Professional Documents
Culture Documents
Chemical Formulae
Chemical Formulae
Uploaded by
Shasha Farzana0 ratings0% found this document useful (0 votes)
35 views4 pages Here are the key pieces of information that can be obtained from the balanced chemical equations:
1. Ba(OH)2 + 2HNO3 → Ba(NO3)2 +2H2O
(i) Barium hydroxide reacts with nitric acid
(ii) The products are barium nitrate and water
2. Cu (s)+ 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq)
(i) Copper (solid) reacts with silver nitrate (aqueous)
(ii) The products are silver (solid), copper nitrate (aqueous)
(iii) The coefficients in the balanced equation indicate the
Original Description:
Original Title
Chemical formulae
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document Here are the key pieces of information that can be obtained from the balanced chemical equations:
1. Ba(OH)2 + 2HNO3 → Ba(NO3)2 +2H2O
(i) Barium hydroxide reacts with nitric acid
(ii) The products are barium nitrate and water
2. Cu (s)+ 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq)
(i) Copper (solid) reacts with silver nitrate (aqueous)
(ii) The products are silver (solid), copper nitrate (aqueous)
(iii) The coefficients in the balanced equation indicate the
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
35 views4 pagesChemical Formulae
Chemical Formulae
Uploaded by
Shasha Farzana Here are the key pieces of information that can be obtained from the balanced chemical equations:
1. Ba(OH)2 + 2HNO3 → Ba(NO3)2 +2H2O
(i) Barium hydroxide reacts with nitric acid
(ii) The products are barium nitrate and water
2. Cu (s)+ 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq)
(i) Copper (solid) reacts with silver nitrate (aqueous)
(ii) The products are silver (solid), copper nitrate (aqueous)
(iii) The coefficients in the balanced equation indicate the
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 4
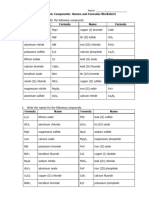
Chemical formulae
Sodium Aluminium Barium
chloride chloride chloride
Magnesium Zinc carbonate Ammonium
okside sulphate
Lead(II) Iron(II) Potassium
nitrate sulphate nitrate
Potassium Iron(III) oxide Sodium
carbonate sulphate
Potassium Tin(II) oxide Calcium
sulphate carbonate
Sodium nitrate Copper(I) Lithium
oxide chloride
Calcium Copper(II) Magnesium
chloride carbonate sulphate
Litium oxide Silver nitrate Aluminium
hydroxide
Zinc sulphate lead (II)
Zinc nitrate
bromide
Iron(II) Copper(II)
Iron(II) nitrate
hydroxide nitrate
iron (III) lead (II) Barium
chloride chloride hydroxide
lead (II) copper (II) potassium
sulphate sulphate iodide
Copper (II)
Silver chloride sodium oxide
chloride
Silver sulphate Barium nitrate Calcium nitrate
Barium Ammonium Magnesium
sulphate chloride chloride
Ammonium Potassium lead (II)
nitrate bromide chloride
Potassium Sodium copper (II)
chloride hydroxide sulphate
Sodium Calcium Argentum
carbonate hydroxide chloride
Calcium Magnesium
Barium nitrate
sulphate carbonate
Magnesium Ammonium
Zinc chloride
nitrate chloride
Aluminium iron (II) Potassium
sulphate chloride bromide
Sodium
Zinc chloride Sodium oxide
hydroxide
iron (II) Calcium
calcium nitrate
chloride hydroxide
lead (II) Magnesium Magnesium
bromide chloride carbonate
copper(II) Zinc bromide Phosphoric
nitrate Asid
Potassium Hydrogen
Ethanoic acid
hydroxide chloride
potassium Iron (III)
Ammonia
iodide sulphate
carbon silver
Sulfur dioxide
monoxide carbonate
Lithium Nitrogen
Oxygen Gas
chloride dioxide
Chlorine Gas Stanum(II)
water
oxide
Bromine Gas iron (II)
carbon dioxide
carbonate
Potassium Potassium
Silver
manganate dichromate(IV
hydroxide
(VII) )
Hydrogen
tin (II) nitrate Nitric acid
peroxide
Vanadium (V) Hydrochloric Sodium
oxide acid thiosulphate
Manganese Sulphuric Asid Hydrogen gas
(II) chloride
hydrogen calcium iron(II)
sulphide phosphate phosphate
Ammonium Iodine Magnesium
carbonate carbonate
Zinc oxide Calcium oxide Magnesium
iodide
magnesium zinc copper
Calcium Iron(III)oxide Aluminium
bromide hydroxide
Copper (II) copper (II) Water
chloride sulphate
Argentum Magnesium
Silver sulphate
chloride oxide
Barium Iron(III) oxide
Barium nitrate
sulphate
Potassium Ammonium Tin(II) oxide
nitrate chloride
Sodium iron (II) Copper(I)
sulphate chloride oxide
Calcium Copper(II)
Sodium oxide
carbonate carbonate
Lithium Silver nitrate
calcium nitrate
chloride
Magnesium Magnesium Silver oxide
sulphate chloride
Magnesium Hydrogen Potassium
fluoride chloride carbonate
Task 1 : write a balanced chemical equation.
