Professional Documents
Culture Documents
SCIENCE Reviewer
SCIENCE Reviewer
Uploaded by
earlyn angelie gopio0 ratings0% found this document useful (0 votes)
8 views2 pagesPure substances can be either elements or compounds. Elements are composed of only one type of atom, while compounds are composed of two or more different types of atoms bonded together. Mixtures contain more than one pure substance that are not chemically bonded and can be either homogeneous, with a uniform composition throughout, or heterogeneous, with a non-uniform composition.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentPure substances can be either elements or compounds. Elements are composed of only one type of atom, while compounds are composed of two or more different types of atoms bonded together. Mixtures contain more than one pure substance that are not chemically bonded and can be either homogeneous, with a uniform composition throughout, or heterogeneous, with a non-uniform composition.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
8 views2 pagesSCIENCE Reviewer
SCIENCE Reviewer
Uploaded by
earlyn angelie gopioPure substances can be either elements or compounds. Elements are composed of only one type of atom, while compounds are composed of two or more different types of atoms bonded together. Mixtures contain more than one pure substance that are not chemically bonded and can be either homogeneous, with a uniform composition throughout, or heterogeneous, with a non-uniform composition.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
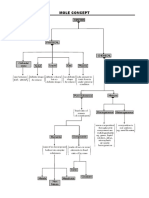
SCIENCE
Substances and Mixtures (page 61-67)
MATTER
Pure Substance Mixtures
Elements Compounds Homogeneous Heterogeneous
Metal Inorganic Solution Colloid
Nonmetal Organic Suspension
Metalloid
Matter- anything that occupies space and has mass.
Pure Substance- type of matter that has a fixed composition. Can be classified as an element or
compound.(1 atom/molecule)
Elements- composed of only one type of atom of an element. Ex. Calcium(Ca), Silver(Ag),
Gold(Au), Sodium(Na), Carbon(C ), Hydrogen(H), Helium(He) and Iron(Fe)
Compounds- composed of two or more atoms. Compounds can be broken down into simpler
compounds or elements by chemical changes or reactions. Ex. Water(H2O), Carbon Dioxide(CO2),
Table Salt(NaCl), Methane(CH4), Ozone(O3), Hydrogen Peroxide(H2O2), Ethylene(C2H4) and
Rust(Fe2O3)
You might also like
- Chemistry SPM DefinitionsDocument3 pagesChemistry SPM DefinitionsUchiha Kimono80% (5)
- Science 7 - CompoundsDocument16 pagesScience 7 - CompoundsKirk KinoNo ratings yet
- Adobe Scan 15-Jul-2023Document1 pageAdobe Scan 15-Jul-2023Rudra RathoreNo ratings yet
- Classification of MatterDocument30 pagesClassification of Mattershasagail100% (1)
- HTTPSBMC - unideb.hupublicdocuments2022!09!132BMCI Lecture Week2 Compounds Mixtures PDFDocument82 pagesHTTPSBMC - unideb.hupublicdocuments2022!09!132BMCI Lecture Week2 Compounds Mixtures PDFayoubNo ratings yet
- Difference Between Elements, Compounds, and Mixtures 7Document7 pagesDifference Between Elements, Compounds, and Mixtures 7jehoc53851No ratings yet
- Classification of Matter: J.T.Ii Olivar, Maed Faculty of Arts and Letters University of Santo TomasDocument7 pagesClassification of Matter: J.T.Ii Olivar, Maed Faculty of Arts and Letters University of Santo Tomasapi-3744104No ratings yet
- Reviewer in General Chemistry 2Document77 pagesReviewer in General Chemistry 2Ana Marie100% (1)
- Notes - Chemistry FoldableDocument21 pagesNotes - Chemistry Foldableapi-271661638No ratings yet
- CLASSIFICATION - OF - MATTER-week 2-pptshwDocument31 pagesCLASSIFICATION - OF - MATTER-week 2-pptshwAlyssa Crizel CalotesNo ratings yet
- Classification of Matters: (Klasifikasi Materi)Document12 pagesClassification of Matters: (Klasifikasi Materi)Fa UziNo ratings yet
- Elements Compounds MixturesDocument18 pagesElements Compounds MixturesDONABEL ESPANONo ratings yet
- Grade 9 Science - Notre Dame College School: John Dalton - The Guy Who Came Up With Particle TheoryDocument25 pagesGrade 9 Science - Notre Dame College School: John Dalton - The Guy Who Came Up With Particle Theorymonsieurkevin30No ratings yet
- Classifying Matter 1q770m1Document3 pagesClassifying Matter 1q770m1Silver RitzNo ratings yet
- Organizing MatterDocument13 pagesOrganizing Matterapi-449002661No ratings yet
- MH1 Che101 02 S2019Document162 pagesMH1 Che101 02 S2019Hazrat AliNo ratings yet
