chapter - 5 - Unit - 5.1loupoo80No ratings yet
- Lab 4 Calorimetry LabDocument6 pagesLab 4 Calorimetry Labapi-458764744No ratings yet
- Wasted Energy and EfficiencyDocument2 pagesWasted Energy and EfficiencyphydotsiNo ratings yet
- Worksheet Energy Changes Chemical Reactions ks3Document5 pagesWorksheet Energy Changes Chemical Reactions ks3trical27 tricalNo ratings yet
- Kinetic and Potential Energy Worksheet PDFDocument3 pagesKinetic and Potential Energy Worksheet PDFMya BNo ratings yet
- Calculating Kinetic and Potential EnergyDocument1 pageCalculating Kinetic and Potential EnergySietina Villanueva100% (1)
- Work and Power Practice ProblemsDocument1 pageWork and Power Practice ProblemsmagiclcjNo ratings yet
- Physics Worksheet For Grade - 10 (Part - 2) Ebenezer School (From K-G Up To Preparatory)Document6 pagesPhysics Worksheet For Grade - 10 (Part - 2) Ebenezer School (From K-G Up To Preparatory)YishakNo ratings yet
- Kinetic and Potential Energy WSDocument3 pagesKinetic and Potential Energy WSKiyu ImanNo ratings yet
- Calculating Work WorksheetDocument1 pageCalculating Work WorksheetMarivic Tanque SedayonNo ratings yet
- Latent Heat QuestionsDocument2 pagesLatent Heat QuestionsSatria HalimNo ratings yet
- Momentum and ImpulseDocument10 pagesMomentum and ImpulseYuniar AmaliaNo ratings yet
- Heat TransferDocument1 pageHeat TransferFernan SibugNo ratings yet
- 3.first Law of ThermodynamicsDocument5 pages3.first Law of ThermodynamicsVarun dhawanNo ratings yet
- Power, Work and Force IIDocument4 pagesPower, Work and Force IIchpwalkerNo ratings yet
- Power, Work, and Force IDocument4 pagesPower, Work, and Force Ichpwalker100% (4)
- Sankey DiagramsDocument2 pagesSankey Diagramsphydotsi100% (2)
- Conservation of Energy QuizDocument2 pagesConservation of Energy QuizJay Noma RamosNo ratings yet
- Kinetic and Potential Energy Worksheet Name: - KEYDocument3 pagesKinetic and Potential Energy Worksheet Name: - KEYBreana Shaw100% (1)
- Work Energy 1Document11 pagesWork Energy 1Bahril IlmiwanNo ratings yet
- Density Calculations ks3 ks4Document2 pagesDensity Calculations ks3 ks4sesma1No ratings yet
- 8.5 Final Review Key PDFDocument4 pages8.5 Final Review Key PDFAlex VongNo ratings yet
- 60 Mins Mains Electricity Exam Qs B+ With AnswersDocument20 pages60 Mins Mains Electricity Exam Qs B+ With AnswersmadhujayanNo ratings yet
- Ippe Round 1Document21 pagesIppe Round 1twometersNo ratings yet
- Problem Set 1-Special Term-Thermo and Fluid MechanicsDocument2 pagesProblem Set 1-Special Term-Thermo and Fluid MechanicsChristian Husmillo ValenzuelaNo ratings yet