1. magnesium + silver nitrate → magnesium nitrate + silver
2. iron(III) chloride + sodium carbonate → iron (III) carbonate + sodium chloride
3.aluminium nitrate → aluminium oxide + nitrogen dioxide + oxygen
4.ammonium chloride + calcium hydroxide → calcium chloride + water + ammonia
5.ammonia + lead (II) oxide → lead + water + nitrogen
6.carbon monoxide + lead(II) oxide → lead + carbon dioxide
7.sulphur dioxide + oxygen → sulphur trioxide
8.copper(II) nitrate + potassium carbonate → copper(II) carbonate + potassium nitrate
9.sulphuric acid + potassium hydroxide → potassium sulphate + water
10.lead(II) carbonate + nitric acid → lead(II) nitrate + carbon dioxide + water
11.hydrogen + iron(III) oxide → iron + water
12.copper(II) oxide + hydrochloric acid → copper(II) chloride + water
13.Lithium + oxygen → lithium oxide
14.sodium + water → sodium hydroxide + hydrogen
15.potassium bromide + chlorine → potassium chloride + bromine
16.calcium hydroxide + carbon dioxide → calcium carbonate + water
17.carbon dioxide + magnesium → magnesium oxide + carbon
18.lead(II) nitrate + potassium iodide → lead(II) iodide + potassium nitrate
19.zinc + copper(II) oxide → zinc oxide + copper
20. sodium + chlorine → sodium chloride
Task 2 : state the informations can be obtained from the equation.
1. Ba(OH)2 + 2HNO3 → Ba(NO3)2 +2H2O
(i)
(ii)
2. Cu (s)+ 2AgNO3 (aq) → 2Ag (s) + Cu(NO3)2 (aq)
(i)
(ii)
(iii)
You might also like
- Chemistry Study Notes Grade 10Document10 pagesChemistry Study Notes Grade 10Jynxx1387% (15)
- Reactions of Copper Experiment 6Document20 pagesReactions of Copper Experiment 6Noranisza Mahmud100% (10)
- Electrovalency Table PDFDocument2 pagesElectrovalency Table PDFPriscaNo ratings yet
- Quiz: Chemical Formulae Ionic Compound Formula Ionic Compound FormulaDocument1 pageQuiz: Chemical Formulae Ionic Compound Formula Ionic Compound FormulaCynthia RoneyNo ratings yet
- Exercise 1: MODUL 1: Formula KimiaDocument5 pagesExercise 1: MODUL 1: Formula KimiaMiesya87No ratings yet
- Module 2 A Topic 1 Ion Formulae & Composite Formulae With DATADocument2 pagesModule 2 A Topic 1 Ion Formulae & Composite Formulae With DATASheikh Ahmad KamalNo ratings yet
- Chemical Formula WorksheetDocument1 pageChemical Formula WorksheetAkash KaleNo ratings yet
- Lukis Struktur AtomDocument4 pagesLukis Struktur Atomu3kiNo ratings yet
- C3 Exercise 1Document8 pagesC3 Exercise 1Noor Liyana Ahmad FuadNo ratings yet
- Bab 1 Formula Sebatian & JMR JawapanDocument2 pagesBab 1 Formula Sebatian & JMR JawapanMaryati KematNo ratings yet
- Writing Ionic FormulaeDocument6 pagesWriting Ionic FormulaeKhondokar TarakkyNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- IonsnameonlyDocument1 pageIonsnameonlyLOUISE RICA LAGAHITNo ratings yet
- Valen CyDocument1 pageValen Cy12&13 SciencesNo ratings yet
- Test 1 Formula of IonsDocument6 pagesTest 1 Formula of IonsSEAW FUI MINGNo ratings yet
- Cations and Anions ListDocument2 pagesCations and Anions Listsamer qaziNo ratings yet
- WS 1 Mole - FormulaDocument6 pagesWS 1 Mole - FormulaSEAW FUI MINGNo ratings yet
- ChemDocument3 pagesChemhayleychan6202007No ratings yet
- OXIDATIONDocument1 pageOXIDATIONAdrian SwiftNo ratings yet
- Common IonsDocument2 pagesCommon Ionsnickloo55No ratings yet
- Cations: Al Aluminium Fe Iron (III) CR Chromium (III)Document2 pagesCations: Al Aluminium Fe Iron (III) CR Chromium (III)NPNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- CationsDocument2 pagesCationsOdd CatNo ratings yet
