Professional Documents
Culture Documents
Alkaline Metals: Characteristics
Alkaline Metals: Characteristics
Uploaded by
Muhammad Ashraf Hafis Bin KamarudinOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alkaline Metals: Characteristics
Alkaline Metals: Characteristics
Uploaded by
Muhammad Ashraf Hafis Bin KamarudinCopyright:
Available Formats
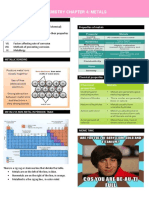
GROUP 1 ELEMENTS
CHARACTERISTICS ATOMIC RADII (nm)
Lowest first ionisation
energy and very high
second ionisation energy.
Soft metal
Metallic character
Low electronegativity
Ionic bond with fixed
oxidation state (+1)
Alkaline metals IONISATION ENERGY
ELECTROPOSITIVE/METALLIC
CHARACTER FLAME
COLOURATION
High tendency to lose
electron to form positive
ions
Metallic character Lowest IE in each
increase down the group period
Decrease down the
Li Na K Rb Cs group
(deep red) (yellow) (lilac) (bluish red) (purplish-blue)
BASIC OXIDES,
OXIDE, PEROXIDE, SUPEROXIDE
HYDROXIDES
ACTION WITH Alkaline
Alkaline
metals
metals ACTION WITH
WATER
HYDROGEN
All the alkali metals react directly with
Water and alkali metals react to
hydrogen to form hydrides which are ionic ACTION WITH HALOGEN form hydrogen and hydroxides.
in nature. While Li responds slowly, Na and the
To create ionic halides, all alkali others respond brutally. For this
metals immediately react with reason, they are stored in paraffin.
From Li to Cs, the ionic nature of
halogen. All are soluble and basic stength
hydrides increases.
Down the group, the reactivity of increases down the group
The stability of hydrides decreases
the halogen towards the alkali
from Li to Cs.
metal rises.
The latter increases from Li to Cs as a
result of the hydrides' strong lowering
actions.
GROUP 2 ELEMENTS
ATOMIC RADII (nm)
CHARACTERISTICS
Low first ionisation energy and
second ionisation energy.
Metallic character
low electronegativity
ionic bond with fixed oxidation state IONISATION ENERGY
(+2)
Alkaline Earth
MELTING POINTS :
Metallic bond depends on
metals Low 1st and 2nd EI
ionic radius, number of
removal of 3rd electron
electron contributed, crystal is harder
BASIC OXYDES AND
layyice structure HYDRATION EI decreases as the
HYDROXYDES ENTHALPY
: group descended
NOTE; Ca has high melting
point due tu d-orbital Decreases going down both EI of group 2 generally
participation in metallic groups higher than group 1
Hydration enthalpy of
bonding
Group 2 is higher than
group 1
THERMAL STABILITY
ACTION WITH HYDROGEN
REACTION OF HYDRIDES
All elements except Be react Increase down the group
All react with water due to Carbonates and hydroxides
directly with hydrogen
strong basic property of of group 1 are more stable
The reactivity increases down
hydride ion than group 2
the group
More stable if charge and
size of ions decreases
REACTION WITH OXYGEN
All alkaline earth metals except
barium form normal oxide
Ba form peroxide Alkaline Earth THE USES OF ELEMENT
CaO (calcium oxide) used in in
metals the manufacture of cement, in
the purification of sugar and
manufacture of dye stuffs
Calcium hydroxide in
FLAME COLOURATION REACTION WITH WATER manufactoring of building
SOLUBILITY
material, and used in white wash
Solubility of hydroxides as disinfectant.