- Substances: Copper CoinDocument4 pagesSubstances: Copper CoinDane BosevNo ratings yet
- Elements, Compounds, and MixturesDocument53 pagesElements, Compounds, and MixturesAsma AkterNo ratings yet
- CLASSIFICATION OF MATTER RevisedDocument23 pagesCLASSIFICATION OF MATTER RevisedTrisha Rae GarciaNo ratings yet
- Topic 1 Stoichiometric RelationshipsDocument49 pagesTopic 1 Stoichiometric RelationshipsMohammad Andrew GhaniNo ratings yet
- Chapter 7Document53 pagesChapter 7rickyNo ratings yet
- CLASSIFICATION OF MATTER RevisedDocument42 pagesCLASSIFICATION OF MATTER RevisedBoni Almueda Valdez Jr.No ratings yet
- 1.some Basic ConceptsDocument18 pages1.some Basic ConceptsMUHAMMAD YASEENNo ratings yet
- Elements, Compounds & MixturesDocument2 pagesElements, Compounds & MixturesHarveyBaitanTabaqueNo ratings yet
- Classification of MatterDocument41 pagesClassification of MatterRicardo Jr. Uy100% (1)
- Chapter 1.1 - 1.3Document30 pagesChapter 1.1 - 1.3Karina Roxana Marquez TumeNo ratings yet
- The Organizational Chart of Matter and The PH LevelDocument8 pagesThe Organizational Chart of Matter and The PH Levelbatoyruby18No ratings yet
- Chemistry: Classifying MatterDocument3 pagesChemistry: Classifying MatterMa. Filipina AlejoNo ratings yet
- Elements Compounds MixturesDocument55 pagesElements Compounds MixturesFatima ?No ratings yet
- General and Physical Chemistry: Bishal GautamDocument12 pagesGeneral and Physical Chemistry: Bishal GautamNirupan neupaneNo ratings yet
- Chemistry Is Matter Around Us Pure Class 9Document7 pagesChemistry Is Matter Around Us Pure Class 9GauraviNo ratings yet
- Sections 1.1, 1.2, 1.3, 1.4,1.5Document17 pagesSections 1.1, 1.2, 1.3, 1.4,1.5gchanjrNo ratings yet
- Classification of Matter RevisedDocument42 pagesClassification of Matter Revisedyazan alsoradiNo ratings yet
- Che101 02 AbiDocument165 pagesChe101 02 Abiashahadat.h2002No ratings yet
- The Classification of Matter: Atoms, Elements, Molecules and CompoundsDocument16 pagesThe Classification of Matter: Atoms, Elements, Molecules and CompoundsChxmmyNo ratings yet
- Pure Substances Vs Mixtures (Recovered)Document11 pagesPure Substances Vs Mixtures (Recovered)Precious BuenafeNo ratings yet
- Chemistry Unit 1 - P1 - MatterDocument21 pagesChemistry Unit 1 - P1 - MatterMelina BazarNo ratings yet
- Elements and CompoundsDocument21 pagesElements and CompoundsAliah FaithNo ratings yet
- Topic 2: Atoms, Elements and Compounds: Najmiyatul Fadilah MohamadDocument23 pagesTopic 2: Atoms, Elements and Compounds: Najmiyatul Fadilah MohamadSamihah YaacobNo ratings yet
- Grade 7 Science: Pure Substances and MixturesDocument30 pagesGrade 7 Science: Pure Substances and MixturesGian Marc EnalisanNo ratings yet
- Chemistry-The IntroductionDocument24 pagesChemistry-The IntroductionHoney Marie BarnuevoNo ratings yet
- Ros Unit 2 Matter and EnergyDocument18 pagesRos Unit 2 Matter and EnergyNina GanapaoNo ratings yet
- Stochiometry-Jeemain Guru PDFDocument19 pagesStochiometry-Jeemain Guru PDFhimanshu yadavNo ratings yet
- Program For Entrance ExamDocument12 pagesProgram For Entrance ExamAkshay ThakurNo ratings yet
- Matter ClassifiedDocument20 pagesMatter Classifiedsandeep.pandeyNo ratings yet
- Classification of MatterDocument5 pagesClassification of Mattercherry shane abanesNo ratings yet
- Aim1 T What Is MatterDocument1 pageAim1 T What Is Matterapi-299809358No ratings yet
- (Klasifikasi Materi) : Classification of MattersDocument12 pages(Klasifikasi Materi) : Classification of MattersnopeyaniNo ratings yet
- Grade 7 Science: Mixtures & SolutionsDocument35 pagesGrade 7 Science: Mixtures & SolutionsKriss HeiNo ratings yet
- Elements Compound and Mixture Chemistry NotesDocument52 pagesElements Compound and Mixture Chemistry Noteseric sivaneshNo ratings yet
- 7 Archimedes 3rd Quarter 2Document100 pages7 Archimedes 3rd Quarter 2annikaclairechuaNo ratings yet
- Classificationofmatter 140910134732 Phpapp01Document4 pagesClassificationofmatter 140910134732 Phpapp01janneeshaaNo ratings yet
- Elements Compound MixtureDocument42 pagesElements Compound MixtureRecilia MarthaNo ratings yet
- Elements, Compounds, & MixturesDocument72 pagesElements, Compounds, & MixturesWendz ArominNo ratings yet
- Elements and AtomsDocument29 pagesElements and AtomsmalaitamanNo ratings yet
- Chemistry Unit 1Document8 pagesChemistry Unit 1aleksia.school8No ratings yet
- Lesson Pure SubstanceDocument25 pagesLesson Pure SubstanceCreamverly ArroyoNo ratings yet
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)