- Cations N AnionsDocument1 pageCations N AnionsgeelatifNo ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Main - Acids Bases and Salts Formulas and Names Cheat SheetDocument2 pagesMain - Acids Bases and Salts Formulas and Names Cheat SheetLorens NorNo ratings yet
- DANH PHÁP HÓA HỌC MỚIDocument6 pagesDANH PHÁP HÓA HỌC MỚILe Huy TranNo ratings yet
- Valency TableDocument1 pageValency TableRitesh SinghNo ratings yet
- Ly Thuyet Hoa 9 Co Ban 2024Document24 pagesLy Thuyet Hoa 9 Co Ban 202415. Võ Thị Mai Khanh. 7A2No ratings yet
- Anion Cation FormulaDocument1 pageAnion Cation FormulaharinistudentNo ratings yet
- Cations AnionsDocument1 pageCations AnionsTiviya Tarini ManiamNo ratings yet
- Chemical Formula of Binary Ionic Compounds US PDFDocument4 pagesChemical Formula of Binary Ionic Compounds US PDFAhmed HammadNo ratings yet
- Chemical Formula WorksheetDocument4 pagesChemical Formula WorksheetMaria adeelNo ratings yet
- Series Toolkit Unit 5 Ionic Charges Chart Cations and AnionsDocument1 pageSeries Toolkit Unit 5 Ionic Charges Chart Cations and AnionsokNo ratings yet
- VIII Chemistry PWS 2Document2 pagesVIII Chemistry PWS 2Ishama ZarintaNo ratings yet
- Common IonsDocument3 pagesCommon IonsabdallaaNo ratings yet
- Nomenclature Exercise AnswersDocument3 pagesNomenclature Exercise AnswersAh TseNo ratings yet
- Common Polyatomic IonsDocument1 pageCommon Polyatomic IonsRoddyNo ratings yet
- List of Common IonsDocument3 pagesList of Common IonsangelonicoNo ratings yet
- Chemical Formula Binary Ionic CompoundsDocument2 pagesChemical Formula Binary Ionic CompoundsRamisNo ratings yet
- Tabel IonDocument1 pageTabel IonAbu KamiliaNo ratings yet
- Metals With More Than One IonDocument2 pagesMetals With More Than One IonPATRICIA JULIANNE CASTAÑETO RIVERANo ratings yet
- Selected Ion ChartDocument1 pageSelected Ion Chartkyle_tosh3382No ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Basic Inorganic Nomenclature FOR IIT-JEE ENTRANCE TEST by S.K.sinha See Chemistry Animations atDocument5 pagesBasic Inorganic Nomenclature FOR IIT-JEE ENTRANCE TEST by S.K.sinha See Chemistry Animations atmyiitchemistry88% (17)
- PH Indicator Acid Neutral BaseDocument6 pagesPH Indicator Acid Neutral BaseYasser ZubaidiNo ratings yet
- Naming Ioniccovalent Compounds Ws 1Document4 pagesNaming Ioniccovalent Compounds Ws 1Pandu MuktiNo ratings yet
- AP Chem Ion List10Document1 pageAP Chem Ion List10AdamNo ratings yet
- Valence SheetDocument1 pageValence SheetQueenie BelleNo ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Compound Name Type of Bond Chemical Formula Alternate Name (If Applicable)Document2 pagesCompound Name Type of Bond Chemical Formula Alternate Name (If Applicable)anon-579447No ratings yet
- Negative Ions (Anions) Positive Ions (Cations)Document1 pageNegative Ions (Anions) Positive Ions (Cations)April Joy BallenerNo ratings yet
- Ion ChartDocument1 pageIon Charthimadrisingh12345No ratings yet
- Valency ChartDocument1 pageValency ChartAdam AzmiNo ratings yet
- Cations AnionsDocument2 pagesCations AnionsAngelica GementizaNo ratings yet
- 2018 - Modul Kimia JKD Sains Tulen (Kimia) Mersing18 PDFDocument24 pages2018 - Modul Kimia JKD Sains Tulen (Kimia) Mersing18 PDFSiti Hajar Abd HamidNo ratings yet
- Nitric Acid: Chemical Process IndustriesDocument13 pagesNitric Acid: Chemical Process Industries78623No ratings yet