increases down the group
Solubility of sulphates
increases up the group
You might also like
- Alkali and Alkali Earth Metals - SRDocument20 pagesAlkali and Alkali Earth Metals - SRMuzahidul IslamNo ratings yet
- F4-C4-Periodic Table of ElementsDocument23 pagesF4-C4-Periodic Table of ElementshkchungNo ratings yet
- Jee S BlockDocument129 pagesJee S BlockAmirtha RajNo ratings yet
- The S-Block Elements - Shobhit NirwanDocument14 pagesThe S-Block Elements - Shobhit NirwanAadarsh PandeyNo ratings yet
- Group Chemistry LEDocument152 pagesGroup Chemistry LERupokNo ratings yet
- Watermark Chemistry Igcse Notes 2 PDFDocument15 pagesWatermark Chemistry Igcse Notes 2 PDFMeerab ShahNo ratings yet
- Chemistry of Representive ElementsDocument26 pagesChemistry of Representive ElementsNatish JaglanNo ratings yet
- S Block ElementsDocument31 pagesS Block ElementsMuhammad AsgharNo ratings yet
- 11th Chemistry Study Materials English MediumDocument13 pages11th Chemistry Study Materials English Mediumprathiksha6660No ratings yet
- RedoxDocument39 pagesRedoxashleytham89No ratings yet
- S Block-1Document46 pagesS Block-1Jeevan KumarNo ratings yet
- Class 11 Chemistry Revision Notes The S-Block ElementsDocument40 pagesClass 11 Chemistry Revision Notes The S-Block ElementsNair SidharthNo ratings yet
- S-Block Elements & Compounds: Group - IDocument46 pagesS-Block Elements & Compounds: Group - Iविशाल जायसवालNo ratings yet
- S-Block Elements 2Document28 pagesS-Block Elements 2Aman SharmaNo ratings yet
- Chemistry of Elements Unit 3.1 8Document45 pagesChemistry of Elements Unit 3.1 8Zyra Erylle Rodriguez CapistranoNo ratings yet
- Chapter 1 PharChemDocument6 pagesChapter 1 PharChemno nameNo ratings yet
- S Block (Landscape)Document8 pagesS Block (Landscape)Drastic Pranksters Inc.No ratings yet
- Metals and Non MetalsDocument15 pagesMetals and Non Metalskebepef613No ratings yet
- Topic 1.3Document1 pageTopic 1.3duneraoreedNo ratings yet
- Chemistry Chapter 4: Metals: by Team Meow Meow (Halimeow and 3 Headed Creature)Document3 pagesChemistry Chapter 4: Metals: by Team Meow Meow (Halimeow and 3 Headed Creature)Bren Jousef BayhonNo ratings yet
- Chapter 4, ChemistryDocument2 pagesChapter 4, ChemistryWilliam ChongNo ratings yet
- 10 S Block Formula Sheets Getmarks AppDocument13 pages10 S Block Formula Sheets Getmarks Appsamsung.galaxy.tab.345cNo ratings yet
- INORGANIC Easy To Learn Handbook EnntuhDocument41 pagesINORGANIC Easy To Learn Handbook Enntuhsakthivelrrs1No ratings yet
- CH 10 ExerciseDocument24 pagesCH 10 ExerciseTr Mazhar PunjabiNo ratings yet
- Chapter 10 S Block Elements NCERT Class 11 SolutionsDocument20 pagesChapter 10 S Block Elements NCERT Class 11 SolutionsZagreus OfficialNo ratings yet
- Alkali Metals NotesDocument16 pagesAlkali Metals Notesboragam.saisharanyaNo ratings yet
- Alkali MetalsDocument32 pagesAlkali MetalsGhana Cintai DiaNo ratings yet
- Form 2 7 Alkali MetalsDocument24 pagesForm 2 7 Alkali MetalsHarshil PatelNo ratings yet
- Chemistry Notes (Metals)Document4 pagesChemistry Notes (Metals)Teo Jia Ming Nickolas75% (4)
- MIDTERMS - InOrgLec - Lesson 1Document4 pagesMIDTERMS - InOrgLec - Lesson 1Ayee Allyra Mocaram EspinosaNo ratings yet
- Alkali MetalsDocument31 pagesAlkali Metalsyamog86345No ratings yet
- 72bd0be0-7231-11ee-a64d-dba66c595fb0Document29 pages72bd0be0-7231-11ee-a64d-dba66c595fb0carla.habib7579No ratings yet