- Chang Chemistry Chapter 3 QuestionsDocument14 pagesChang Chemistry Chapter 3 QuestionsBlanche Dauz100% (1)
- 01-Metals & Non MetalsDocument23 pages01-Metals & Non Metalssandeep kumar yadavNo ratings yet
- October 2017 (IAL) QP - Paper 4 Edexcel Chemistry A-LevelDocument28 pagesOctober 2017 (IAL) QP - Paper 4 Edexcel Chemistry A-LevelZarin NawarNo ratings yet
- Uop303 97Document7 pagesUop303 97Anix DiazNo ratings yet
- Presentation MSDS Nitric AcidDocument19 pagesPresentation MSDS Nitric Acidtatoo1No ratings yet
- 0654 s03 QP 3Document24 pages0654 s03 QP 3Saurabh ShresthaNo ratings yet
- Lecture: 38 Calcium Ammonium Nitrate Dr. N. K. PatelDocument4 pagesLecture: 38 Calcium Ammonium Nitrate Dr. N. K. PatelAnonymous NPKBxgyUAH100% (1)
- Chemical ResistanceDocument23 pagesChemical ResistancegarrybieberNo ratings yet
- Analysis of Heavy Metals in TeaDocument11 pagesAnalysis of Heavy Metals in Teaendalek50% (2)
- Stuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListDocument1 pageStuff I Should Know For The Ap Test But Do Not Know Yet: Ions ListShubham MangalNo ratings yet
- Previous Year Board Exam QuestionsDocument19 pagesPrevious Year Board Exam QuestionsRishabh AgarwalNo ratings yet
- Using Beer's Law To Determine Mass Percent of CuDocument3 pagesUsing Beer's Law To Determine Mass Percent of CuMuhammad MukhtarNo ratings yet
- E 353 - 93 R00 - RTM1MW - PDFDocument33 pagesE 353 - 93 R00 - RTM1MW - PDFDavid AriasNo ratings yet
- United States Patent (19) : Fisher, Both of Chester, All of VaDocument4 pagesUnited States Patent (19) : Fisher, Both of Chester, All of VaCh PrasadNo ratings yet
- IGCSE Chemistry Paper 0620 - s12 - QP - 63 PDFDocument12 pagesIGCSE Chemistry Paper 0620 - s12 - QP - 63 PDFjanova100% (1)
- Reverse Electroplating of SilverDocument2 pagesReverse Electroplating of SilverMohammad Umer AsgherNo ratings yet
- DST-4000 Data Sheet SAVILLEXDocument2 pagesDST-4000 Data Sheet SAVILLEXBriggitte FloresNo ratings yet
- Expt7 - Something-to-Fume-About-Cigarette - (Cigarette-Smoking-and-Air-Pollution)Document7 pagesExpt7 - Something-to-Fume-About-Cigarette - (Cigarette-Smoking-and-Air-Pollution)Rex BayonaNo ratings yet
- hn3 6 PDFDocument18 pageshn3 6 PDFKarina ElizabethNo ratings yet
- Water-Soluble Chlorides Present As Admixtures in Graded Aggregate Road MixesDocument3 pagesWater-Soluble Chlorides Present As Admixtures in Graded Aggregate Road MixesAhmed AbidNo ratings yet
- Absorber:: Reactions: N O 2no + O 3NO + H O + NoDocument4 pagesAbsorber:: Reactions: N O 2no + O 3NO + H O + NoShivam PandyaNo ratings yet
- FinetekDocument25 pagesFinetekWesley WesloqNo ratings yet
- Canadian Business English Canadian 7th Edition Guffey Solutions ManualDocument35 pagesCanadian Business English Canadian 7th Edition Guffey Solutions Manualpeanutsofteniscd1n100% (31)
- Testing Precious Metals C. M. Hoke Screen Readable.Document92 pagesTesting Precious Metals C. M. Hoke Screen Readable.Richard.nl100% (2)
- Dissolution of Uranium Dioxide in Nitric Acid MediaDocument13 pagesDissolution of Uranium Dioxide in Nitric Acid MediaMuhammad Riaz, 0092-3138432432No ratings yet
- File 1245136933 1705632985 S8 Worksheets Unit 8 Chemical ReactionsDocument12 pagesFile 1245136933 1705632985 S8 Worksheets Unit 8 Chemical ReactionspkshousingspvtltdNo ratings yet
- Nitric Acid Power PointDocument20 pagesNitric Acid Power Pointعلی محمد قادر خضرNo ratings yet