- S - Block Elements, Class 11Document13 pagesS - Block Elements, Class 11Ashish kumarNo ratings yet
- S BlockDocument53 pagesS BlockhappyNo ratings yet
- Inorganic-Chemistry ShortDocument41 pagesInorganic-Chemistry ShortAshwina JaikrishnanNo ratings yet
- S-Block Elements: Earth Metals. These Are So Called Because Their Oxides and Hydroxides Are Alkaline in NatureDocument8 pagesS-Block Elements: Earth Metals. These Are So Called Because Their Oxides and Hydroxides Are Alkaline in NatureAgamGoelNo ratings yet
- Atomic Radius: S-Block Elements The Elements Variation in Physical PropertiesDocument8 pagesAtomic Radius: S-Block Elements The Elements Variation in Physical PropertiesH.r. IndiketiyaNo ratings yet
- Periodic Table of Elements ChapterDocument10 pagesPeriodic Table of Elements ChapterReo RandoNo ratings yet
- The S-Block ElementsDocument34 pagesThe S-Block ElementsPrakhar TandonNo ratings yet
- S-Block Notes-1Document26 pagesS-Block Notes-1Kishore SurampalliNo ratings yet
- S Block ElementsDocument8 pagesS Block ElementsSwati Jadhav100% (3)
- 8B Group 1 2Document14 pages8B Group 1 2pediaNo ratings yet
- Ncert Solutions For Class 11 Chemistry Jan11 Chapter 10 The S Block ElementsDocument16 pagesNcert Solutions For Class 11 Chemistry Jan11 Chapter 10 The S Block Elementserfgtrgv vfvvvNo ratings yet
- 908 - CH 11 Alkali MetalsDocument42 pages908 - CH 11 Alkali MetalsPutri FatimahNo ratings yet
- S - and P-Block ElementsDocument8 pagesS - and P-Block Elementssameenrashid410No ratings yet
- Chemistry: Video Lectures Questions and Answers Problems Discussion (NEET, JEE)Document19 pagesChemistry: Video Lectures Questions and Answers Problems Discussion (NEET, JEE)Yahya RajputNo ratings yet
- 1CONCEPTS SUMMARY WITH QUESTIONS - Docx 2Document17 pages1CONCEPTS SUMMARY WITH QUESTIONS - Docx 2haiqaNo ratings yet
- Group 1 ElementsDocument5 pagesGroup 1 ElementsLeong Kit WaiNo ratings yet
- The Group 1 Elements: The Alkali Metals: "Read in The Name Your God Who Created " Chemistry of ElementsDocument44 pagesThe Group 1 Elements: The Alkali Metals: "Read in The Name Your God Who Created " Chemistry of ElementsFajar Sa'bandiNo ratings yet
- Chemical Changes LearnITDocument16 pagesChemical Changes LearnITIoana IonNo ratings yet
- IGCSE Chemistry Lecture 16 - Group 1 ElementsDocument12 pagesIGCSE Chemistry Lecture 16 - Group 1 Elementsnazzlor08No ratings yet
- Chapter 7: CorrosionDocument97 pagesChapter 7: CorrosionjavierNo ratings yet
- Subject: Chemistry Class: XI Chapter: The S-Block Elements Top ConceptsDocument10 pagesSubject: Chemistry Class: XI Chapter: The S-Block Elements Top ConceptsRISHI KEJRIWALNo ratings yet
- S BlockDocument20 pagesS BlockMeenakshi SuhagNo ratings yet
- S Block ElementsDocument16 pagesS Block Elementsyashvir.lko4963No ratings yet
- The S-Block ElementsDocument7 pagesThe S-Block ElementsSteveMathewKuruvillaNo ratings yet
- Complete S Block ElementsDocument108 pagesComplete S Block ElementsDrushya SalunkeNo ratings yet
- S-Block ElementDocument31 pagesS-Block ElementSiva ChamlingNo ratings yet
- Inorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionFrom EverandInorganic Hydrides: The Commonwealth and International Library: Chemistry DivisionNo ratings yet
- Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and TestingFrom EverandCorrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and TestingNo ratings yet
- Chemistry End of Term Revision Term 2Document18 pagesChemistry End of Term Revision Term 2sohaila ibrahimNo ratings yet
- JR VSA Q and A Wtih Star Marks (2023) PDFDocument42 pagesJR VSA Q and A Wtih Star Marks (2023) PDFShaik irfan basha Shaik irfan bashaNo ratings yet
- Data Booklet FinalDocument2 pagesData Booklet FinalLiqi FengNo ratings yet
- New Energy Technologies Issue 10Document77 pagesNew Energy Technologies Issue 10blameitontherain9877100% (1)
- General Chemistry: Chapter 2:atoms, Molecules, and IonsDocument13 pagesGeneral Chemistry: Chapter 2:atoms, Molecules, and IonsPhuong Nghi TruongNo ratings yet
- Solution Manual For Matrix Analysis of Structures 2nd Edition by KassimaliDocument36 pagesSolution Manual For Matrix Analysis of Structures 2nd Edition by Kassimalisecrecy.tetradic.0s46al100% (50)
- Inorganic Pharmaceutical Chemistry Page 1 of 19Document19 pagesInorganic Pharmaceutical Chemistry Page 1 of 19Mayrigen DominguezNo ratings yet
- PMC MDCAT Test 31 AugustDocument36 pagesPMC MDCAT Test 31 AugustEsha UwuNo ratings yet
- NEET Chemistry SyllabusDocument10 pagesNEET Chemistry SyllabusGaurav MittalNo ratings yet
- Study On Ash Fusion Temperature Using Original and Simulated Biomass AshesDocument6 pagesStudy On Ash Fusion Temperature Using Original and Simulated Biomass AshesPrakash WarrierNo ratings yet
- Is Coarse Aggregate and Fine AggregateDocument21 pagesIs Coarse Aggregate and Fine AggregateVishow SharmaNo ratings yet
- P.E.S. Pu CollegeDocument6 pagesP.E.S. Pu CollegeSamrudh BhaskarNo ratings yet
- Jee M ADocument70 pagesJee M AMallikarjuna SarmaNo ratings yet
- Advanced Chemistry For You Answers PDFDocument50 pagesAdvanced Chemistry For You Answers PDFpauljkt1No ratings yet
- Technique of Answering SPM Chemistry: Disediakan Oleh Chong Pei SiDocument8 pagesTechnique of Answering SPM Chemistry: Disediakan Oleh Chong Pei SichongpeisiNo ratings yet
- 2nd Year Short Questions Complete BookDocument99 pages2nd Year Short Questions Complete BookZain ZuhabNo ratings yet
- F.Y.B.Sc. Chemistry Syllabus PDFDocument26 pagesF.Y.B.Sc. Chemistry Syllabus PDFBhushan jadhavNo ratings yet
- HONORS Chapter 5 Test 2010 PDFDocument13 pagesHONORS Chapter 5 Test 2010 PDFAnonymous QfYZQyNo ratings yet
- Chemistry Notes Form 4 PDF Free PDFDocument28 pagesChemistry Notes Form 4 PDF Free PDFJane DoeNo ratings yet
- Chemistry SPM SyllabusDocument5 pagesChemistry SPM SyllabusAcyl Chloride HaripremNo ratings yet
- Nota Kimia Ting.4 Bab4Document9 pagesNota Kimia Ting.4 Bab4fauzan_evandraNo ratings yet
- Chemistry Formulas - List of Chemistry FormulasDocument34 pagesChemistry Formulas - List of Chemistry FormulasGirdhar TiwariNo ratings yet
- Question Bank On S-BLOCK ELMENTSDocument7 pagesQuestion Bank On S-BLOCK ELMENTSSnehaNo ratings yet
- Mod 2 BA PhysicsDocument412 pagesMod 2 BA PhysicsBenitoKameloNo ratings yet
- Chapter 10 The S-Block ElementsDocument18 pagesChapter 10 The S-Block ElementsYash PlayNo ratings yet
- Chemistry MetalsDocument100 pagesChemistry MetalsLilia Suruceanu RogojinaruNo ratings yet
- S-Block Elements L7Document11 pagesS-Block Elements L7Aryaman VyasNo ratings yet
- Chemistry Perfect Score Module Form 4 Set 2Document19 pagesChemistry Perfect Score Module Form 4 Set 2alanisln100% (1)